Inter-center reproducibility of standard and advanced echocardiographic parameters in the EACVI-AFib echo registry
Abstract
Aim
we sought to test the inter-center reproducibility of 16 echo laboratories involved in the EACVI-Afib Echo Europe.
Methods
This was done on a dedicated setting of 10 patients with sinus rhythm (SR) and 10 with persistent atrial fibrillation (AF), collected by the Principal Investigator. Images and loops of echo-exams were stored and made available for labs. The tested measurements included main echo-Doppler parameters, global longitudinal strain (GLS) and peak atrial longitudinal strain (PALS).
Results
Single measures interclass correlation coefficients (ICCs) of left ventricular mass and ejection fraction were suboptimal in both patients with SR and AF. Among diastolic parameters, ICCs of deceleration time were poor, in particular in AF (=.50). ICCs of left atrial size and function, besides optimal in AF, showed an acceptable despite moderate concordance in SR. ICC of GLS was .81 and .78 in SR and AF respectively. ICCs of PALS were suitable but lower in 4-chamber than in 2-chamber view. By depicting the boxplot of the 16 laboratories, GLS distribution was completely homogeneous in SR, whereas GLS of AF and PALS of both SR and AF presented a limited number of outliers. GLS mean ± SE of the 16 labs was 19.7 ± .36 (95% CI: 18.8-20.4) in SR and 16.5 ± .29 (95% CI: 15.9-17.1) in AF, whereas PALS mean ± SE was 43.8 ± .70 (95% CI: 42.3-45.3) and 10.2 ± .32 (95% CI: 9.5-10.9) respectively.
Conclusion
While the utilization of some standard-echo variables should be discouraged in registries, the application of GLS and PALS could be largely promoted because their superior reproducibility, even in AF.
1 INTRODUCTION
The importance of multimodality imaging approach, and in particular of echocardiography, to the heart in patients with atrial fibrillation (AF) is recognized.1 The European Association of Cardiovascular Imaging (EACVI) AFib Echo Europe Registry is a multi-center European observational, cross-sectional registry which has been designed with the aim of evaluating the relationships of structural and functional parameters obtainable by transthoracic echocardiography with thromboembolic and bleeding risk profiles in patients with any kind (paroxysmal, persistent and permanent) of non-valvular atrial fibrillation (AF).2 By this registry, it is expectable to collect data on the echocardiographic phenotype of patients with AF and to test the level of agreement of different echocardiographic measurements with the available risk scores. Currently, twenty European centers decided to participate and are active in patient's enrollment.
When designing an echocardiographic registry involving several echocardiographic laboratories, quality control is fundamental to reduce measurement variability among laboratories. Accordingly, operators should have a certified expertise by applying appropriate procedures for data acquisition, storage and interpretation. Standardized approaches involving operator, equipment and training should be well established. The EACVI takes particular attention to these aspects, encouraging training programs and standardized procedures of centers devoted to research.3 In this view, the control of the inter-center reader reproducibility of different laboratories involved in a registry is of paramount importance. A low variability measurement is strongly warrant to increase the consistency of the studied parameters among the participating centers.
The objective of this study was to test the inter-center reproducibility of main standard and advanced echo-Doppler variables among the echocardiographic laboratories involved in EACVI Afib Echo Europe. This was done on a dedicated setting of patients with both sinus rhythm (SR) and history of paroxysmal AF and persistent AF, made available in the data-storing system of the echo lab of the Principal Investigator (PI).
2 METHODS
EACVI AFib Echo Europe Registry has been designed with the aim of assessing the relationship of echocardiographic measurements of left atrial (LA) size and function, left ventricular (LV) geometry, systolic and diastolic function with the clinical scores of thromboembolic and bleeding risks as evaluated by using CHA2DS2VASc and HASBLED scores. This is a multicenter registry involving 20 reference European echocardiographic laboratories, chosen among the accredited echo labs under the guidance of the European National Societies and Working Groups, along a period initially established to be of 6 months and subsequently prolonged for additional 6 months. The Registry was initially approved by the Ethical Committee of the PI (Federico II University Hospital of Naples, Italy, protocol 8/18) and then by the other participating centers. After obtaining their informed consent, all consecutive patients with non-valvular permanent, persistent or paroxysmal AF (with heart rate ranging between 60 and 100 bpm) and undergoing a transthoracic echocardiography exam will be enrolled in the echocardiographic labs. Exclusion criteria will be as follow: previous catheter or surgical ablation, LA appendage occlusion, cardiac surgery or percutaneous non-coronary interventional procedures, moderate-to-severe aortic and mitral valve stenosis, severe aortic and mitral valve regurgitation, aortic and mitral prosthesis, sepsis and inadequate quality echo images.
Before beginning the enrollment, the PI supplied a brief illustrative echocardiographic tutorial explaining how to perform all the measurements required in the registry (in agreement with the most recent EACVI recommendations)3, 4 to all participating centers in order to standardize and homogenize all the measurements obtained.
This training was preliminary to the acquisition by the same PI of echocardiographic images and raw-DICOM loops of 10 cases with SR and history of paroxysmal AF and 10 with permanent AF, randomly selected from a preliminary list of the 50 cases of patients with permanent AF or paroxysmal AF history. Echocardiographic acquisitions were performed according to the ASE/EACVI Chamber quantification recommendations and the ASE/EAE Expert consensus on myocardial mechanics.4, 5 These cases were transferred on a cloud secured storage platform (drop box) in order to make these available for the reading of all the participating centers. Parasternal long-axis M-mode screen of the left ventricle and left atrium, imaging of Doppler mitral inflow and pulsed Tissue Doppler of septal and lateral mitral annulus, as well as video-clips of apical long-axis, 4- and 2-chamber views were all stored in the drop box.
-
standard primary echo parameters (according to the American Society of Echocardiography (ASE)/EACVI recommendations on chamber quantification4 such as:
measurements of LV mass (septal and posterior wall thickness and LV internal end-diastolic diameter), and of LV ejection fraction (EF) (end-diastolic and end-systolic volume by Simpson biplane)
measurements of LA size: LA antero-posterior diameter, LA maximal and minimal volumes,
-
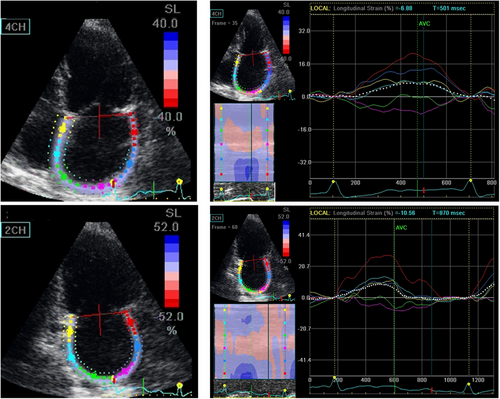
advanced echo parameters by using speckle tracking echocardiography such as LV global longitudinal strain (GLS)—as the average of 17-model regional strain recorded in apical long-axis, 4- and 2-chamber views—and peak atrial longitudinal strain (PALS), as the average of apical 4- and 2-chamber views. Regional LV longitudinal strain and GLS as well as LA regional strain and PALS measurements were performed according to the majority of EACVI/ASE/Industry Task Force standard criteria.7, 8 LA regional strain and PALS were measured at end-diastole (ECG trigger at the upslope of the R-wave), and the narrowest ROI was chosen in order to adequately track thin LA walls. PALS was obtained as the average of regional LA strain measured in apical 4- and 2-chamber views (Figure 1). Measurements of GLS and PALS were performed automatically, with the possible adjustment of border traces when judged to be needed by the reader. All the reported echocardiographic measurements were averaged from three cardiac cycles in patients in sinus rhythm and from five cardiac cycles during AF. Of note, the reproducibility of PALS was also tested separately in apical 4- and 2-chamber views.
2.1 Statistical analyses
Continuous data were expressed as mean ± standard deviation (SD). The reproducibility among the involved laboratories was tested by calculating single measures intra-class correlation coefficients (ICCs) and 95% confidence intervals (CI), separately on the data set of patients in SR and in those with AF. In fact, in the present study in which several echo labs were involved in the measurement reproducibility processes, the “single measures ICC” appear to be more appropriate than the “average measures” to verify the reliability of a scale that is scored by just several raters at one occasion. When using this kind of analysis, since ICC ranges from 0 to 1, an ICC close to 1 indicates high agreement of measurements whereas a low ICC close to zero means that measurements are not concordant. Accordingly, values <.5 are indicative of poor reliability, values between .5 and .75 indicate moderate reliability, values between .75 and .90 indicate good reliability, and values >.90 indicate excellent reliability. In addition, the distribution of specific variables in the individual echo labs was assessed by boxplot models. The standard error (SE) of the mean and the upper and lower 95% confidence interval (CI) among the involved echo laboratories was also used to calculate the range of variance for specific variables.
All statistical analyses were performed by the SPSS software, release 12 (SPSS Inc, Chicago, IL).
3 RESULTS
Sixteen echocardiographic laboratories (6 from Italy: Federico II University of Naples, San Raffaele Hospital and Niguarda Hospital of Milan, Le Scotte University Hospital of Siena, Madonna del Soccorso Hospital of San Benedetto del Tronto, and San Giovanni di Dio e Ruggi d'Aragona of, Salerno; 3 from Belgium: Centrum voor hart –en Vaatziekten, UZ Brussel, University of Liege Hospital, and OLV Clinic of Aalst; 2 from France: La Timone Hospital of Marseille, and Pontchaillou University Hospital of Rennes; 1 from Portugal: Hospital Da Luz of Lisbon: 1 from Croatia: University Hospital Center of Zagreb; 1 from Germany: University Hospital of Leipzig; 1 from Romania: “Prof. Dr. CC. Iliescu” Hospital of Bucharest; 1 from Turkey: University of Baskent, Ankara) participated to the reproducibility tests. The demographic characteristics of the two study groups (SR and AF) are summarized in Table 1.
| Sinus rhythm = 10 | ||
|---|---|---|
| Variable | Mean ± SD | Range |
| Sex (F/M) | 5/5 | |
| BMI (kg/m2) | 23.5 ± 3.4 | 18.2–27.7 |
| Systolic BP (mm Hg) | 116.7 ± 8.7 | 110.0–130.0 |
| Diastolic BP (mm Hg) | 70.0 ± 5.0 | 60.0–80.0 |
| Mean BP (mm Hg) | 85.6 ± 5.0 | 76.7–93.3 |
| Heart rate (bpm) | 68.0 ± 7.8 | 55.1–80.2 |
| Atrial fibrillation = 10 | ||
|---|---|---|
| Variable | Mean ± SD | Range |
| Sex (F/M) | 5/5 | |
| BMI (kg/m2) | 28.5 ± 4.8 | 22.1–36.4 |
| Systolic BP (mm Hg) | 125.0 ± 10.8 | 110.0–140.0 |
| Diastolic BP (mm Hg) | 79.5 ± 7.6 | 65.0–90.0 |
| Mean BP (mm Hg) | 94.7 ± 8.5 | 80–106.7 |
| Heart rate (bpm) | 76.2 ± 13.8 | 80.2–99.3 |
- Abbreviations: BMI, body mass index, BP, blood pressure.
Table 2 reports the reproducibility analysis of parameters of LV geometry and function. The single measures ICCs of LV mass (=.67) and LV EF (=.49) were suboptimal in both patients with SR and AF. Among diastolic parameters, the ICCs of E velocity DT were poor, in particular in patients with AF (=.50). The ICCs of GLS was .81 and .78 in patients with sinus rhythm and AF respectively.
| Patients in sinus rythm | |||
|---|---|---|---|
| Single measures | |||
| Parameter | ICC | 95% CI | P |
| LV Mass | .67 | .47–.88 | <.0001 |
| LV EF | .49 | .29–.77 | <.0001 |
| LV GLS | .81 | .65–.93 | <.0001 |
| E/A ratio | .72 | .53–.90 | <.0001 |
| DT | .61 | .40–.85 | <.0001 |
| e’ septal | .73 | .55–.90 | <.0001 |
| e’ lateral | .85 | .71–.95 | <.0001 |
| E/e’ ratio | .87 | .73–.97 | <.0001 |
| Patients in atrial fibrillation | |||
|---|---|---|---|
| Single measures | |||
| Parameter | ICC | 95% CI | P |
| LV Mass | .42 | .23–.72 | <.0001 |
| LV EF | .40 | .20–.75 | <.0001 |
| LV GLS | .78 | .61–.93 | <.0001 |
| E/A ratio | .72 | .53–.90 | <.0001 |
| DT | .50 | .29–.78 | <.0001 |
| e’ septal | .77 | .59–.92 | <.0001 |
| e’ lateral | .71 | .52–.89 | <.0001 |
| E/e’ ratio | .75 | .57–.91 | <.0001 |
- Abbreviations: CI, confidence interval, DT, deceleration time; EF, ejection fraction, GLS, global longitudinal strain, ICC, inter-class correlation coefficient, LV, left ventricular.
Table 3 lists the single measures ICC of LA size and function. The single measures ICCs, besides being optimal in patients with AF, showed an acceptable despite moderate concordance in patients with SR. Notably, the single measures ICCs of PALS were lower in 4-chamber than in 2-chamber view in the SR and in AF setting as well.
| Patients in sinus rhythm | |||
|---|---|---|---|
| Single measures | |||
| Parameter | ICC | 95% CI | P |
| LAD | .72 | .53–.89 | <.0001 |
| LAV max | .73 | .54–.90 | <.0001 |
| LAV min | .71 | .52–.89 | <.0001 |
| PALS 4CH | .71 | .51–.89 | <.0001 |
| PALS 2CH | .75 | .57–.91 | <.0001 |
| PALS AVG | .75 | .57–.91 | <.0001 |
| Patients in atrial fibrillation | |||
|---|---|---|---|
| Single measures | |||
| Parameter | ICC | 95% CI | P |
| LAD | .75 | .57–.91 | <.0001 |
| LAV max | .89 | .78–.97 | <.0001 |
| LAV min | .87 | .74–.96 | <.0001 |
| PALS 4CH | .71 | .51–.91 | <.0001 |
| PALS 2CH | .81 | .64–.94 | <.0001 |
| PALS AVG | .81 | .66–.94 | <.0001 |
- Abbreviations: CI, confidence interval, ICC, inter-class correlation coefficient, LAD, left atrial antero-posterior diameter; LAV max, maximum left atrial volume; LAV min, minimum left atrial volume; PALS 4CH, peak systolic atrial longitudinal strain in 4 apical chambers view; PALS 2 CH, peak atrial longitudinal strain in 2 chambers apical view; PALS AVG, average peak atrial systolic longitudinal strain (between 4CH and 2 CH).
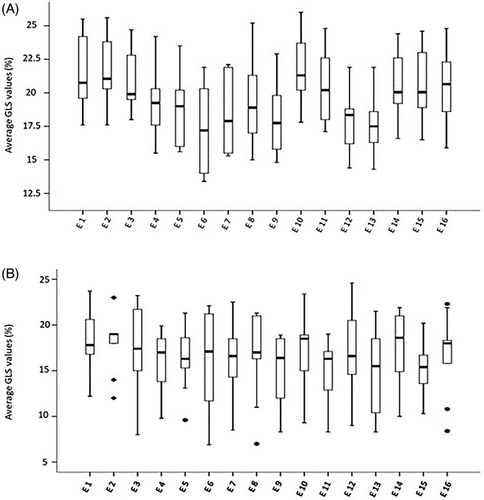
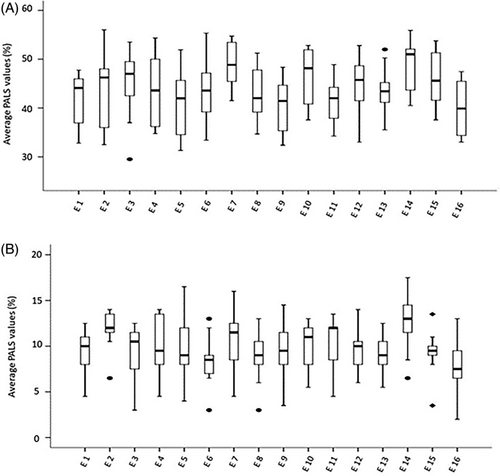
Figures 2 and 3 depict the boxplot distribution of GLS and PALS respectively in the 16 echo laboratories involved in the reproducibility tests. GLS distribution was completely homogeneous in SR patients, with a limited number of outliers in patients with AF. PALS presented a limited number of outliers in both patients with SR and AF. In addition, GLS mean ± SE of the 16 labs was 19.7 ± .36% (95% CI: 18.8-20.4) in patients in SR and 16.5 ± .29% (95% CI: 15.9-17.1) in patients with AF, whereas PALS mean ± SE was 43.8 ± .70% (95% CI: 42.3-45.3) and 10.2 ± .32% (95% CI: 9.5-10.9) respectively (data not in Figures). These findings show the very narrow range of variability of GLS and PALS, in both patients with SR and AF.
4 DISCUSSION
The analysis of inter-center reproducibility is a preliminary, fundamental condition for the start up and development of echocardiographic registries in which the measurements taken in different laboratories are collected and combined together for statistical end-points. To the best of our knowledge, we are the first to present this kind of reproducibility in separate settings of patients with SR and AF, before starting the recruitment of the EACVI AFibEcho Registry.2 Noteworthy, the reproducibility tests were performed by accredited, highly selected echocardiographic labs with consolidated expertise in both standard and advanced echocardiography. The tests were preceded by the supply of a tutorial provided by the PI to all the participating centers in order to homogenize both the acquisition and the reading procedures. Last but not least, a single vendor was chosen for both the imaging acquisition on the echocardiographic machines and the measurement reading by a dedicated work-station with the same updated release. This strict methodology and harmonization between co-investigators obviously limits the extension of the results to the overall panorama of the echocardiographic machines but also strengths the amount of the data collected in different echocardiographic laboratories.
The findings of the present study demonstrate that the inter-center reading reproducibility of echocardiographic Doppler parameters chosen is consistent with the aims of the EACVI AFib Echo Registry.2 In this context, the single measures ICCs were optimal for the majority of parameters whereas others showed a moderate concordance and only one (transmitral E velocity DT) a poor concordance.
Our data confirmed the suboptimal, large variability of some parameters such as LV mass and LV EF obtainable by standard echocardiography. The calculation of LV mass implies a geometric assumption and the use of 2D guided M-mode or direct 2D echocardiographic linear primary measurements, each on one with its own intrinsic variability.9 Accordingly, LV mass has a recognized large inter-observer variability (SE = 30.2 g, 95% CI: width = 59 g) and a poor inter-study (test–retest) reproducibility, with SDs of the difference between successive measurements ranging from 22 to 40 g (95% CI: 45−78 g).10 These findings are worse compared to previous results obtained by echo labs particularly devoted in this kind of measurement.11 In the present study, the single measures ICCs of LV mass showed moderate variability in the SR setting (.67), which became poor in patients with AF (.42). The large RR variability caused by AF can obviously provoke a detrimental impact on the primary linear measurements needed to determine LV mass. The poor reproducibility of 2D-echocardiographic derived LV EF is also well known and now confirmed in the present study, single measures ICCs being .49 in patients with SR and .40 in those with AF respectively. LV EF reproducibility was previously tested and both +7% of inter-observer reading variability12 and +10% of test-retest variability13 were observed. The suboptimal reproducibility of E velocity DT (single measures ICCs = .61 and .50 in patients with sinus rhythm and AF respectively) could also have been expected. Diastolic time intervals such as isovolumic relaxation and DT have shown a larger variability than Doppler-derived diastolic velocities in both multicenter and inter-study experiences.14, 15 This variability was obviously amplified in the setting of AF, due the RR variability own of this arrhythmia.16 Conversely, the reproducibility of the other diastolic parameters chosen for the EACVI-Afib Echo Registry was moderate (transmitral E/A ratio) to good (septal and lateral e’ velocity, E/e’ ratio). The reproducibility of GLS was good in patients with SR (single measures ICC = .81) and remained stable even in the AF setting (ICC = .78). In relation with its relative operator independency, GLS had previously shown a substantial good variability,17, 18 which is lower when compared with LV EF also in test-retest evaluations.13, 19 Of interest, the precision in GLS measurements has been recently shown to be improved after training, regardless the experience, in a multi-center study on an oncologic population.20 Besides LV EF, the use of GLS has been recently promoted as a key parameter of LV systolic function in the EACVI standardization of the adult echocardiography report.21
The reproducibility tests of LA size and function provided further information. According to analysis of single measure ICCs, the variability of all the chosen parameters, including PALS, was lower in patients with AF than in those with SR. Notably, the single measures ICCs of minimal LA volume, in particular in the SR setting (=.71), were lower than those of maximal LA volume. It is conceivable that the absence of an ECG reference point when measuring minimal LA volume, might have reduced the consistency of this measurement in comparison with the determination of maximal LA volume, which is addressed by the R wave onset at the ECG trace. The lower variability of PALS in permanent AF can be considered only partially unexpected. AF is a condition associated to larger LA cavity and easier cavity border detection and also to an intrinsically smaller deformation of LA walls than the SR state. It is also of interest that single measures ICCs of PALS were lower in apical 4- than in 2-chamber view. The tracing of LA cavity borders and the consequent regional strain of the 4-chamber view can be difficult to obtain accurately in correspondence of the atrial septum drop-out,7 a characteristic which could induce a lower grade of accuracy of apical 4-chamber derived PALS we found. An ASE/EACVI consensus document of the EACVI/ASE Industry Task Force to standardize deformation imaging has recently promoted the possible use of the single 4-chamber view for calculating PALS,8 a choice which does not seem to be supported from our findings. However, the reproducibility of average PALS based on single measures was good in both patients with SR (ICC = .75) and AF (ICC = .81). These results confirm the data previously found in single center studies,22-24 extending the information to a multicenter investigation. Of note, the good reproducibility of PALS we found reinforces the clinical value of this parameter which has been successfully used to characterize LA remodeling in patients experiencing paroxysmal AF25 and has a recognized prognostic role in the general population.26
Noteworthy, the suitable reproducibility of GLS and PALS was further strengthened by the boxplot models depicted in Figures 1 and 2—which showed a substantially homogeneous distribution of both speckle tracking derived parameters—and by the low SE and the very narrow range of 95% CI, independently on the rhythm condition.
In conclusion, the present study provides important information on the parameters which will be of a great importance in the analyses of EACVI Afib Echo Registry and also adds interesting insights on the general use of echo-Doppler measurements in multicenter registries. While the utilization of some traditional standard echocardiographic variables, currently applied in large epidemiologic and interventional trials—in particular LV mass and LV EF—should be discouraged in registries not provided of a central reading by a dedicated echocardiographic core lab, the application of advanced echocardiographic parameters obtainable by speckle tracking echocardiography (GLS and PALS) could be largely preferred because of their relatively operator independence, which facilitates the achievement of a suitable reproducibility even in patients with AF, a critical clinical setting in which the accuracy of measurements is mandatory.
ACKNOWLEDGMENTS
Special mention to: Maurizio GALDERISI disappeared before the end of the work that has been built with the EACVI and in close collaboration with Ciro SANTORO. After 2-year, it is probably important to publish this contribution and to remind the crucial role that Maurizio had for the EACVI and for the cardiac imaging community. Funding: European Association of Cardiovascular Imaging.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restriction.