Estimating the cost-effectiveness of intermittently scanned continuous glucose monitoring in adults with type 1 diabetes in England
Section: health economics
Abstract
Objective
We previously showed that intermittently scanned continuous glucose monitoring (isCGM) reduces HbA1c at 24 weeks compared with self-monitoring of blood glucose with finger pricking (SMBG) in adults with type 1 diabetes and high HbA1c levels (58–97 mmol/mol [7.5%–11%]). We aim to assess the economic impact of isCGM compared with SMBG.
Methods
Participant-level baseline and follow-up health status (EQ-5D-5L) and within-trial healthcare resource-use data were collected. Quality-adjusted life-years (QALYs) were derived at 24 weeks, adjusting for baseline EQ-5D-5L. Participant-level costs were generated. Using the IQVIA CORE Diabetes Model, economic analysis was performed from the National Health Service perspective over a lifetime horizon, discounted at 3.5%.
Results
Within-trial EQ-5D-5L showed non-significant adjusted incremental QALY gain of 0.006 (95% CI: −0.007 to 0.019) for isCGM compared with SMBG and an adjusted cost increase of £548 (95% CI: 381–714) per participant. The lifetime projected incremental cost (95% CI) of isCGM was £1954 (−5108 to 8904) with an incremental QALY (95% CI) gain of 0.436 (0.195–0.652) resulting in an incremental cost-per-QALY of £4477. In all subgroups, isCGM had an incremental cost-per-QALY better than £20,000 compared with SMBG; for people with baseline HbA1c >75 mmol/mol (9.0%), it was cost-saving. Sensitivity analysis suggested that isCGM remains cost-effective if its effectiveness lasts for at least 7 years.
Conclusion
While isCGM is associated with increased short-term costs, compared with SMBG, its benefits in lowering HbA1c will lead to sufficient long-term health-gains and cost-savings to justify costs, so long as the effect lasts into the medium term.
What's new?
What is already known?
- Most people living with type 1 diabetes have above-target HbA1c levels.
What this study found?
- In people with type 1 diabetes with high HbA1c (58–97 mmol/mol, 7.5%–11.0%) intermittently scanned continuous glucose monitoring (isCGM) was cost-effective compared with self-monitoring of blood glucose with finger pricking (SMBG) with a dominant effect in those with HbA1c >75 mmol/mol (9%).
- People need to use isCGM for around 7 years for it to be cost-effective.
Implications of the study
- Our data support the NICE 2022 recommendations to offer a choice of rtCGM or isCGM to all people living with type 1 diabetes.
1 INTRODUCTION
Most people living with type 1 diabetes have above-target HbA1c levels,1 increasing the risk of long-term complications and lifetime healthcare costs. An intermittently scanned continuous glucose monitoring device (isCGM) consists of a subcutaneous glucose sensor displaying glucose levels on a mobile phone app or hand-held reader, reducing the need to perform painful self-monitoring of blood glucose with finger pricking (SMBG). Previous evaluations of isCGM focusing on its use to reduce hypoglycaemic events in people with well-controlled type 1 diabetes, suggest it is effective2 and cost-effective.3
Use of isCGM devices improves HbA1c in people with type 1 diabetes under routine care conditions,4 but data from randomised controlled trials (RCTs) were limited prior to our trial.5 The National Institute for Health and Care Excellence (NICE) recommends clinicians offer a choice of rtCGM or isCGM for adults with type 1 diabetes in England.6 Funding for, and access to isCGM is variable across the UK. These devices cost more than SMBG. The objective of this study was to evaluate the long-term cost-effectiveness of isCGM compared with SMBG in people with type 1 diabetes with high HbA1c, from the English National Health Service (NHS) healthcare payer perspective.
2 METHODS
2.1 The technology under investigation
The Freestyle Libre 2 (FSL2, [Abbott Diabetes Care]) isCGM system includes optional alarms to alert users of hypoglycaemia or hyperglycaemia. The comparator is SMBG.
2.2 Randomised controlled trial
We conducted an open label, multicentre, randomised (1:1), parallel-group trial at seven UK specialist diabetes clinics and one primary care centre (FLASH-UK study, ClinicalTrials.gov NCT03815006). The protocol was approved by Greater Manchester West Research Ethics Committee on 21/03/2019 (Reference 19/NW/0081).7 People 16 years or above with type 1 diabetes and HbA1c 58–97 mmol/mol (7.5%–11.0%), either on continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI) were eligible. Trial information (List of study investigators at participating clinical sites, trial inclusion and exclusion criteria, schedule of study visits, and CONSORT diagram) is provided in Appendices S1–S4.
2.3 Cohort characteristics and trial results
Participants were randomly assigned to isCGM (intervention group, n = 78) or SMBG (usual-care group, n = 78). At baseline, participants had mean (SD) age 44 (15) years, duration of diabetes 21 (13) years; 44% were women and 98% were white. At 24 weeks, the adjusted mean HbA1c between-group difference was −5 mmol/mL (95% confidence interval (Cl: −8 to −3) (−0.5 percentage points[CI], −0.7 to −0.3;); p < 0.001).5
2.4 EQ-5D-5L
Quality-adjusted life years (QALYs) were derived from EQ-5D-5L values at baseline, and 24 weeks after randomisation.8 QALYs were calculated by attaching available utility weights to health states generated from EQ-5D-5L, using area under the curve methods, assuming a linear change between time points, controlling for baseline. NICE's current guidance is to use a mapping algorithm to match health states to their corresponding utility values.9 For the estimation of mean QALYs in the isCGM and the SMBG arms, a regression-based adjustment was carried out.10 This generated predictions for adjusted QALYs, estimation of the QALY difference between treatment arms, and controlled for baseline utility values.
2.5 Within-trial costs
The cost of the intervention consisted of devices related to the FSL system (reader, sensors, training, reagent strips and lancets for finger puncture). For the SMBG arm, the costs were reagent strips and lancets.
We collected information about primary and secondary healthcare resource use (outside of the trial specific assessment pathway) up to 24 weeks post-randomisation. A summary of parameters is provided in Table 2, full details in Appendix S6.
Person-level costs were generated by combining trial-based resource use with published unit costs. Unit costs were obtained from up-to-date publicly available UK sources.11-13 Costs were expressed in 2020/21 prices. Costs were compared between groups using bootstrap estimates for credibility intervals.
A complete-case analysis was used owing to the low percentage of missing data (<15% overall and < 15 percentage points for between-group differences).
2.6 Economic evaluation
The economic evaluation was conducted to determine the difference in costs and outcomes generated for isCGM compared with SMBG, from the perspective of the NHS in England following standard quality design and reporting criteria.14 The longer term effect of isCGM was estimated via a comparison between the isCGM and SMBG arm, using a decision model to estimate long-term cost and health effects. A commercially available, validated model, the IQVIA Center for Outcomes Research and Effectiveness (CORE) Diabetes Model(IQVIA CDM) version 9.5 (IMS Health, Danbury), was used.15 This internet-based simulation model predicts the long-term health outcomes and costs associated with type 1 diabetes management, comprising 15 sub-models that simulate diabetes-related complications, non-specific mortality and costs over time. As the model simulates individuals over time, it updates risk factors and complications to account for disease progression.
Inputs used cohort characteristics, intervention costs and HbA1c effectiveness estimates from the trial.5 Where those data were not collected in the trial, data were taken from published sources (Appendices S7 and S8). Subgroup analyses (according to baseline HbA1c level, treatment modality and participation in structured education) used specific HbA1c baseline and effectiveness from the trial; we held all other inputs constant.
2.6.1 Hypoglycaemia events
We imported the event rates of severe hypoglycaemic events (SHEs) from the UK Hypoglycaemia Study group study,16 using published values of 320 SHEs per 100 patient years.3 Foos et al. reported that the proportion of SHEs not requiring medical assistance (SHE1 events) was 88%, and those requiring medical assistance (SHE2 events) was 12%.17 This was applied to the Bilir overall SHE rate. The conservative assumption was made that isCGM had no effect on SHE rates.18 We imported the event rates of non-severe hypoglycaemic events from the IMPACT trial19 (isCGM: 4897 per 100 patient years; SMBG: 6760 per 100 patient years). We imported the proportion of nocturnal hypoglycaemic events from the IMPACT trial of 0.25 for isCGM and 0.27 for SMBG.19
2.6.2 Intervention costs
The insulin total daily dose and use of lancets and test strips per day for each arm was obtained from the trial and extrapolated to 1 year. The most common insulin regimen in the trial was MDI therapy. We assumed patients in the isCGM and the SMBG arm follow a similar insulin regimen, see Appendix S7.
2.6.3 Event costs
The costs included in the model are for: management (primary prevention of complications); diabetes-related complications (including hypoglycaemic events and DKA); treatment of diabetes (including intervention cost) and other hospital costs. These were taken from published sources, see Appendix S8.
2.6.4 Quality of life (QoL)
For underlying QoL of people with type 1 diabetes and the disutility associated with hypoglycaemic events and long-term complications, we utilised default utility values in IQVIA CDM, where appropriate (Appendix S8).
Process utility, the extent to which a person's QoL may be affected by the use of technology, independent of the associated outcomes, should be quantified where possible.20 A UK study estimated process utility for isCGM compared with SMBG at +0.03.21 We included this benefit in our base-case and explored the impact of excluding it in sensitivity analysis.
2.6.5 Incremental analysis
All costs and effects were discounted by 3.5%, as recommended by the UK Treasury. Probabilistic estimates of costs and outcomes for each comparator were generated using this model, allowing the derivation of mean incremental costs and outcomes, with 95% credibility intervals. Incremental cost-effectiveness ratios (ICERs) and cost-effectiveness acceptability curves (CEACs) (Appendix S10) were constructed to show the probability that the intervention is cost-effective for different willingness-to-pay (WTP) thresholds.
This assumes that people using isCGM will retain the magnitude of HbA1c benefit, compared with SMBG, that was observed in the trial for the full duration of the model, and that people continued to use the isCGM for lifetime (incurring lifetime costs and benefits), the model being run for 80 years. This is consistent with NICE NG17. However, given that true future treatment effects are not known, we undertook scenario analyses to explore cost-effectiveness when benefit does not persist indefinitely.20
Planned exploratory subgroup analyses were performed for the primary outcome measure and included: Baseline HbA1c: 58–75 mmol/mol (7.5%–9.0%); >75–97 mmol/mol (>9.0%–11%); Treatment modality: MDI or CSII; Prior structured education course: yes or no.
- Internal peer review by clinical and economic modelling experts.
- Scrutiny of the implemented model coding and formulae.
- Checking the accuracy of all model inputs against sources.
Appendix S9 summarises the completed Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS) checklist.14
3 RESULTS
Within-trial EQ-5D-5L and resource use data were obtained from 69 (isCGM) and 63 (SMBG) participants. (see Appendix S5 for baseline participant characteristics of this cohort).
3.1 EQ-5D-5L
Within-trial EQ-5D-5L results showed a non-significant adjusted incremental QALY gain of 0.006 (95% CI: −0.006 to 0.017) for isCGM compared with SMBG. See Table 1.
| isCGM (n = 69) | SMBG (n = 63) | |
|---|---|---|
| Mean utility (SD) | Mean utility (SD) | |
| EQ-5Da | ||
| Baseline | 0.838 (0.188) | 0.880 (0.121) |
| Follow-up (24 weeks) | 0.820 (0.217) | 0.835 (0.171) |
| QALYs | ||
| 0–24 weeks | 0.383 (0.086) | 0.396 (0.061) |
| Difference (unadjusted) | −0.013 (95% CI: −0.039 to 0.013) | |
| Difference (adjustedb) | 0.006 (95% CI: −0.006 to 0.017) | |
- Abbreviations: isCGM, intermittently scanned continuous glucose monitoring; QALYs, quality-adjusted life-years; SMBG, self-monitoring of blood glucose.
- a EQ-5D-5L with crosswalk to EQ-5D-3L, time period is 24 weeks; therefore, the maximum number of QALYs any participant can accrue during the study is 24/52 (0.46) QALYs (i.e. perfect health at baseline and the 24-week follow-up).
- b Adjusted for baseline EQ-5D values using seemingly unrelated regression in the same system of equations as total costs and diabetes-related costs.
3.2 Within-trial costs
Participant-level resource use and unit costs used are reported in Appendix S6. The mean (SD) 24-week patient-level within-trial cost in the isCGM arm was £955 (£638) and SMBG arm £408 (£202) with an incremental total adjusted cost of £548 (95% CI: 381–714) per participant. See Table 2.
| Cost per patient | isCGM (n = 69) | SMBG (n = 63) |
|---|---|---|
| £, Mean (SD) | £, Mean (SD) | |
| isCGM sensora | 420.00 (0.00) | NA |
| SMBG tests (costs of reagent strips, and lancets for finger puncture) | 51.98 (62.76) | 248.71 (117.97) |
| Insulin doses | 215.51 (105.32) | 195.99 (97.28) |
| Routine visits (including initial training in isCGM or SMBG) | 118.00 (0.00) | 118.00 (0.00) |
| Hospital admissions for diabetic ketoacidosis | 2.34 (19.44) | 0.00 |
| Severe Hypoglycaemia episodes | 1.03 (8.61) | 0.45 (546.65) |
| Ketosis events (not requiring hospital admission) | 0.00 | 2.45 (16.51) |
| General practice visits | 21.31 (45.09) | 14.11 (24.28) |
| Nurse visits | 1.25 (2.36) | 1.76 (4.87) |
| General practice home visits | 6.46 (53.96) | 0.00 |
| Nurse home visits | 23.68 (192.12) | 0.00 |
| Hospital visits | 58.11 (258.83) | 25.46 (141.73) |
| Phone calls (primary care) | 5.99 (12.38) | 5.59 (11.77) |
| Emergency department visits | 6.27 (25.48) | 12.02 (39.42) |
| Ad-hoc outpatient visits | 69.18 (166.06) | 31.77 (88.15) |
| Paramedic calls | 6.04 (35.24) | 0.00 |
| Total costs | 955.22 (637.68) | 407.64 (202.10) |
| Diabetes-related costs b | 825.36 (196.70) | 347.48 (131.71) |
| Unadjusted difference (Total) | £547.58 (95% CI: £381.46 to £713.70) | |
| Unadjusted difference (Diabetes-related) | £477.88 (95% CI: £419.67 to £536.09) | |
| Adjusted difference (Total)c | £547.84 (95% CI: £392.30 to £703.38) | |
| Adjusted difference (Diabetes-related)d | £475.65 (95% CI: £417.94 to £533.35) | |
- Abbreviations: isCGM, intermittently scanned continuous glucose monitoring; NA, not applicable; QALYs, quality-adjusted life-years; SD, Standard Deviation; SMBG, self-monitoring of blood glucose.
- a In the NHS in England, a mobile phone app was provided with the provision of sensors with zero added cost. A reader is provided free of charge (by the company) for anybody without a compatible smartphone or who does not want to use a smartphone.
- b Diabetes-related codes included only.
- c Adjustment for baseline characteristics (age, gender, baseline EQ-5D, baseline HbA1c, number of hypoglycaemic episodes, number of daily glucose tests, Clarke score, GOLD score, history of microalbuminuria, history of retinopathy), using seemingly unrelated regression.
- d Adjustment for baseline characteristics using seemingly unrelated regression with Baseline EQ-5D transformed from EQ-5D-5L to EQ-5D-3L using crosswalk values.
3.3 Long-term economic evaluation
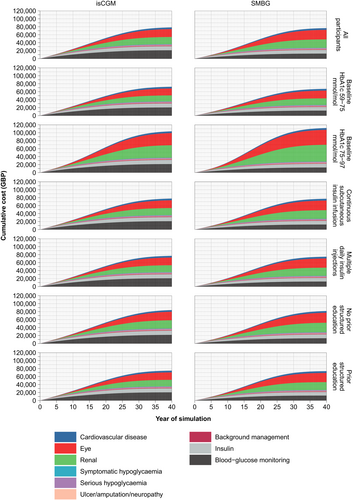
Figure 1 shows the accumulation of discounted costs in the base-case model. isCGM is associated with lifetime acquisition costs of £21,049 per person, compared with £12,771 for SMBG. It results in higher lifetime insulin costs (£10,372 vs. £9377 from SMBG). These additional expenditures are offset by reduced costs of long-term diabetic complications (£43,518 for isCGM vs. £50,927 for SMBG).
In the base case, isCGM generates over 0.4 more QALYs than SMBG, generating an ICER of below £5000 per QALY gained (Table 3). The probability that isCGM would be considered an effective use of resources is over 95% when QALYs are valued at £20,000 each, and close to 100% at the higher threshold of £30,000/QALY.
| Analysis | Costs (GBP) | Effects (QALYs) | ICER (GBP/QALY) | Incremental net health benefit (QALYs) | Probability isCGM is cost-effective | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| isCGM | SMBG | Difference (95% CI) | isCGM | SMBG | Difference (95% CI) | 1 QALY := £20,000 | 1 QALY := £30,000 | 1 QALY := £20,000 | 1 QALY := £30,000 | ||
| Base case | |||||||||||
| Ongoing HbA1c benefit; hypo benefit; process benefita | 79,034 | 77,080 | 1954 (−5108 to 8904) | 10.043 | 9.607 | 0.436 (0.195–0.652) | 4477 | 0.339 (−0.033 to 0.702) | 0.371 (0.082–0.643) | 0.959 | 0.991 |
| Alternative scenarios assuming ongoing HbA1c benefit | |||||||||||
| No hypo benefit; process benefita | 79,034 | 77,080 | 1954 (−5108 to 8904) | 9.975 | 9.607 | 0.368 (0.126–0.583) | 5310 | 0.270 (−0.101 to 0.635) | 0.303 (0.014–0.575) | 0.911 | 0.975 |
| Hypo benefit; no process benefita | 79,034 | 77,080 | 1954 (−5108 to 8904) | 9.983 | 9.607 | 0.376 (0.137–0.592) | 5191 | 0.279 (−0.093 to 0.643) | 0.311 (0.022–0.581) | 0.918 | 0.978 |
| No hypo benefit; no process benefit | 79,034 | 77,080 | 1954 (−5108 to 8904) | 9.915 | 9.607 | 0.308 (0.069–0.523) | 6345 | 0.210 (−0.162 to 0.575) | 0.243 (−0.046 to 0.512) | 0.862 | 0.948 |
| Other sensitivity analyses (assuming hypo benefit, process benefit and ongoing HbA1c benefit) | |||||||||||
| Discount rate 0% | 147,762 | 145,629 | 2133 (−11,907 to 15,513) | 15.812 | 14.951 | 0.861 (0.330–1.318) | 2479 | 0.754 (0.048–1.438) | 0.790 (0.264–1.292) | 0.982 | 0.997 |
| Discount rate 5% | 62,863 | 60,863 | 2001 (−3524 to 7457) | 8.539 | 8.195 | 0.343 (0.156–0.509) | 5825 | 0.243 (−0.056 to 0.531) | 0.277 (0.040–0.496) | 0.936 | 0.987 |
| Time Horizon 10 years | 24,275 | 20,994 | 3280 (1104 to 5620) | 5.255 | 5.105 | 0.150 (0.086–0.215) | 21,823 | −0.014 (−0.156–0.122) | 0.041 (−0.067–0.144) | 0.432 | 0.766 |
| Time Horizon 20 years | 51,967 | 49,689 | 2279 (−2658 to 7464) | 8.312 | 8.058 | 0.254 (0.114–0.395) | 8985 | 0.140 (−0.149 to 0.407) | 0.178 (−0.035 to 0.377) | 0.833 | 0.946 |
| Time Horizon 40 years | 78,506 | 77,079 | 1427 (−5292 to 8483) | 10.014 | 9.594 | 0.420 (0.196–0.647) | 3397 | 0.349 (−0.034 to 0.711) | 0.372 (0.084–0.660) | 0.962 | 0.995 |
| Subgroup analyses (assuming hypo benefit, process benefit and ongoing HbA1c benefit) | |||||||||||
| HbA1c | |||||||||||
| 58–75 mmol/mol (7.5–9%) | 72,885 | 67,574 | 5311 (−1066 to 11,417) | 9.887 | 9.587 | 0.301 (0.063–0.530) | 17,673 | 0.035 (−0.297 to 0.379) | 0.123 (−0.144 to 0.394) | 0.576 | 0.807 |
| >75–97 mmol/mol (>9–11%) | 104,454 | 112,468 | −8014 (−17,170 to 875) | 10.087 | 9.492 | 0.595 (0.383–0.803) | Dominant | 0.996 (0.542–1.490) | 0.863 (0.543–1.210) | 1.000 | 1.000 |
| Treatment modality | |||||||||||
| CSII | 78,406 | 77,752 | 654 (−6316 to 7891) | 10.069 | 9.600 | 0.469 (0.240–0.701) | 1394 | 0.436 (0.070–0.800) | 0.447 (0.182–0.725) | 0.990 | 0.997 |
| MDI | 77,549 | 75,244 | 2306 (−4374 to 9110) | 9.922 | 9.507 | 0.414 (0.183–0.648) | 5565 | 0.299 (−0.044 to 0.687) | 0.337 (0.062–0.623) | 0.949 | 0.995 |
| Prior participation in structured education | |||||||||||
| Yes | 75,343 | 73,982 | 1361 (−5494 to 8248) | 9.775 | 9.313 | 0.462 (0.230–0.689) | 2946 | 0.394 (0.038–0.766) | 0.417 (0.146–0.708) | 0.985 | 0.999 |
| No | 84,893 | 82,368 | 2524 (−4938 to 10,433) | 10.425 | 10.049 | 0.376 (0.151–0.607) | 6715 | 0.250 (−0.188 to 0.657) | 0.292 (−0.042 to 0.607) | 0.873 | 0.955 |
- a Process benefit refers to the assumption that process utility for isCGM compared with SMBG is +0.03 QALYs per year.21
3.4 Sensitivity analysis
Use of isCGM would need to reduce HbA1c for around 5 years for isCGM to have an ICER better than £30,000/QALY and around 7 years to fall below £20,000/QALY, if hypoglycaemia and process-utility benefits are included (Figure 2). If we remove one or both of these benefits, a difference in HbA1c lasting up to 10 years may be necessary to achieve an ICER of better than £20,000/QALY.
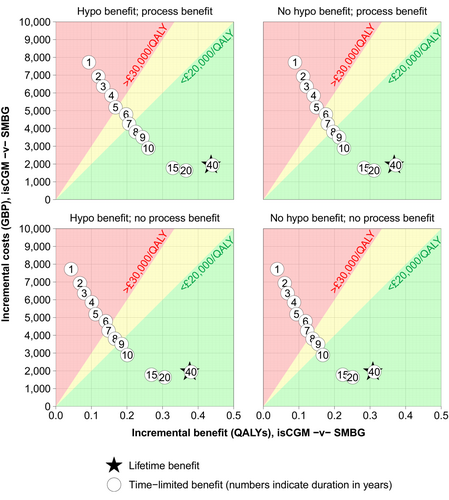
The ICER rises marginally above £20,000/QALY when the model is limited to 10 years, which is insufficient time for the short-term glucose control with which isCGM is associated to translate into all expected long-term benefits.
The QALY gain is lower when we remove the modelled additional benefits of reduced hypoglycaemic episodes and process-utility gain, but ICERs are no higher than £6500/QALY.
3.5 Subgroup analysis
In all subgroups (Table 3), isCGM meets NICE's lower cost-effectiveness threshold of £20,000/QALY, compared with SMBG. In people with higher HbA1c (>75 mmol/mol [>9%]) at baseline, isCGM becomes cost-saving (Figure 1).
4 DISCUSSION
The within-trial differences in cost (£548) reflect increased acquisition costs of isCGM compared with SMBG. As expected, there was very little difference in health status at 24 weeks between the arms, as the anticipated effects of better HbA1c on health status would not be evident in such a short timescale.
In the base-case analysis of the long-term economic evaluation, isCGM was cost-effective compared with SMBG at a threshold of £20,000 per QALY. The cost-effectiveness results were robust across different scenarios, apart from: a 10-year or less time horizon and the effect of isCGM lasting equal to, or less than, 7 years of use.
Our RCT was the first robust study to show that isCGM with optional alarms in adults with type 1 diabetes can safely reduce HbA1c levels. This is the first health economics study using primary data from an RCT of isCGM in adults with type 1 diabetes, where accurate data on quality of life and clinical outcomes have been collected, and the long-term costs and benefits of isCGM have been estimated in this cohort. The mean (SD) number of sensor scans carried out by participants per full 24 hours was 11.00 (6.2).5 This is similar in magnitude to the mean (SD) number of scans reported in a UK real-world cohort of 12.9 (14.1).22
Recent work suggests that CGM is cost-effective compared with SMBG among people with type 1 diabetes in the UK.23 A review of the cost-effectiveness of technologies in adults with type 1 diabetes did not find any studies of isCGM in adults with high HbA1c levels but still concluded that glucose sensors were cost-effective.24 One Canadian modelling study of people with type 1 diabetes and HbA1c of 8.1% reported a cost per QALY of $17,488 CAD for isCGM compared with SMBG.25 NICE, Healthcare Improvement Scotland and Health Technology Wales suggest that isCGM is cost-effective compared with SMBG in type 1 diabetes.26-28 However, in the absence of primary data in an appropriate cohort, each of these reports relied on the results of the IMPACT trial, from people with type 1 diabetes with an HbA1c of less than 7.5%, which did not find a significant reduction in HbA1c.2 These analyses estimated isCGM to be cost-effective on the basis of reductions in non-severe hypoglycaemic events and SMBG use, rather than any reduction in HbA1c.
The economic analysis carried out by NICE generated a cost per QALY of £10,157 for isCGM compared with SMBG, the benefit accruing from assumed lower hypoglycaemia rates with isCGM.27 Our analysis has the benefit of the results of the FLASH-UK RCT, so when we incorporated the HbA1c reduction demonstrated in our trial, this generated a lower ICER of £4477 per QALY.
There are important limitations with our approach, one of which being that we have relied on the results of the FLASH-UK RCT, one trial of 156 patients with a 24-week time horizon.
There was uncertainty around the model input parameters. Most importantly, we assumed that the reduction in HbA1c at 24 weeks was sustained, with continued use (and associated cost) of isCGM. Therefore, multiple sensitivity analysis scenarios were carried out for this specific parameter, suggesting how long isCGM use and its benefits would need to continue to demonstrate cost-effectiveness compared with SMBG. Given the evolving nature of medical technology, input parameters into a future economic evaluation of isCGM may need to be re-examined, as it is likely that certain cost components will change over time, such as purchase costs. The comparators may also change, for example, CGM may become easier to use and less costly. In addition, in the NHS in England, the technologies for reading the sensors have zero cost, whereas this may not be the case in other healthcare settings.
The use of an established decision-model to estimate long-term effects of HbA1c control is both a strength and a weakness of our analysis. IQVIA CDM is a trusted simulation that has been extensively validated against empirical data,15 including in head-to-head comparisons at the Mount Hood Challenge.29 Many decision-makers place reliance on its predictions, including NICE. Critically, it enables us to project the lifetime implications of short-term benefits, which is not possible using empirical data alone. But it is not perfect. While most of its predictions derive from type 1-diabetes-specific evidence, some aspects (e.g. background diabetes mortality) rely on equations from a type 2 diabetes population (UKPDS). It has a fixed cycle-length, dictating that we applied the 24-week results from our trial at a 1-year time point. No other accessible models currently provide a better solution to these issues. Future work for this study team would be to explore structural uncertainty by using different models.
Another limitation of the economic evaluation was the need to incorporate hypoglycaemia data from sources other than the trial, so we made the conservative assumption that isCGM had no effect on SHE rates.18 Event rates of non-severe and nocturnal hypoglycaemic events were taken from the IMPACT trial because this trial provided a measure of relative occurrence between isCGM and SMBG in type 1 diabetes, although the cohort had better controlled disease than our cohort.19
The key limitation of the within-trial analysis was that there was 15% missing data for costs and EQ-5D parameters, assumed to be missing at random, but this may have biased the results of the within-trial analysis, especially as there were more missing data in the SMBG arm.
It is important for policymakers and payers to have clinical and cost information when making reimbursement decisions. A budget impact analysis from a UK nationwide audit of people with type 1 diabetes suggested that isCGM is £168 per person per year more expensive to provide or purchase than SMBG,18 with a small cost avoided (£21) due to changes in HbA1c. This provides some real-world estimates of the budget impact of isCGM in the short-term but does not allow policymakers and payers to understand the additional costs of isCGM in the context of longer term benefits, and does not focus on people with type 1 diabetes with high HbA1c.
This study has relevance beyond the UK setting. Policymakers and payers around the world have to consider how best to invest in diabetes care and minimise unmet need. Access to cost-effective equipment for self-monitoring of glucose in people with type 1 diabetes is essential to reduce the clinical and economic impact of diabetes and its complications.
5 CONCLUSION
In people with type 1 diabetes with high HbA1c (58–97 mmol/mol [7.5%–11%]), isCGM was cost-effective compared with SMBG for a willingness-to-pay threshold of £20,000/QALY. In the subgroup of people with highest baseline HbA1c (>75–97 mmol/mol [>9%–11%]) it was cost-saving. Our results suggest that isCGM needs to maintain its effect for around 7 years to achieve cost-effectiveness.
AUTHOR CONTRIBUTIONS
LL, EGW and ME conceptualised the RCT. RAE conceptualised the economic evaluation. LL, EGW, ME, IC, PN, SN, RE, CS, HT contributed to the grant application. MB was the Lead Clinical Trial Manager. CS, AK and VT are responsible for statistical analysis. All authors contributed to protocol development at various stages. LL, EGW, ME, IC, PN, SN, HT, GER, SL, NK, CK provided site oversight and were responsible for study conduct at each site. RE & GAR are responsible for the health economic analysis. RAE and LL wrote the first draft of the manuscript and all authors reviewed and had the opportunity to comment on the content prior to submission. The corresponding author confirms that all co-authors are ICMJE recommendation compliant for the submission of this manuscript. No professional writers have been engaged for the preparation of this manuscript.
FUNDING INFORMATION
This work was supported by Diabetes UK grant number 18/0005836. The device manufacturer played no part in design, conduct or any other aspects of the study. The study devices were paid by the National Health Service (NHS) UK. Work was supported by the NIHR Cambridge Biomedical Research Centre. The University of Cambridge has received salary support for MLE from the National Health Service in the East of England through the Clinical Academic Reserve.
CONFLICT OF INTEREST STATEMENT
EGW has received personal fees from Abbott Diabetes Care, Astra Zeneca, Dexcom, Eli Lilly, Embecta, Glooko, Insulet, Medtronic, Novo Nordisk, Roche, Sanofi, Ypsomed. LL has received personal fees from Abbott Diabetes Care, Dexcom, Insulet, Medtronic, Novo Nordisk, Sanofi Diabetes Care. ME has received personal fees from Abbott Diabetes Care, Eli Lilly, Medtronic, Dexcom, Novo Nordisk, Astra Zeneca, Zucara. SN has received personal fees from QUIN, Roche, Abbott Diabetes Care, Insulet, Astra Zeneca. NK has received personal fees from Abbott, Eli Lilly, Novo Nordisk, Astra Zeneca, Napp, Sanofi. PN has acted as a clinical expert for NICE Medtech innovation briefing MIB110 relating to FreeStyle Libre system. GER has received personal fees from Abbott Diabetes UK, Novo Nordisk, Sanofi Diabetes Care and Eli Lilly. RAE has received personal fees from Janssen and Takeda. GAR, MB, KBK, GG, CJS, CK, VT, AK declare no competing interests.




