The Ambulatory Medical Assistance (AMA) programme during active-phase treatment in patients with haematological malignancies: A cost-effectiveness analysis
Funding information: The study was funded by the Leon Berard Cancer Center, Lyon, France.
Abstract
Context
The need for patient navigator is growing, and there is a lack of cost evaluation, especially during survivorship.
Objective
The objective of this study is to evaluate the cost-effectiveness of an Ambulatory Medical Assistance (AMA) programme in patients with haematological malignancies (HM).
Design
A cost-effectiveness analysis of the AMA programme was performed compared to a simulated control arm.
Setting
An interventional, single-arm and prospective study was conducted in a French reference haematology–oncology centre between 2016 and 2020.
Participants
Adult patients were enrolled with histologically documented malignant haematology, during their active therapy phase, and treated either by intravenous chemotherapy or oral therapy.
Methods
An extrapolation of the effectiveness was derived from a similar nurse monitoring programme (CAPRI study). Cost effectiveness of the programme was evaluated through adverse events of Grade 3 or 4 avoided in different populations.
Results
Included patient (n = 797) from the AMA programme were followed during 125 days (IQR: 0–181), and adverse events (Grade 3/4) were observed in 10.1% of patients versus 13.4% in the simulated control arm. The overall cost of AE avoided was estimated to €81,113, leading to an ICER of €864.
Conclusion
The AMA programme was shown to be cost-effective compared to a simulated control arm with no intervention.
1 INTRODUCTION
Haematological malignancies (HM) encompass a variety of disease such as lymphoma, lymphoma B, multiple myeloma, Hodgkin's lymphoma (LH), chronic lymphocytic leukaemia (CLL) and other forms of lymphoma. They represent 13 new cases per 100,000 habitants worldwide (Defossez et al., 2019). About two-thirds of HM are lymphomas (LH and non-HL).
HM, and specifically aggressive lymphomas, are mostly treated with standard cytotoxic chemotherapy. While chemotherapy is associated with improvement in overall survival, it can induce life-threatening adverse events (AEs) (Habermann et al., 2006; Pfreundschuh et al., 2008). Severe neutropenia is the most critical treatment-related AE. Frequent but less severe AEs also include constipation, nausea, vomiting, mucositis and neuropathy (15% in the GELA study) (Coiffier et al., 2002).
The AEs reduce quality of life and sometimes lead to delays in chemotherapy schedules or even treatment discontinuation. This results in decreases in relative dose intensity (RDI) associated with a reduction in treatment efficacy and of HM survival (Hirakawa et al., 2010).
The AEs generally appear during the days following treatment administration when patients are back home and thus are generally managed by phone calls where the patients call the oncology unit and less commonly the primary care provider. Unscheduled patient calls lack reliability and urgency and cause inappropriate use of healthcare provider time (Formica et al., 2009; Marcus, 2002). The ability to guarantee the continuity of care, especially in oncology, is a major challenge to optimise the organisation of the health system.
Patient navigator (PN) programmes were developed in the United States in the 1990s as an organisational innovation to meet some of these challenges (Valaitis et al., 2017). PN programmes present a wide variety in terms of targeted patients, disease focus and implementation, but they all aim to achieve a patient-centred care approach to create a link between patients, their families, health services and primary care (Peart et al., 2018).
To improve the management of AEs in oncology, and more generally continuity of care for cancer patients, the Ambulatory Medical Assistance (AMA) programme was developed a decade ago in the Toulouse oncologic centre. This programme was described and evaluated in previous pilot studies (Compaci et al., 2011, 2015). The programme was a telephone-based follow-up procedure, based on scheduled calls to the patient's home by a certified oncology nurse. The aims of AMA were to provide healthcare quality, to promote therapeutic adherence (by encouraging patients and by providing strict control of their plans of care) and therapeutic education and to maintain patients at home, improve psychological support and use medical resources appropriately.
The first pilot study, in patients with large B-cell lymphoma, showed that most patients considered that AMA was an important contributor to their safety as well as better understanding of their disease and treatment plan. Nearly one-third of phone calls resulted in significant modifications of the planned supportive therapies, and substantial medical time was saving from oncologist (Compaci et al., 2011).
Based on these promising outcomes, a retrospective study was conducted to evaluate the impact of AMA programme on RDI in patients with CLL. The AMA programme tended to decrease the risk of dose reductions [RDI < 80%: 41.4% in non-AMA vs. 20.7% in AMA patients (p = 0.09)] and was significantly associated a reduction of the risk of reduced RDI (OR = 0.22, IC95% 0.05–0.84, p = 0.04) (Ysebaert et al., 2019).
Globally, systematic reviews showed PN improve clinical outcomes for a variety of chronic diseases and at different continuum (Bernardo et al., 2019). However, only few studies have been conducted on the economic evaluation of PN, and they focused only on colorectal cancer care. Although there have been conceptual models developed for evaluating the cost-effectiveness of PN programmes, there has been a lack of cost evaluation in PN studies (Gerves-Pinquie et al., 2018; Ramsey et al., 2009; Whitley et al., 2011).
The objective of this study was to evaluate the cost-effectiveness of the AMA programme specifically in patients with HM, carried out at the Léon Bérard cancer centre in the haematology unit.
2 METHODS
2.1 Setting and participants
Patients were enrolled in the study from March 2016 to March 2020 and were followed in the haematology unit from the specialised cancer centre of Léon Bérard (France). Patients were eligible according to the following criteria: age greater than or equal to 18 years and a histologically documented malignant haematology (lymphoma, lymphoma B, multiple myeloma, HL, CLL and other forms of lymphoma). Patients were all enrolled during their active therapy phase, treated either by intravenous chemotherapy or oral therapy (oral targeted agents). Participants had to have access to and ability to use a telephone. The study was approved by the Ethical Committee of Léon Bérard Center and compliant to regulatory on post hoc research studies. All patients gave written informed consent, according to the institutional review board procedure.
2.2 Intervention
The AMA programme was previously described (Compaci et al., 2011, 2015) and evaluated in a retrospective study in another French specialised oncologic centre for patients with HM (Ysebaert et al., 2019). This pilot study is an interventional, prospective, non-controlled and single-centre study.
All patients had a 1-h first visit with an oncologist before starting their therapy. This was followed by an additional visit with an oncology certified nurse. Three nurses were specifically dedicated to the AMA programme. The nurse visit aimed to describe modalities of care (planning of hospitalisation dates, biological follow-up), means of prevention and detection of side effects, emergency patient call procedure (oncology unit hot line, oncologist and AMA nurse e-mails) and finally the AMA programme. AMA consisted in calling patients at home at a set time and day twice a week. The duration of the call was on average 10 min. The AMA follow-up was stopped after the completion of all chemotherapy cycles, after 6 months in average.
This personalised follow-up aimed at coordinating inpatient and outpatient cares through different interventions: anticipate and manage risk at home; manage and detect as early as possible the toxicities that can be generated by treatment; ensure proper compliance with treatment; break isolation; and rationalise care. By this mean, they could encourage patients to visit their general practitioner (GP) or other supportive cares such as nutritionist, psychologist or social workers.
2.3 Comparator
To evaluate the cost-effectiveness of the programme, the effect size of a similar programme, the CAPRI programme, was applied to simulate an AMA controlled arm based. The CAPRI programme was used because of its similarities in terms of monitoring programme with the AMA programme and was previously described elsewhere (Gervès-Pinquié et al., 2017). Briefly, the CAPRI programme consisted in an intervention of nurse navigators in adult patients with cancer treated with oral anticancer agents in a specialised oncologic centre (Gustave Roussy, France). Nurses provided regular phone follow-ups to assess toxicities and manage symptoms, adherence and supportive care needs. Also, patients could use the digital CAPRI interface (Internet platform and mobile app) accessible to assigned outpatient healthcare professionals who were enabled to visualise and record all of their consultations. The intervention lasted 6 months maximum per patients, and patients were followed for 6 additional months.
The CAPRI programme was evaluated in a prospective, single-centre, randomised and controlled (clinical trial information: NCT02828462). The CAPRI study compared randomised patients to intervention or standard of care in a 1:1 basis. The RCT provided scientific evidence that a system combining digital technology and new human organisation significantly improves the clinical follow-up of patients treated with oral anticancer therapies. Intervention group showed significantly higher RDI (primary outcome) (93.4% vs. 89.4%, p = 0.04), the overall Grade 3–4 AEs were less frequent (27.6% vs. 36.9%, p = 0.02), and the number of hospital admissions was lower in the CAPRI group (15.1% vs. 22%, p = 0.04) (Mir et al., 2020).
2.4 Outcomes
Outcomes included for this cost-effectiveness study were focused on AEs having considerable impact on the organisation of care and leading to hospitalisation. Based on patient phone calls, AEs of Grades 3 and 4 were collected and classified into the following categories: haematology (neutropenia, anaemia, thrombopenia), digestive (diarrhoea, constipation, nausea, vomiting), neuropathy and dermatology (skin rash, mucositis). The AEs of Grades 1 and 2 were negligible from an economic perspective and therefore were excluded from the scope of this study. Additional supportive care related to pain, nutrition, psychology, medico-social, palliative or general order was also included and provided by the CAPRI trial.
2.5 Effect size
To elaborate a simulated control arm for the AMA programme, the generalisation of the efficacy outcome of Grade 3–4 AE from the CAPRI programme was applied. The intervention effect compared to standard of care was derived from the RCT evaluating the CAPRI programme. Despite some differences in terms of population, the generalisation of the effect size from CAPRI programme was used to simulate the AMA controlled arm. Populations were comparable in terms of demographics, and some differences remained regarding primitive tumours (Supplementary tables) and type of treatment prescribed where more cytotoxic chemotherapy was prescribed in the AMA programme (Table 1). No other potential confounding factors was identified.
| Variable | AMA | CAPRI | |||
|---|---|---|---|---|---|
| Total cohort | Minimum follow-upa (1M-FU) | Minimum interventionb (INT) | Case | Control | |
| Number of patients | 797 | 690 | 215 | 272 | 287 |
| Age (mean, SD) | 62.8 (17) | 61.5 (18) | 65.2 (16) | 59.8 (14) | 60.5 (13) |
| Male (n, %) | 432 (54) | 338 (49) | 97 (45) | 116 (43) | 113 (39) |
| Follow-up, days | 162 | 176 | 180 | 166 | 164 |
| [Median, IQR] | [110–182] | [135–188] | [145–180] | [74–179] | [87–180] |
| Death (n, %) | 35 (4.4) | 34 (4.9) | 19 (8.8) | 85 (31.3) | 87 (30.3) |
| Time to death, days (median, IQR) | 156 | 157 | 167 | 174 | 190 |
| [87–258] | [87–258] | [121–449] | [101–242] | [108–285] | |
| Therapy (n, %) | |||||
| Cytotoxic (IV) | 577 (72) | 519 (75) | 85 (40) | - | - |
| Cytotoxic (oral) | - | - | - | 109 (40) | 109 (38) |
| Targeted (oral) | 219 (27) | 170 (25) | 130 (60) | 163 (60) | 177 (62) |
- a Patient with at least 2-month follow-up.
- b Patients with at least one intervention made by nurses.
The same category of Grade 3–4 AEs from the CAPRI study was used. Scope of AEs was similar between both studies over these categories. We used the relative risks from CAPRI, assuming similar effect size, and applied it on the AMA arm to estimate an AMA control arm. In CAPRI, patients having at least one of Grade 3–4 AEs of the four categories in the intervention group were significantly less frequent than in the control group [22.8% vs. 30.3%, RR = 1.33 (1.05–1.61)]. Increased proportion of patients with supportive care was also extrapolated from the CAPRI study [43.8% vs 35.2%, RR = 0.80 (0.60–1.01)] and apply directly in both AMA arms. An additional increase rate of supportive cares resulting from nurses' support was applied to AMA intervention versus control arm [43.8% vs. 35.2%, RR = 1.24 (0.99–1.68)]
2.6 Costs
The cost of the AMA programme was based on fixed costs for the overall programme set-up and per patient costs (variable costs), both from the payer perspective. A 5-year time horizon was used, with 2020-euro currency and no discount rate. Costs items of the AMA programme are presented in Table 2. Among the fixed costs, a cost of structure of €100 per month was included and was derived from the annual national micro-costing study (ATIH, 2020). Additional €100 per month was included corresponding to furnishings, phones, pagers and computers. A cost of training sessions (€1200) for oncology specialisation was considered for three nurses.
| Costs | Definition | Source | |
|---|---|---|---|
| Adverse event | |||
| Haematological | €2260 | Neutropenia, anaemia, thrombopenia | DRG tariffs 2019 |
| Gastrologic | €1457 | Diarrhoea, constipation, nausea, vomiting | DRG tariffs 2019 |
| Neurologic | €2710 | Neuropathy | DRG tariffs 2019 |
| Dermatologic | €1944 | Skin rash, mucositis | DRG tariffs 2019 |
| Supportive care | €53 | Tariff for specialist visit | AMELI |
| AMA programme | |||
| Nurse salary | €15 | Net hourly wage | INSEE |
| Calls per patient | 48 | Two calls per week for a 6-month follow-up | AMA experience |
| Time per call | 10 mn | Average time spent per call with a patient | AMA experience |
| Structure | €100 | Monthly cost | ENC |
| Furnishings | €100 | Monthly cost | Centre estimation |
| Training | €1200 | Cost of training for oncology certification | Training in Léon Bérard |
| Annual total cost | €22,152 | Total 5-year cost for the entire cohort (n = 797) | |
For the entire cohort (n = 797), a 10-min call in average was conducted twice a week for each patient for an approximately 6-month period. Hourly salary of nurses was based on the median national gross salary of €15 per hour. The cost of the AMA programme was estimated to €110,760 for the period of 5 years, corresponding to an annual cost of around €22,000. A sensitivity analysis was carried with +/−20% of the total cost of the programme.
Costs included in the analyses are presented in Table 2. Costs of Grade 3–4 AE were based on DRG tariffs for hospitalizations (payer perspective). For each category, types of AE were weighted average by the number of admissions in 2019 and their associated tariffs. An additional cost of supportive care was valued with the tariff for a specialist visit for the intervention arm.
2.7 Statistical analyses
Analyses were conducted for the entire AMA cohort and for two subpopulations: a subpopulation with a minimum of 1-month follow-up (1M-FU) (which is one of the exclusion criteria of CAPRI study) and patients with at least one direct intervention made by nurses (INT). For comparability purposes, demographics and basic medicals were described for each population and for the intervention and the control arm of the CAPRI study. All-cause death occurring during the follow-up and time to death were reported. Risk ratios and their 95% confidence intervals were reported for each category of AE from the CAPRI study. Only the RR of patients with at least one Grade 3–4 AE was applied to simulate the AMA control arm. Increased proportion of patients with supportive care was also extrapolated from the CAPRI study and apply directly in both AMA arms.
Incremental cost-effectiveness ratio (ICER) was calculated for Grade 3–4 AEs avoided. The ICER, defined as the difference in cost between the AMA intervention arm and the simulated control arm divided by the number of Grade 3–4 AEs avoided, was estimated from a static model. Progression-free survival (PFS) and overall survival (OS) were not considered in the study because of the short follow-up period of the programme (6 months). Sensitivity analyses were conducted for RR of grade 3–4 AEs and for the total cost of the AMA programme. A subgroup analysis was conducted in patients prescribed targeted oral therapy.
3 RESULTS
3.1 Description of the populations
The entire AMA cohort included 797 patients, of 63 years of age in average, and 54% were male (Table 1). Median follow-up was 5.3 months (162 days, IQR: 110; 182), and 35 deaths (4.4%) occurred during the programme. Targeted oral therapy was prescribed for 27% of patients (n = 219), and the remaining was prescribed intravenous chemotherapy (72%, n = 577). The two subpopulations had similar proportion of males, similar ages and similar follow-up. However, in the INT subpopulation, with at least one direct intervention, patients had a majority of targeted oral agents (60%).
Patients included in the CAPRI cohort had similar age, similar proportion of males and similar median follow-up. Patients were prescribed oral therapy only, with around 60% having targeted therapy and 40% cytotoxic chemotherapy.
3.2 Outcomes
Description of outcomes is presented in Table 3. At least one AE of Grade 3–4 occurred in 80 patients, which represent 10.1% of the entire cohort and accounted for a total of 117 events (1.46 events per patient). Haematological AEs accounted for 12.6% of events, 1.6% were digestive events, 0.4% were dermatologic, and 0.1% were neurologic. The 80 patients with AEs were included the two subpopulations, representing 15.5% and 37.2%, respectively, for 1M-FU and INT subpopulations.
| Adverse events (%) | CAPRI | AMA | Control (simulated) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case | Control | RR (95%CI) | Total | 1M-FU | INT | Total | 1M-FU | INT | |
| Patients with AE (%) | 22.8 | 30.3 | 0.75a (0.62–0.95) | 10.1 | 11.6 | 37.2 | 13.4 | 15.4 | 49.4 |
| Total number of AE | 115 | 152 | 0.76 (0.67–0.87) | 117 | 117 | 117 | 155 | 155 | 155 |
| Average number per patient | 1.85 | 1.75 | - | 1.46 | 1.46 | 1.46 | 1.46 | 1.46 | 1.46 |
| AE category | |||||||||
| Haematological | 12.1 | 11.8 | 1.03 (0.70–1.90) | 12.6 | 14.5 | 46.5 | 12.3 | 14.1 | 45.4 |
| Digestive | 6.3 | 8.0 | 0.79 (0.53–1.50) | 1.6 | 1.9 | 6.0 | 2.1 | 2.4 | 7.7 |
| Neurologic | 0.7 | 2.8 | 0.25 (0.18–0.41) | 0.1 | 0.1 | 0.5 | 0.5 | 0.6 | 1.9 |
| Dermatologic | 3.7 | 7.7 | 0.48 (0.36–0.74) | 0.4 | 0.4 | 1.4 | 0.8 | 0.9 | 2.9 |
| Patients with supportive careb | 43.8 | 35.2 | 1.24 (0.99–1.68) | 43.8 | 43.8 | 43.8 | 35.2 | 35.2 | 35.2 |
- a Only this RR was used to simulate the control arm.
- b Care related to pain, nutrition, psychology, medico-social, palliative or general order.
In the CAPRI study, the intervention and the control arm had 22.8% (n = 62) and 30.3% (n = 87) of patients (RR = 1.33, 95%CI: 1.05–1.61), respectively, with at least one AE of Grade 3–4. The total number of AEs was 115 events in the interventional arm (1.85 events per patient) and 152 events in the control arm (1.75 events per patient).
The AMA control arm was simulated from the unique RR of increased proportion of patients with at least one AE of Grade 3–4, which led to an estimated 13.4% of patients (n = 107, for the entire cohort), accounting for a total of 155 estimated events (1.46 events per patients).
3.3 Costs
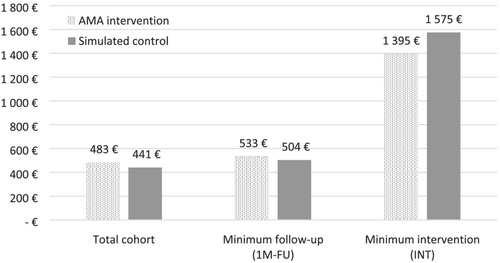
Costs of the Grade 3–4 AEs and cost of the AMA programme are presented in Table 4. On the entire cohort, the cost of AEs for the 5 years of programme was estimated to €253,479 (€320 per patient) and €334,593 (€422 per patient) for the intervention and the control arms respectively. The cost of the AMA programme was estimated to €110,760 (€140 per patient) for the 5 years. On the two subpopulations, cost of AEs per patients ranged from €367 to €1179 and from €485 to €1556 for the intervention and the simulated control arms, respectively. Costs of supportive care were estimated to €23 and €19 per patient for intervention and control arms, respectively. Average total costs per patient, including AE-related costs, cost of the AMA programme and cost of supportive care, are presented in Figure 1.
| AMA intervention programme | Simulated control arm | |||||
|---|---|---|---|---|---|---|
| Total | 1M-FU | INT | Total | 1M-FU | INT | |
| Patients with AE (n, %) | 80 (10.1) | 80 (15.5) | 80 (37.2) | 107 (13.4) | 107 (15.4) | 107 (49.4) |
| Number of AEs | 117 | 117 | 117 | 155 | 155 | 155 |
| Cost by AE category | ||||||
| Haematology | €226,002 | €226,002 | €226,002 | €275,759 | €273,308 | €275,759 |
| Gastrologic | €18,936 | €18,936 | €18,936 | €30,085 | €31,060 | €30,085 |
| Neurologic | €2710 | €2710 | €2710 | €13,565 | €14,005 | €13,565 |
| Dermatologic | €5831 | €5831 | €5831 | €15,183 | €15,675 | €15,183 |
| AE-related costs | €253,479 | €253,479 | €253,479 | €334,593 | €334,048 | €334,593 |
| AE-related cost per patient | €320 | €367 | €1179 | €422 | €485 | €1556 |
| Cost of AMA programme | €110,760 | €98,400 | €41,400 | - | - | - |
| Cost of AMA programme per patient | €140 | €143 | €193 | |||
| Cost of supportive care | €18,409 | €16,018 | €4991 | €14,794 | €12,873 | €4011 |
| Cost of supportive care per patient | €23 | €23 | €23 | €19 | €19 | €19 |
| Total costs (payer perspective) | €382,648 | €367,897 | €299,870 | €349,387 | €347,465 | €338,604 |
| Total costs (payer perspective) per patient | €483 | €533 | €1395 | €441 € | €504 | €1575 |
3.4 Cost-effectiveness
We estimated that the AMA programme avoided 38 AEs (0.05 per patient for the entire cohort) accounting for 26 patients without AEs and generating savings of €81,113 in terms of AEs for the entire cohort (€102 per patient) (Table 5). The ICER, defined as the cost difference per Grade 3–4 AE avoided, was estimated to €864 for the entire cohort. In subpopulations, ICER was estimated to €531 in patient with 1-month minimum follow-up, and the AMA intervention arm was dominant over the simulated control arm for patients with at least one intervention from nurses.
| Total | 1M-FU | INT | |
|---|---|---|---|
| AE-related cost difference | €−81,113 | €−81,113 | €−81,113 |
| AE-related cost difference per patient | €−102 | €−118 | €−377 |
| Difference in total costs | €33,261 | €20,432 | €−38,733 |
| Difference in total cost per patient | €42 | €30 | €−180 |
| Number of AEs avoided (n) | 38 | 38 | 38 |
| Number of AEs avoided per patient (n) | 0.05 | 0.06 | 0.18 |
| Cost-effectiveness | |||
| ICER per AE avoided | €864 | €531 | Dominant |
Sensitivity analyses are presented in Table 6. The optimistic scenario considering a 38% reduction (lower bounds, RR = 0.62) estimated that the programme was dominant on the entire population and the two subpopulations. The pessimistic scenario allowing a 5% reduction in patients with Grade 3–4 AE (upper bounds, RR = 0.95) estimated an ICER of €17,691 on the entire population. An increase of 20% of the total cost of the AMA programme led to an estimated ICER of 1440€. In patient with targeted oral therapy only, the intervention was dominant on the entire population and subpopulations.
| Total | Follow-up | Info | |
|---|---|---|---|
| Patients with Grade 3–4 AE (lower scenario, RR = 0.62) | |||
| Total cost difference per patient | €−47 | €−73 | €−509 |
| AE avoided per patient | 0.09 | 0.10 | 0.33 |
| ICER per AE avoided | Dominant | Dominant | Dominant |
| Patients with Grade 3–4 AE (upper scenario, RR = 0.95) | |||
| Total cost difference per patient | €131 | €131 | €147 |
| AE avoided per patient | 0.01 | 0.01 | 0.03 |
| ICER per AE avoided | €17,691 | €15,498 | €5384 |
| Total cost of AMA programme (lower scenario, −20%) | |||
| Total cost difference per patient | €14 | €1 | €−219 |
| AE avoided per patient | 0.05 | 0.06 | 0.18 |
| ICER per AE avoided | €289 | €20 | Dominant |
| Total cost of AMA programme (lower scenario, +20%) | |||
| Total cost difference per patient | €70 | €58 | €−142 |
| AE avoided per patient | 0.05 | 0.06 | 0.18 |
| ICER per AE avoided | €1440 € | €1042 | Dominant |
| AMA targeted oral therapy only | |||
| Total cost difference per patient | €−357 | €−494 | €−7 |
| AE avoided per patient | 0.23 | 0.30 | 0.10 |
| ICER per AE avoided | Dominant | Dominant | Dominant |
4 DISCUSSION
This study aimed at estimating the cost-effectiveness of the AMA programme (Outpatient Medical Assistance) in patients with haematological malignancies from the payer perspective in a single centre.
The complexity of care, the hyperspecialisation of units and the multiplication of call lines all contribute to considering the incoming call as an imperfect organisational modality. In this context, an alternative model based on a programmed outgoing call, directed from the care unit to the patient, from a trained nurse has been implemented with the AMA programme.
The programme assumed that the application of the PN concept to the active phase of treatment in haematology could improve the safety and effectiveness of care. The objective was to improve the monitoring of patients during the active phase of their treatment: anticipate and manage risk at home; manage and detect as early as possible the toxicities that may be caused (fever, mucositis, digestive problems, asthenia, pain, neutropenia, anaemia, etc.) by chemotherapy; ensure good compliance with treatment; break the isolation; and rationalise care.
To our knowledge, only one study has reported the cost-effectiveness of a PN during survivorship (active treatment phase), in patients with Stage 3 colorectal cancer (Bernardo et al., 2019; Blakely et al., 2015). The need to evaluate PN from a cost-effectiveness perspective is warranted, although their feasibility is made complex by the lack of control intervention.
The cost-effectiveness of the programme was made possible by simulating a control arm from a similar PN programme, the CAPRI programme and its intervention effect on Grade 3–4 AEs (RR = 0.75, 95%CI = 0.61, 0.95) compared to standard of care evaluated in a RCT. Based on a 25% reduction of patients with at least one AE of Grade 3–4, 38 events were avoided over the 5 years, leading to estimated savings of around €80,000 in terms of AEs. The AMA programme was estimated to costs around €110,000 for the 5 years. This study showed that the AMA programme was cost-effective (ICER = €864) on the entire cohort of patients included during the 5-year period and dominant on the subpopulation of patients receiving at least one intervention from nurses.
Sensitivity analyses showed that the conservative scenario with a 5% reduction of patients with Grade 3–4 AEs would lead to an increase of total cost of €131 per patient and an ICER estimated to €17,691. The cost of the AMA programme had little impact on the cost-effectiveness, where a 20% increase of the total cost led to an estimated ICER of €1440 in the total cohort. Analyses of the two subpopulations were optimistic, leading to better ICER. They focused on patients followed for at least 1 month and on patients with at least one intervention from nurses.
Results of this study should be considered conservative given that OS and PFS were not taken into account. Patients were followed during 6 months only and impact on PFS and OS could not be evaluated on this short-term window. It has been demonstrated that PN and Grade 3–4 AEs have a substantial impact on RDI (Mir et al., 2020). Also, intermediate results from a similar AMA programme (the OMA1 program) in France showed promising results in terms of OS and PFS (Ysebaert et al., 2019, 2020).
In the CAPRI study, patients with malignant haematology or solid tumour and with oral therapy (cytotoxic or targeted agent) were randomised based on sex, age and type of primary tumour. Similarly, to the AMA programme, patients were followed by certified nurses. Some differences in the PN relied on the availability of an online platform for CAPRI patients to notify AEs. Investigators indicated that this platform was poorly used, only once in average per patient, and may not participate to intervention effect.
Populations between the AMA programme and the CAPRI study had some differences in term of nature of cancer. In the CAPRI study, only 12 patients had a malignant haematology, involving some differences in terms of therapy and care support. Oral chemotherapy was prescribed for 40% of patients in the CAPRI study, where 72% of patients were under intravenous chemotherapy in the AMA programme. Despite this difference in terms of cytotoxic therapy versus targeted agent, patients in the AMA programme were most likely to visit every 2–3 weeks the care centre and being followed regularly. The fact that, in the minimum intervention subpopulation, more patients were prescribed oral targeted agent may support this point.
The design of the study was the main limitation as it was a non-controlled and single-centre study.
The lack of a randomised controlled group and the single centre were the main limitations of the study that led us to use a simulated control arm. A well-designed RCT conducted in several centres is needed to capture the precise effect size of the AMA programme and to confirm our results.
The scope of evaluation was limited to four categories of Grade 3–4 AEs. AEs of Grade 1–2 were not considered in this study because there had a possible under-reporting due to the design of the study. A substantial part of patients was prescribed intravenous chemotherapy, and it was assumed that some AEs of Grade 1–2 may have been managed during administration visit and not directly reported in the registry. Additionally, Grade 1–2 AEs have a negligible impact on organisation of care.
The cost of wastage due to early discontinuation of treatment was not considered in the study. This assumption was a limitation not favourable for the AMA programme due to the very high cost of oral targeted therapies that were prescribed in almost a third of patients.
The scope of costs was derived from Whitley et al. (2011) and included wages, training, structure and furnishings cost. Supervision by haematologist was considered to be part of current practice within the specialised centre. Other possible costs were negligible (costs for recruitment, hiring and orientation of the programme, administrative support services).
The ability of the AMA programme to achieve its objectives depends on the patient's understanding, perception and appropriation of the management system. Therefore, the programme also consists in identifying the representation and the perception of the management system as well as the impact on the attitude and behaviour of the patients.
Encouraging results from the AMA programme shows the need to guide the patient towards greater autonomy and more active attitudes, in particular on the dimensions of ‘personal interaction’ and ‘feedback’, and to develop training sessions for nurses on patient empowerment. The concepts of patient empowerment, patient-centred care and therapeutic patient education are all part of this perspective of improving the care pathway to ensure an effective seamless system.
5 CONCLUSION
Economic evaluation of PN in survivorship is limited. This study provides a cost-effectiveness evaluation of the AMA programme, a telephone-based follow-up procedure, with scheduled calls to the patient's home by a certified oncology nurse. The AMA intervention programme was compared to a controlled arm simulated from an intervention effect measured in a RCT of a similar PN, the CAPRI programme. Based on a 25% reduction in Grade 3–4 AEs, ICER per event avoided was estimated to €824. In the worst-case scenario, based on a 5% reduction in Grade 3–4 AEs, the ICER was estimated at €17,691. These results showed the AMA programme is cost-effective and should be implemented in routine in other oncologic centres. More research needs to be conducted through a RCT to evaluate the impact of AMA programme on RDI, PFS and OS.
CONFLICT OF INTEREST
Authors have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




