B-type natriuretic peptide and severity of cognitive disorder
Abstract
Background
Natriuretic peptides have been linked to cognitive disorder in previous studies. The aim of this study was to examine the association between the severity of cognitive disorder and the levels of B-type natriuretic peptide (BNP) in an older general population.
Material and methods
This study is a part of the larger population-based, multidisciplinary Kuopio 75+ health study. A total of 601 subjects aged 75 or older participated in the study. A subgroup of 126 individuals was diagnosed with cognitive disorder, and the severity of the disease was assessed. The participants were tested for BNP. Analysis of covariance was carried out to study the relationship between BNP and the stage of cognitive disorder.
Results
The association between the level of cognitive disorder and BNP resembled an inverse U-shaped curve, with higher levels of BNP observed among participants with mild cognitive disorder when compared to cognitively intact participants or counterparts with more severe cognitive disorder. This effect remained after adjustment for age (P = 0·02). However, association between BNP and level of cognitive disorder was lost in further adjustment with covariates connected to the levels of BNP.
Conclusion
The previously reported elevation of natriuretic peptides among individuals with diagnosed cognitive disorder was found only in people with milder stages of the disorder.
Introduction
In recent studies, natriuretic peptides, markers of direct cardiovascular stress, have outperformed blood pressure as an indicator of a cognitive disorder, cognitive decline and future cognitive disorder in older people 1-4. Both ageing and declining cognitive function are connected to several mechanisms with potential to reduce the stress on the heart chambers and thus decrease the blood levels of natriuretic peptides, such as the lowering of blood pressure, dehydration and reduced physical activity 5-8.
The relationship between blood pressure and cognitive decline is age dependent. In the middle age, hypertension has been found to predict cognitive dysfunction 9, 10, but in later life, low blood pressure associates with cognitive decline 11, 12. The levels of natriuretic peptides are known to increase with ageing 13-15. Furthermore, the prevalence and progression of cognitive disorder increase with ageing 16, 17. We have recently shown that the association between natriuretic peptides and cognitive disorder may be age dependent 18.
The possible association between the progression of cognitive disorder and natriuretic peptides – both age dependent and possibly linked to each other via many pathways – has not been previously addressed. We launched this study to examine the relationship between the severity of cognitive disorder and levels of B-type natriuretic peptides (BNP) in an older general population.
Material and Methods
Study population
This study is a part of the larger population-based, multidisciplinary Kuopio 75+ health study focusing on the clinical epidemiology of diseases as well as medications and the functional capacity of elderly persons aged 75 years or older. The target population was a stratified random sample (n = 700) of all the inhabitants of the City of Kuopio in Eastern Finland who were aged 75 years or more on 1 January 1998 (N = 4518).
The cohort included 700 participants. A total of 79 persons refused to take part in the study, five could not be contacted and 15 expired before the examination could take place. The remaining 601 participants formed the final study population. They attended a structured clinical examination and an interview conducted by a geriatrician and trained nurse during the year 1998. As a part of the diagnostic process, brain imaging either by computer tomography (CT) or magnetic resonance imaging (MRI) was carried out for all participants with a suspicion of dementive illness, but no brain imaging in the medical history. Mini-Mental State Examination (MMSE) test was performed guided by an experienced nurse. Cognitive disorder was diagnosed as Alzheimer's disease, vascular dementia, dementia with Lewy bodies or dementia due to other medical conditions according to the DSM-IV criteria and the consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies 19 by an experienced neurogeriatrician (R.S.). Cognitive disorder severity staging was assessed according to the DSM-III R criteria.
A geriatrician and trained nurse interviewed and examined the participants at the outpatient clinic of the municipal hospital concerning their medical history and use of medications and recorded the medications they were currently taking. If a participant was unable to visit the study site, the nurse and geriatrician visited the home to perform the interview and examination. Medical records from the municipal health centre, home nursing service, local hospitals and the Kuopio University Hospital were also available. Baseline clinical and demographic data were also recorded. Depressive symptoms were assessed with the Zung self-rated depression scale (SDS) in Finnish 20. Diabetes was defined as a diagnosis of diabetes in the medical records or a fasting plasma glucose level of 7·0 mM or more. Other cardiovascular conditions were recorded from medical records. Systolic and diastolic blood pressures were measured twice, and the average of the measurements was recorded.
Written informed consent was obtained from the study participants or their relatives as stipulated in the Declaration of Helsinki. The study was approved by the ethics committee of the Hospital District of Northern Savo and the Kuopio University Hospital.
Laboratory analyses
The blood samples for the natriuretic peptide analysis were withdrawn with other blood samples into chilled tubes containing 1·5 mg of K2-EDTA per mL of blood after the patient had stayed in a supine position for 30 min at 8 a.m. The procedure for the BNP analysis has been described previously 18. Other laboratory analyses were carried out at the clinical laboratory of Kuopio University Hospital using standard laboratory techniques.
Statistical analyses
Baseline characteristics are given for the participants divided into four groups by their status and stage of cognitive disorder. Comparisons of baseline characteristics between the groups with no cognitive disorder and those with different stages of cognitive disorder were made with the aid of anova or the Kruskal–Wallis test for continuous variables, based on whether the distribution was Gaussian. BNP levels were highly skewed and therefore logarithmically transformed in the analysis. A further comparison of BNP levels in each cognitive disorder group was corrected by the LSD post hoc method. The chi-squared test was used for categorical data.
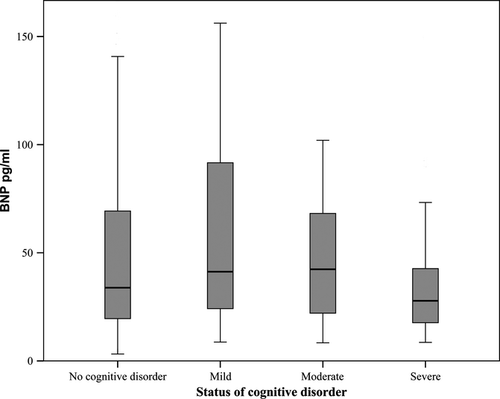
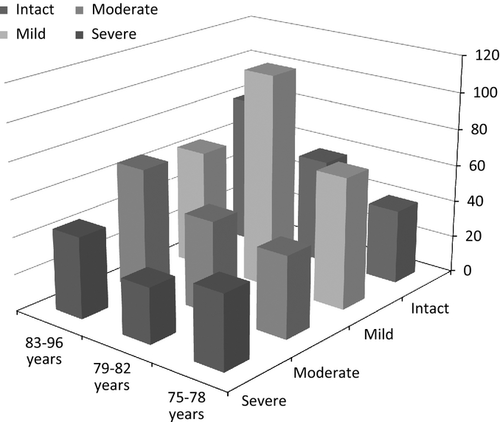
The impact of age, sex, creatinine clearance, systolic blood pressure, use of angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARB), use of diuretics and history of heart failure on BNP was calculated using linear regression analysis. All of these factors are known to have an effect on blood levels of BNP 21, 22. The association between the level of cognitive disorder and BNP resembled an inverse U-shaped curve in a box plot (Fig. 1), and therefore, the relationship was evaluated with age-adjusted analysis of covariance (ancova). Using the above-listed parameters with physiological association with BNP as covariates, a further ancova model was constructed to assess how these factors impacted the relationship between BNP and the level of cognitive disorder. A comparison of the levels of BNP at different stages of cognitive disorder in each age tertile was calculated with anova (Fig. 2). P < 0·05 was considered statistically significant. All analyses were performed with SPSS (release 17.0).
Reporting of the study conforms to the STROBE statement 23.
Results
General characteristics
A total number of 601 participants were included in this study. The general characteristics of the participants as divided into four groups by their status of cognitive disorder are presented in Table 1. Systolic and diastolic blood pressure was higher, the renal function was better, and the use of antihypertensive medication was the most common among individuals with no cognitive disorder. Those with cognitive disorder were older and had lower cholesterol and body mass index than their counterparts with no diagnosed cognitive disorder.
| Cognitive intact persons, n = 464 | Mild cognitive disorder, n = 48 | Moderate cognitive disorder, n = 50 | Severe cognitive disorder, n = 39 | P-value | |
|---|---|---|---|---|---|
| Age, mean (SD) | 80·0 (4·3) | 83·1 (5·7) | 82·5 (4·1) | 84·2 (4·5) | < 0·001 |
| Female, n (%) | 336 (72·4) | 37 (77·1) | 38 (76·0) | 32 (82·1) | 0·528 |
| Blood pressure systolic, mean (SD) mmHg | 153·1 (24·2) | 142·0 (20·9) | 132·3 (21·8) | 139·3 (23·4) | < 0·001 |
| Blood pressure diastolic, mean (SD) mmHg | 76·6 (10·8) | 72·9 (11·0) | 71·8 (11·1) | 72·7 (11·3) | 0·002 |
| Creatinine clearance, mean (SD) mL/min | 52·9 (15·4) | 43·6 (14·7) | 42·8 (14·6) | 40·4 (10·8) | < 0·001 |
| Smoking, n (%) | 121 (26·1) | 7 (14·6) | 13 (26·0) | 4 (10·3) | 0·352 |
| Education, years (SD) | 6·9 (3·5) | 5·9 (3·2) | 5·4 (2·1) | 4·0 (0·0) | 0·030 |
| MMSE, median (IQR) | 27 (24–29) | 19 (17–21) | 14 (12–17) | 0 (0–7) | < 0·001 |
| ZUNG, median (IQR) | 39 (33–45) | 42 (36–49) | 39 (34–50) | 46·5 (41–52) | 0·023 |
| BMI (SD) | 26·5 (4·5) | 24·9 (4·1) | 23·1 (3·8) | 24·8 (3·7) | < 0·001 |
| Lipids | |||||
| Cholesterol, mean (SD) mM | 5·7 (1·2) | 5·4 (1·2) | 5·2 (1·1) | 5·1 (1·0) | 0·001 |
| HDL cholesterol, mean (SD) mM | 1·5 (0·4) | 1·3 (0·4) | 1·4 (0·3) | 1·2 (0·3) | < 0·001 |
| Triglycerides, mean (SD) mM | 1·5 (0·8) | 1·4 (0·6) | 1·6 (1·3) | 1·5 (0·8) | 0·910 |
| Medication | |||||
| ACE inhibitors/ARBs, n (%) | 96 (26·1) | 6 (12·5) | 9 (18·0) | 2 (5·1) | 0·068 |
| Diuretics, n (%) | 161 (34·7) | 24 (50·0) | 24 (48·0) | 12 (30·8) | 0·051 |
| Beta-blockers, n (%) | 205 (44·2) | 18 (37·5) | 18 (36·0) | 6 (15·4) | 0·004 |
| Calcium channel blockers, n (%) | 83 (17·9) | 6 (12·5) | 1 (2·0) | 2 (5·1) | 0·006 |
| Statins, n (%) | 23 (5·0) | 0 (0) | 0 (0) | 0 (0) | 0·070 |
| Previous illnesses | |||||
| TIA, n (%) | 66 (14·2) | 9 (18·8) | 12 (24·0) | 5 (12·8) | 0·266 |
| Stroke, n (%) | 47 (10·1) | 6 (12·5) | 11 (22·0) | 8 (20·5) | 0·030 |
| Diabetes, n (%) | 94 (20·3) | 8 (16·7) | 8 (16·0) | 7 (17·9) | 0·834 |
| Atrial fibrillation, n (%) | 70 (15·1) | 10 (20·8) | 10 (20·0) | 6 (15·4) | 0·630 |
| Heart failure, n (%) | 113 (24·4) | 18 (37·5) | 20 (40·0) | 11 (28·2) | 0·034 |
| Peripheral vascular disease, n (%) | 50 (10·8) | 5 (19·0) | 4 (8·0) | 3 (7·7) | 0·876 |
| Myocardial infarction, n (%) | 149 (32·1) | 16 (33·3) | 22 (44·0) | 12 (30·8) | 0·394 |
| Cardiovascular disease, n (%) | 255 (55·0) | 28 (58·3) | 33 (66·0) | 24 (61·5) | 0·431 |
- ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; HDL, high-density lipoprotein; IQR, interquartile range; MMSE, Mini-Mental State Examination score; SD, standard deviation; TIA, transient ischaemic attack.
In univariable linear regression models, age, creatinine clearance, the use of diuretics, the use of ACE inhibitors/ARBs and a previous history of heart failure had a significant association with the level of BNP, while systolic blood pressure and sex were not significantly connected to BNP (Table 2).
| Beta (SE) | P-value | |
|---|---|---|
| Sex | −0·005 (0.035) | 0·901 |
| Age | 0·229 (0.003) | < 0·001 |
| Systolic blood pressure | −0·056 (0.001) | 0·180 |
| Creatinine clearance | −0·262 (0.001) | < 0·001 |
| Use of ACE inhibitors/ARBs | 0·172 (0.039) | < 0·001 |
| Use of diuretics | 0·130 (0.032) | 0·002 |
| History of heart failure | 0·318 (0.033) | < 0·001 |
- ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide.
The impact of the level of cognitive disorder on BNP is presented in Fig. 1. The association between the level of cognitive disorder and BNP resembled an inverse U-shaped curve, with participants suffering from mild cognitive disorder having higher levels of BNP in comparison to cognitively intact participants or counterparts with a more severe cognitive disorder (anova P = 0·077). In the post hoc LSD analysis, the levels of BNP were significantly higher in mild cognitive disorder compared with severe cognitive disorder (P = 0·012). In the same analysis, the levels of BNP showed a tendency towards being higher in mild cognitive disorder than in cognitively intact participants (P = 0·050). Other comparisons of the levels of BNP between the groups were not statistically significant.
In the age-adjusted ancova, the association between the level of cognitive disorder and the levels of BNP was significant (P = 0·02). We created a further multivariable ancova model where factors known to have a connection with BNP – age, sex, the use of ACE inhibitors or diuretics as well as creatinine clearance, mean systolic blood pressure and the history of heart failure – were included as covariates. In this model, BNP was no longer associated with the stage of cognitive disorder (P = 0·254).
The study population was divided into age group tertiles for further analysis: 75–78 years (n = 235), 79–82 years (n = 175) and 83 years or older (n = 191). The levels of BNP for each age tertile divided by their status of cognitive disorder are presented in Fig. 2. The levels of BNP resembled a U-shaped curve, being highest in the youngest and middle-age tertiles of the groups with mild cognitive disorder (anova P = 0·494 and P = 0·219, respectively). This U-shape tendency was lost in the oldest age tertile where the highest levels of BNP were detected among the cognitively intact participants (P = 0·047).
Discussion
Our main finding was that the association of cognitive disorder and BNP, a marker of cardiovascular stress, is dependent on the severity of cognitive disorder and resembles an inverse U-shaped curve, which is a novel observation. The blood levels of BNP were highest among participants with a mild form of cognitive disorder. In this group, BNP was higher than in the cognitively intact group, as expected based on previous studies 1-3, 24, 25. Individuals with more severe forms of cognitive disorder had lower levels of BNP when compared to those with mild cognitive disorder. Surprisingly, in patients with severe cognitive disorder, the levels of BNP were numerically even lower, albeit without statistical significance, than in cognitively intact participants.
If the study population was divided into age tertiles, the inverse U-shaped association between BNP and dementia stage was lost in the oldest tertile where the highest level of BNP was found in the cognitively intact group. This age dependency is in concordance with our earlier study where the association between cognitive disorder and BNP was shown to vary by age 18. Moreover, in concert with earlier studies, the presence and severity of cognitive disorder were connected to higher age, short education, low cholesterol and renal dysfunction 16, 26-30. In line with the previous studies, age and renal dysfunction were also connected to higher levels of BNP 21.
Blood pressure and cognitive disorder
In an older population, low diastolic blood pressure is related to an increased risk of developing a cognitive disorder 31, and the presence of cognitive disorder has been associated with low blood pressure 8. In the present study, the individuals included in the groups with any level of cognitive disorder had significantly lower systolic and diastolic blood pressure when compared to their cognitively intact counterparts.
Natriuretic peptides and cognition
Natriuretic peptides are secreted from the heart muscle cells in response to cardiovascular stress, and clinically, they are used in the diagnosis and follow-up of heart failure 21. The association between elevated levels of blood natriuretic peptides and both cognitive dysfunction and cognitive disorder has been demonstrated in several studies 2, 4, 24, 25, 32. In a study performed on the present older general population, BNP was shown to be superior to traditional cardiovascular risk factors in its predictive value for future cognitive disorder 3. In one study, elevated MR-proANP predicted the conversion of mild cognitive impairment (MCI) into AD in patients aged < 72 years, but not among older individuals 1. In our recent study, we demonstrated that the association between BNP and cognitive disorder was age dependent: the relationship was valid only among those under 79 years of age 18.
Older people often have an impaired ability to sense thirst 5, and chronic dehydration is common among the elderly, especially among cognitively impaired individuals 6. In a study with 890 patients suffering from the final phase of dementia, cachexia and dehydration were the immediate cause of death in 35·2% of all deaths occurred 33. Dehydration decreases the filling pressures and stretch of the left ventricle, which in turn attenuates the secretion of natriuretic peptides 21 and could partly explain why the individuals with a progressed cognitive disorder in our material had a decrease in their BNP levels. Also, history of heart failure was more frequent among subjects with mild or moderate cognitive disorder than in those cognitively intact or with severe cognitive disorder. This may also explain lower levels of BNP among those with severe cognitive disorder.
BNP is excreted from the body through the kidneys, and renal dysfunction is known to elevate the levels of natriuretic peptides 21. Dehydration, related to cognitive disorder, may explain, at least partly, the lowered creatinine clearance. In addition, creatinine clearance is known to decrease with ageing even without diagnosed renal disease 34, 35. In the present study, participants with cognitive disorder were older than the cognitively intact subjects, which may, for its part, explain the lowered creatinine clearance in those with a diagnosed cognitive disorder.
Study limitations, strengths and conclusions
As a study limitation, the number of participants in the subgroups was low, which decreased the power to detect significant differences in blood BNP levels between the groups. However, the total number of participants with a diagnosed cognitive disorder was superior to earlier studies on natriuretic peptides and cognitive dysfunction 2, 24, 25, 32, 36. The participants of the study were over 75 years of age, which is the clinically most relevant age group. The extrapolation of the results to other age groups or other ethnic groups besides Caucasians should be carried out with caution. The strengths of our study include the population-based approach and extensive characterization of the study population. The diagnoses and classification of cognitive disorders were carried out by an experienced geriatrician (R.S.), and brain imaging by CT or MRI was used routinely.
In conclusion, the previously reported elevation of natriuretic peptides among individuals with a diagnosed cognitive disorder occurs only in individuals with milder stages of the disorder. Comorbidities and behavioural changes connected to progressed cognitive disorder are the probable causes of the decrease in BNP levels that occurs in a more progressed cognitive disorder.
Acknowledgements
This work was supported by the Finnish Cultural Foundation.
Conflict of interest
The authors have no conflicts to disclose. The sponsors had neither a role in the analysis or interpretation of these data, nor in the content of the manuscript.
Author contributions
M.H. wrote the manuscript, designed the study and analysed the data. T.N. and T.K. designed the study, analysed the data, provided important intellectual comments on the manuscript and supervised. R.K. and R.S. collected the data and provided important intellectual comments on the manuscript. S.H. provided important intellectual comments on the manuscript and analysed the data. O.V. provided important intellectual comments on the manuscript.
Address
Department of Clinical Neurophysiology, Helsinki University Central Hospital, Haartmaninkatu 4, FI-00290 Helsinki, Finland (M. Hiltunen); Department of Internal Medicine, Päijät-Häme Central Hospital, Keskussairaalankatu 715850 Lahti, Finland (M. Hiltunen, R. Kettunen, T. Kerola); Department of Internal Medicine, Päijät-Häme Central Hospital, Keskussairaalankatu 715850 Lahti, Finland (T. Nieminen); Division of Cardiology, Helsinki University Central Hospital, Haartmaninkatu 4, FI-00290 Helsinki, Finland (T. Nieminen); Faculty of Pharmacy, Kuopio Research Centre of Geriatric Care, University of Kuopio, Yliopistonranta 1 C, P.O. Box 1627, FI-70210 Kuopio, Finland (S. Hartikainen); Leppävirta Health Centre, Savonkatu 17, 79100 Leppävirta, Finland (S. Hartikainen); Division of Geriatrics, School of Public Health and Clinical Nutrition, University of Kuopio, P.O. Box 1627, FI-70211 Kuopio, Finland (R. Sulkava); Department of Neurology, Kuopio University Hospital, P.O. Box 100, FI-70029 Kuopio, Finland (R. Sulkava); Department of Physiology, Faculty of Medicine, Biocenter Oulu, University of Oulu, P.O. Box 5000, FI-90014 Oulu, Finland (O. Vuolteenaho).




