Structured group education programme and accompanying mHealth intervention to promote physical activity in women with a history of gestational diabetes: A randomised controlled trial
Abstract
Aims
Assess effectiveness of a hybrid intervention targeting physical activity in women with prior gestational diabetes.
Methods
Randomised controlled trial with parallel arms. 293 women (35.1 ± 5.1 years; 40% ethnic minority) recruited from two hospitals and randomised to routine care or hybrid lifestyle intervention comprising two group sessions and access to a mobile web app. Primary outcome was a change in objectively measured physical activity at 12 months. Secondary outcomes included self-efficacy for exercise, quality of life and anxiety and depression. Linear regression compared outcome measures between groups.
Results
83% of intervention participants attended at least one group session, of who 66% registered to use the app. There was a non-significant increase in physical activity at 12 months (between-group difference of 0.95 mg [95% CI: −0.46 to 2.37]), equivalent to approximately 500 steps per day. Intervention participants reported higher self-efficacy for exercise (0.54, 95% CI: 0.05 to 1.102; p = 0.029), lower anxiety (−0.91, 95% CI: −1.74 to −0.09; p = 0.031), and higher quality of life (0.05, 95% CI: 0.004 to 0.09; p = 0.032), compared to controls.
Conclusions
The intervention improved confidence in exercise and quality of life. Further research is needed to improve participant engagement with physical activity interventions in multi-ethnic populations with a history of gestational diabetes.
What is already known?
- Risk of type 2 diabetes is increased 10-fold in women who have had gestational diabetes.
- Strategies are needed to reduce the progression of gestational diabetes to type 2 diabetes.
What this study has found?
- Randomised controlled trial of a hybrid intervention showed improvements in exercise self-efficacy, anxiety and quality of life in 293 women with post-gestational diabetes.
What are the implications of the study?
- mHealth component of the intervention has the potential for low cost and scalability but strategies are needed to improve engagement.
1 INTRODUCTION
Gestational diabetes (GDM) affects around 5%–20% of pregnancies depending on the population, screening criteria and diagnostic criteria used.1 GDM increases the risk of developing type 2 diabetes (T2DM) by at least 10-fold2 and doubles the risk of cardiovascular disease.3 Nearly 50% of women with GDM have pre-diabetes or T2DM within 10 years of index pregnancy,4 highlighting the importance of strategies for preventing progression from GDM to T2DM. The National Institute for Health and Care Excellence (NICE) recommends lifestyle advice and a fasting glucose or HbA1c test at 13 weeks post-partum, followed by an annual HbA1c test in primary care.5 If diagnosed with prediabetes (HbA1c 42 to 49 mmol/mol [6.0% to 6.4%]), guidance on preventing T2DM should be offered.
Large prevention trials have shown that intensive lifestyle interventions reduce T2DM incidence by up to 50% in people with impaired glucose regulation6 and lifestyle interventions delivered within routine clinical settings can elicit weight loss and reduction in diabetes risk.7 Consequentially, diabetes prevention programmes have been implemented internationally8, 9 but none specifically target those with a history of GDM. Randomised controlled trials (RCT) are limited, often of small size and not including multi-ethnic populations. In the majority of studies, the primary outcome is weight loss and physical activity is self-reported.10
The study objective was to assess the clinical and cost-effectiveness of a hybrid intervention, incorporating both face-to-face and mHealth components and targeting physical activity, in a multi-ethnic population of women with a history of GDM.
2 SUBJECTS, MATERIAL AND METHODS
2.1 Study design
This was a two-centre, parallel-design RCT with stratified randomisation. Ethical approval was granted by East Midlands–Derby Research Ethics Committee, UK (16/EM/0488). Recruitment took place between July 2017 and July 2018. The study was prospectively registered (ISRCTN 17299860) and the protocol has been reported.11
2.2 Participant eligibility criteria
Women aged ≥18 years on GDM registers of two hospitals in England (Leicester and Nuneaton) were sent postal invitations if they had a diagnosis of GDM during any pregnancy in the previous 5 years. Exclusion criteria were inability to speak or read English, current pregnancy or diagnosis of type 1 or type 2 diabetes, cancer, severe mental illness, previous intervention for obesity, lack of access to the internet or participation in another clinical trial in the preceding 12 weeks.
2.3 Randomisation and blinding
Eligible participants were randomised to intervention or control arms (1:1), stratified by age (<30 years; ≥30 years) and ethnicity (White European; other) using a variable block size with concealed allocation sequence produced prior to study commencement. The sequence was generated by an independent statistician and allocation was carried out by an independent researcher. Blinding of participants was not possible, but staff analysing the primary outcome accelerometer data were blinded to group allocation.
2.4 Study intervention
Control participants were given a diabetes prevention booklet routinely used in primary care. Intervention participants were invited to take part in the Baby Steps programme, comprising two group education sessions and an accompanying mHealth intervention. The programme was developed by a multi-disciplinary team with substantial co-production from multi-ethnic patient and stakeholder groups following an iterative pathway comprising stages of design, testing and refinement.11
2.4.1 Group sessions
These were based on the Let's Prevent Diabetes programme, which has been shown to be effective at improving physical activity behaviour in individuals at increased T2DM risk12 and is based on robust theoretical frameworks and the philosophy of patient empowerment.13, 14 Two group-based sessions were delivered 2 weeks apart. The first session discussed opportunities to make lifestyle changes to reduce the risk of developing T2DM, focusing on physical activity. Participants were encouraged to set a target of an additional 30 min of moderate activity per day and given a wrist-worn activity tracker to aid monitoring. A link and access code to the mHealth component of the programme were provided at the end of the first session. During the second session, physical activity was revisited and diet as a modifiable risk factor was discussed. Participants completed an action plan which they were encouraged to review regularly.
2.4.2 mHealth intervention
The mHealth component was a mobile web application11, 15 intended to (1) provide interactive bite-sized information resources in a number of formats to supplement the group sessions and (2) motivate the participant to become more active. The activity tracker could be connected to the app to monitor and review the daily step count. ‘Leader boards’ allowed participants to compete against each other and participants were able to set goals and record information such as body weight. Automated messages related to goal setting, goals achieved and setting of new challenges were sent at regular intervals. Participants could join a team or global chat forum to share challenges and experiences with peers.
2.5 Measurement outcomes
Baseline data were collected after participants had provided written informed consent. Follow-up data were collected at 6 months (by post) and 12 months. Clinic visits were run by trained research nurses following standard operating procedures.
2.5.1 Primary outcome
The primary outcome was a change in daily average acceleration (proxy for overall physical activity), from baseline to 12 months measured using the wrist-worn GENEActiv accelerometer (Activinsights Ltd.). Higher values of average acceleration (milli-gravitational units [mg]) represent a more physically active day. The change was calculated as mean daily physical activity (mg) at 12 months minus mean physical activity (mg) at baseline. Participants wore the accelerometer continually on their non-dominant wrist for eight consecutive days and recorded their sleep and wake times in a diary at baseline, 6 and 12 months. Data were processed with R-package GGIR version 1.9, (http://cran.r-project.org).16 This included auto-calibration relative to local gravity, detection of non-wear, and calculation of average acceleration corrected for gravity (Euclidean Norm minus 1 g) averaged over 5 s epochs. Files with post-calibration error >0.01 g (10 mg), or no valid days (≥16 h wear time per day, irrespective of weekday/weekend) were excluded.
Interpretation of primary outcome
Average acceleration captures all movement undertaken, with greater intensity or longer duration of movement resulting in greater acceleration. Triangulation of data sources has recently suggested that a difference in average acceleration over a 24 h day of 0.8–1 mg approximates a difference of ~500 steps/day.17 Based on associations with mortality, it has been proposed that 1 mg represents the minimum clinically meaningful difference for overall physical activity in an inactive population.17 Changes to the primary outcome are interpreted on this basis.
2.5.2 Secondary outcomes
A number of physical activity secondary outcomes were generated from the GENEActiv data (Table 1). Other secondary outcomes were anthropometric measures (body weight, body mass index (BMI), hip and waist circumferences), clinical measures (blood pressure, resting heart rate) and biochemical measures (glycated haemoglobin [HbA1c] and lipid profile). These data were collected at baseline and 12 months. Questionnaires were completed at baseline, 6 and 12 months. These contained the Recent Physical Activity Questionnaire (RPAQ),18 Health-Related Quality of Life (EQ-5D-5L),19 Hospital Anxiety and Depression Scale (HADS),20 and Jenkins Self-Efficacy-for-Exercise-Scale.21 Utility values were generated from the EQ-5D-5L using reported estimates from crosswalk methodology mapping EQ-5D-5L to EQ-5D-3L value sets.22 The Five-A-Day Consumption and Evaluation Tool (FACET) was used as a measure of fruit and vegetable intake.23
| Characteristics | Control n = 150 | Intervention n = 143 | Total n = 293 |
|---|---|---|---|
| Age (years) | 34.8 (4.7) | 35.5 (5.5) | 35.1 (5.1) |
| Ethnicity, No. (%) | |||
| White | 89 (59) | 87 (61) | 176 (60) |
| South Asian | 53 (35) | 45 (31) | 98 (34) |
| Others | 8 (6) | 11 (8) | 19 (6) |
| Smoking statusa, No.(%) | |||
| Non-smokers | 134 (89) | 137 (96) | 271 (92) |
| Current smokers | 16 (11) | 6 (4) | 22 (8) |
| Alcohol consumption, No. (%) | |||
| Non-drinker | 58 (39) | 51 (36) | 109 (37) |
| ≤2–4 times a month | 69 (46) | 73 (51) | 142 (49) |
| >2–4 times a month | 23 (15) | 19 (13) | 42 (14) |
| Body weight (kg) | 78.3 (19.7) | 76.6 (16.8) | 77.5 (18.3) |
| Body mass index (kg/m2) | 29.5 (6.5) | 28.8 (5.7) | 29.2 (6.1) |
| BMI cat. for White & othersb, No.(%) | |||
| Normal | 22 (23) | 23 (23) | 45 (23) |
| Overweight | 26 (27) | 34 (35) | 60 (31) |
| Obese | 49 (50) | 41 (42) | 90 (46) |
| BMI cat.for South Asianc, No.(%) | |||
| Normal | 10 (19) | 8 (18) | 18 (18) |
| Overweight | 19 (36) | 12 (27) | 31 (32) |
| Obese | 24 (45) | 25 (56) | 49 (50) |
| Waist (cm) | 95.9 (14.1) | 94.8 (13.5) | 95.4 (13.8) |
| Hip (cm) | 109.0 (13.5) | 108.9 (13.5) | 109.0 (13.4) |
| Waist-to-hip ratio (cm) | 0.88 (0.07) | 0.87 (0.07) | 0.88 (0.07) |
| Systolic blood pressure (mm Hg) | 112 (13) | 111 (11) | 112 (12) |
| Diastolic blood pressure (mm Hg) | 77 (9) | 76 (8) | 77 (9) |
| Heart rate (bpm) | 73 (9) | 74 (10) | 73 (10) |
| Total cholesterol (mmol/L) | 4.8 (0.9) | 4.8 (0.9) | 4.8 (0.9) |
| Triglycerides (mmol/L) | 1.5 (0.8) | 1.5 (0.8) | 1.5 (0.8) |
| HDL-c (mmol/L) | 1.4 (0.4) | 1.4 (0.3) | 1.4 (0.4) |
| LDL-c (mmol/L) | 2.8 (0.8) | 2.7 (0.8) | 2.8 (0.8) |
| TC-HDL ratio (mmol/L) | 3.6 (1.0) | 3.6 (1.0) | 3.6 (1.0) |
| HbA1c (mmol/mol) | 36.8 (3.7) | 37.7 (3.8) | 37.2 (3.8) |
| HbA1c (%) | 5.5 (0.3) | 5.6 (0.4) | 5.6 (0.4) |
| Pre-diabetesd, No. (%) | 16 (11) | 22 (15) | 38 (13) |
| GDM diagnosed, No. (%) | |||
| 1 occasion | 116 (77) | 109 (76) | 225 (77) |
| 2 occasions | 28 (19) | 29 (20) | 57 (20) |
| ≥3 occasions | 6 (4) | 5 (4) | 11 (4) |
| Time since most recent GDM (months) | 22 (17) | 21 (17) | 22 (17) |
| Months since most recent GDM, No. (%) | |||
| ≤12 months | 56 (38) | 60 (42) | 116 (40) |
| >12 months and ≤24 months | 35 (24) | 30 (21) | 65 (22) |
| >24 months | 58 (38) | 53 (37) | 111 (38) |
| Number of children, No. (%) | |||
| 1 child | 50 (33) | 44 (31) | 94 (32) |
| 2 children | 55 (37) | 72 (50) | 127 (43) |
| ≥3 children | 45 (30) | 27 (19) | 72 (25) |
| Family history of diabetes (first-degree relatives) | |||
| Type 2 diabetes, No. (%) | 83 (55) | 73 (51) | 156 (53) |
| Gestational diabetes, No. (%) | 12 (8) | 15 (10) | 27 (9) |
| Physical activity (from GENEActiv data) | |||
| Daily average acceleration (mg) | 29.7 (7.2) | 28.3 (6.4) | 29.0 (6.8) |
| Av. accel for most active 30 min (mg) | 78.4 (37.8) | 73.7 (26.4) | 76.1 (32.9) |
| MVPA 1 min bout (min) | 32.7 (27.1) | 27.2 (21.2) | 30.1 (24.6) |
| ≥150 min MVPA per week, No. (%) | 82 (57) | 70 (53) | 152 (55) |
| Sedentary time per day (<40 mg) (min) | 666 (92) | 676 (108) | 671 (100) |
| Sleep duration per night (min) | 380 (53) | 381 (57) | 380 (55) |
| Number of valid measurement (days) | 6.8 (0.5) | 6.7 (1.0) | 6.8 (0.8) |
- Note: MVPA = moderate to vigorous intensity physical activity (threshold is ≥100 mg) accrued in 1-min bouts. Missing data: 1 hip; 2 total cholesterol; 2 HDL cholesterol; 5 LDL cholesterol; 2 triglycerides; 1 hypertension; 17 overall accelerometer variables; 21 sedentary time; 21 sleep duration; 0 all other variables.
- a Ex-smokers were included in the non-smokers category.
- b For White and others: Normal (BMI < 25), Overweight (BMI ≥ 25 & BMI ≤ 30), Obese (BMI > 30).
- c For South Asians: Normal (BMI < 22.5), Overweight (BMI ≥ 22.5 & BMI ≤ 27.5), Obese (BMI > 27.5).
- d HbA1c ≥ 6.0 and <6.5 % (≥42 and <46 mmol/mol).
2.6 Sample size
In order to detect a significant difference in average acceleration of 2.1 mg, which is equivalent to the increase in overall physical activity that would result from ~30 min of light walking per day,24 assuming a standard deviation of 5.3 mg,25 a power of 80% and significance level of 5%, the sample size required 202 participants. Allowing for a 20% loss to follow-up and 10% non-compliance of the GENEActiv monitor, 290 participants (145 per arm) needed to be recruited.
2.7 Statistical analysis
A statistical analysis plan was predefined. Categorical baseline variables were presented by the group as numbers (percentages) and continuous variables as means (standard deviations).
The primary outcome was analysed using a linear regression model with change from baseline in overall daily physical activity at 12 months as the dependent variable and randomisation group as the explanatory variable, adjusted for stratification factors (age and ethnicity), change in wear time between baseline and 12 months and baseline value. Participants who had worn the accelerometer for at least one valid day were included; sensitivity analyses were carried out using two, three and four valid days.
The main analysis was conducted with a modified intention to treat where the participants with missing data for any of the included covariates were excluded. Sensitivity analyses were conducted on intention-to-treat (using multiple imputations) and per-protocol basis (only participants who attended at least one education session were included in the intervention group). Multiple imputation was carried out in Stata (Version 15), missing values were replaced with simulated values using 100 imputations, with analysis carried out on each set. Rubin's formula26 was used to combine these into a single set of results. The imputation model included the variables used in the subsequent regression analysis (overall physical activity at 12 months, randomisation group, stratification factors and overall physical activity at baseline). Given the slight non-normal distribution of the residuals for our primary outcome analysis, a post hoc exploratory analysis was conducted using square root transformation for change in daily physical activity level at 12 months.
Subgroup analyses were performed to look at whether the intervention effects were different between the following pre-specified subgroups of baseline characteristics: median age (< & ≥35 years), number of GDM episodes (=1 & ≥2), ethnicity (White, south Asian & others), BMI categories (normal, overweight & obese) and parity (=1 & ≥2). The adjusted interaction between treatment and subgroups was used to assess differences in outcome by subgroup and results were presented as a forest plot. Adjustments were made for stratification factors and baseline overall physical activity and change from baseline in accelerometer wear time. The effect of level of engagement in the intervention on the primary endpoint was also assessed, adjusted and unadjusted. Additional subgroup analyses explored differences by site, time since GDM (< & ≥1 year) and meeting weekly MVPA targets (< & ≥150 min). These additional analyses were not pre-specified and should be viewed as hypothesis-generating. Secondary outcomes were analysed in a similar manner to the primary outcome, and the analysis was repeated for each time point (6 and 12 months). Statistical significance was assessed at the 5% level with a 95% confidence interval, and all analyses were completed using Stata (v.15).
Cost-effectiveness was assessed by fitting a simple probabilistic decision-analytic model using the software WinBUGS, to generate an incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (generated from EQ-5D-5L), and a cost-effectiveness acceptability curve (CEAC). Total costs of the intervention for 143 participants included costs for staff, teaching materials, participant costs, refreshments, and hosting the mobile app. The time horizon modelled was that of the duration of the trial (12 months). Sensitivity analyses were also run, assuming the difference in quality of life was maintained for 6 months and for 3 years.
3 RESULTS
The flow of participants through the study is shown in Figure 1. Of 3581 women invited, 530 (14.8%) returned a reply slip expressing interest. 304 (57.4%) provided consent and 293 were randomised (143 intervention and 150 control). Baseline characteristics are shown in Table 1 and were similar in both arms (35.1 ± 5.1 years, 40% ethnic minority, BMI of 29.2 ± 6.1).
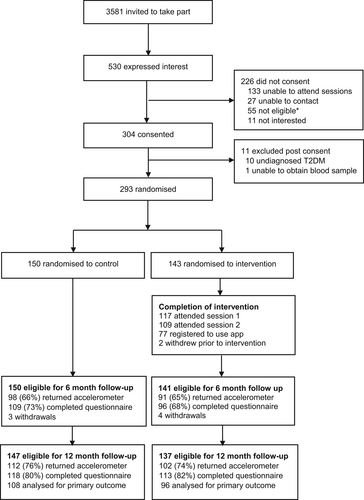
Of the 143 intervention participants, 117 (83%) attended the first group session and 109 (77%) attended both sessions. After attending Session 1, 77 participants (66%) registered to use the mobile web app. Level of engagement with components of the app varied, for example, 62 (82%) linked their fitness tracker to the app, 23 (30%) viewed at least one monthly Booster Session and 23 (30%) used the chat forum. Attendance at group sessions and subsequent registration with the app was higher in white Europeans compared to ethnic minorities (89% vs. 71% and 75% vs. 48% respectively).
No significant difference in change in overall physical activity (i.e., daily average acceleration) in the complete case analysis was seen between intervention and control groups at 12 months. There was a between-group difference in daily average acceleration of 0.95 mg (95% CI: −0.46 to 2.37), approximating 500 steps per day (Table 2). Similar results were obtained in the intention-to-treat and per protocol analyses (Table 2). When transforming the primary outcome data to account for the slight non-normal distribution of the residuals, comparable results were found (Table S1). Sensitivity analysis based on minimum wear criteria of 2, 3 and 4 days showed comparable results (Table S2). Various sub-group analyses showed no significant interaction effects of the intervention on the primary outcome (Figure S1). The level of engagement in the intervention did not alter the effect of the intervention on the primary outcome (Figure S2).
| Number of participants | Mean change from baseline | Adjusted difference at follow-upa | ||||
|---|---|---|---|---|---|---|
| Control (n = 150) | Intervention (n = 143) | Control | Intervention | Coefficient (95% CI) | p-value | |
| Modified intention to treatb | ||||||
| Change physical activity (mg) | 108 | 96 | −0.02 | 1.30 | 0.95 (−0.46 to 2.37) | 0.185 |
| Square root transformation | −0.07 (−0.43 to 0.30) | 0.722 | ||||
| Intention to treatc | ||||||
| Overall physical activity (mg) | 150 | 143 | −0.10 | 1.30 | 0.97 (−0.40 to 2.33) | 0.165 |
| Per protocold | ||||||
| Overall physical activity (mg) | 108 | 88 | −0.02 | 1.11 | 0.82 (−0.63 to 2.26) | 0.266 |
- a Adjusted for stratification factors: age (<30 vs. ≥30) and ethnicity (White vs. Other); change from baseline in accelerometer wear time and baseline value of outcome.
- b Participants with missing outcome data or missing variables required for the model adjustment were excluded.
- c Missing data imputed using multiple imputation (n = 100).
- d Participants who did not engage in at least one group session of the programme have been excluded from the intervention arm.
Secondary outcomes analysis of physical activity measures from accelerometer data (Table S3), self-reported physical activity (Table S4), and anthropometric and clinical outcomes (Table S5) showed no statistically significant between-group differences apart from lower self-reported home-based physical activity at 12 months in the intervention arm (−0.17 kcal/kg/day, 95% CI: −0.34 to 0.00: p = 0.185). Self-Efficacy-for-Exercise and quality of life scores were significantly higher in the intervention compared to control group at 12 months (0.54, 95% CI: 0.05 to 1.02; p = 0.029) and (0.05, 95% CI: 0.004 to 0.09; p = 0.032), respectively (Table 3). The anxiety score was lower at 12 months in the intervention group compared to the control (−0.91, 95% CI: −1.74 to −0.09; p = 0.031) (Table 3).
| Number of participants | Mean change from baseline | Adjusted difference at follow-upa | ||||
|---|---|---|---|---|---|---|
| Control n (150) | Intervention n (143) | Control | Intervention | Coefficient (95% CI) | p-value | |
| Self-efficacy for exercise (SEE)b | ||||||
| SEE—6 months | 108 | 94 | −0.72 | −0.29 | 0.45 (−0.03 to 0.94) | 0.069 |
| SEE—12 months | 118 | 113 | −0.18 | 0.14 | 0.54 (0.05 to 1.02) | 0.029 |
| Hospital anxiety and depression (HADS)c | ||||||
| Anxiety 6 months | 107 | 94 | 1.03 | 0.26 | −0.69 (−1.60 to 0.21) | 0.132 |
| Anxiety 12 months | 116 | 113 | 0.66 | −0.37 | −0.91 (−1.74 to −0.09) | 0.031 |
| Depression—6 months | 109 | 96 | 1.25 | 0.91 | −0.37 (−1.20 to 0.46) | 0.285 |
| Depression—12 months | 118 | 113 | 0.27 | −0.16 | −0.48 (−1.25 to 0.29) | 0.222 |
| EuroQoL EQ-5D-5Ld | ||||||
| Index score—6 months | 109 | 96 | −0.04 | −0.07 | −0.02 (−0.06 to 0.02) | 0.329 |
| Index score—12 months | 117 | 113 | −0.07 | −0.02 | 0.05 (0.004 to 0.09) | 0.032 |
| VAS scale—6 months | 109 | 95 | −2.66 | 0.15 | 2.77 (−1.81 to 7.34) | 0.234 |
| VAS scale—12 months | 118 | 112 | 0.16 | 3.62 | 3.01 (−0.90 to 6.91) | 0.131 |
| Fruit & vegetable intake (FACET)e | ||||||
| FACET—6 months | 109 | 96 | 0.12 | −0.01 | −0.12 (−0.23 to 0.00) | 0.050 |
| FACET—12 months | 118 | 113 | 0.09 | 0.11 | 0.02 (−0.10 to 0.14) | 0.750 |
- a Adjusted for stratification factors: age (<30 vs. ≥30) and ethnicity (White vs. Other); and baseline value. Participants with missing outcome data or missing variables required for the model adjustment were excluded.
- b SEE; Scale for all responses ranges from 0 = not confident to 10 = very confident; total scores range from 9 to 90 with higher scores indicating higher self-efficacy for exercise.
- c HADS; responses were for over the last week; scale for anxiety and depression responses range from 0 to 3; total raw scores for both anxiety and depression range from 0 to 21, 0 representing feeling normal and 21 representing anxiety or depression.
- d EQ-5D-5L; Higher scores indicating good health status.
- e Number of portions ranges from 0 to 4+.
The total intervention delivery cost (excluding research costs) was estimated as £35,751.55 for 143 participants (£250 per participant) (Table S5), whilst the mean adjusted difference in quality of life at 12 months was 0.05 (95% Credible Interval (CrI): 0.004 to 0.09). Assuming this difference was representative of the value throughout the 12-month period, the ICER estimates a cost per QALY gained of £4937 (95% CrI: £2525 to £24,780), and gives a 95% probability of the intervention being cost-effective at the £20,000 willingness-to-pay threshold. Assuming the difference in quality of life was maintained for just 6 months, cost per QALY was estimated as £9873 (95% CrI: £5050 to £49,570) and for 3 years as £1373 (95% CrI £841 to £8261).
Nine serious adverse events (6 control, 3 intervention) were recorded. None were related to the study intervention and one event led to the participant being withdrawn from the study.
4 DISCUSSION
Although the study did not show a significant improvement in objectively measured physical activity at 12 months, there were significant improvements in self-efficacy-for-exercise scores, anxiety levels and quality of life in the intervention group. This improvement in quality of life is cost-effective at the £20,000 willingness-to-pay threshold. Engagement in both components of the intervention was lower in ethnic minority participants.
The following were key strengths of the study. The programme was a bespoke intervention, collaboratively developed with various stakeholder groups.11 The study was a two-centre RCT involving a multi-ethnic population, with 33% of participants being South Asians, who are at higher risk of GDM and T2DM,27 thus increasing generalisation to the wider UK population. Objectively measured physical activity was an important strength.
There were a number of limitations to the study. We observed an improvement in the average daily acceleration of 0.95 mg, which approximates 500 steps/day and a change of this size has recently been suggested as clinically meaningful.17 However, our study, which was designed prior to establishing this lower level of a clinically meaningful difference, was powered to detect a difference of 2.1 mg. Consequently, the likelihood of a type-2 error in the primary outcome is high, especially when considering the positive effects seen for key secondary outcomes. This study was not powered to detect differences in all the measured outcomes and adjustments were not made for multiple testing, but all the results are reported and borderline p-values are interpreted taking into account the overall pattern of the results. Individual results should therefore be interpreted with caution. The mean time since GDM diagnosis was 21 months, with 40% of participants outside 2 years of diagnosis and there is tentative evidence that starting an intervention during pregnancy or the early post-partum period is more beneficial.28 At baseline, 55% of participants were meeting international weekly physical activity guidelines of 150 min of moderate to vigorous physical activity.29 This may have limited their capacity for benefit in response to the intervention. However sub-group analyses comparing these groups (Figure S1) showed no difference. The numbers in each sub-group were small, and a larger trial would be needed to draw these conclusions with confidence. The study was designed using the MRC complex intervention framework30 and hence it is not possible to know which of the components has worked. An in-built qualitative study31 has provided some insights from the participants on ‘what worked for them’. In future studies, it would be beneficial to build in a Realist Evaluation32 to delineate what element of the intervention worked and for whom.
Comparison with the literature is difficult as most lifestyle interventions in this population group have targeted post-partum weight loss,10 with self-reported physical activity as a secondary outcome. Mothers after Gestational Diabetes in Australia (MAGDA; n = 573) and Gestational Diabetes' Effects on Moms (GEM; cluster randomised trial; n = 2280) studies33, 34 are the largest trials reported, with both studies delivering the intervention during the 12-month post-partum period. GEM found an increase in self-reported vigorous physical activity of 15 min a week while MAGDA used self-reported physical activity to compare the numbers achieving physical activity goals but found no difference. Although a number of studies have given participants a pedometer to help them monitor their activity levels as part of an intervention, very few studies have objectively measured physical activity as an outcome. One small study35 used pedometers to measure physical activity and showed ‘a trend to increased physical activity’.
A possible reason for the lack of improvement in physical activity was the degree of engagement with the mobile web app, which is a key component of the programme. Only 66% of those who attended group sessions (i.e., 54% of intervention participants) registered to use the app and engagement in some of the components of the app was low. Lack of engagement with an intervention has been a problem in other trials in this population group. In MAGDA, 66% attended an initial face-to-face appointment and 53% attended the appointment and one or more of five group sessions. In GEM, where the intervention was telephone-based, only 50% of participants completed one or more of the telephone sessions.
Barriers to lifestyle intervention in this population include a low perceived risk of diabetes, competing demands of work and family and lack of childcare.36 The individual's self-reported motivation for exercise was significantly higher in the intervention group and there were significant reductions in self-reported anxiety and improvements in quality-of-life measures, which contributed to the overall cost-effectiveness of the programme. A small systematic review of overweight/obese women of reproductive age, albeit without a history of GDM, confirmed that lifestyle interventions significantly reduced anxiety scores.37
mHealth interventions have the potential for low cost and high efficacy, particularly when targeting large populations, but are currently limited in GDM and clinical practice due to barriers relating in part to data quality and security.38 More research is needed to develop and evaluate interactive and scalable online interventions for a multi-ethnic population of women with a history of GDM. As well as developing culturally adapted versions, strategies are needed to improve the acceptance rates of lifestyle RCTs and encourage engagement with the intervention, particularly in high-risk ethnic minority groups where engagement in the intervention was lower.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support of the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health and Research Care East Midlands, the NIHR Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre. The authors would like to thank the research staff involved in running the study and delivering the programmes at the two sites.
FUNDING INFORMATION
This research was funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health and Research Care East Midlands (CLAHRC-EM), now recommissioned as the NIHR Applied Research Collaboration East Midlands (ARC-EM), and the East Midlands Academic Health Science Network. Funders played no role in the design and delivery of the study.
The views expressed in this paper are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
CONFLICT OF INTEREST STATEMENT
All Leicester-based authors are or have previously been engaged in research developing and testing Diabetes Self-Management Education and Support programmes such as the DESMOND suite of programmes, one of which is Baby Steps. University Hospitals of Leicester NHS Trust supports the implementation of Baby Steps in Wales and Scotland as part of a national roll-out. In England, the National Diabetes Prevention Programme is the programme of choice, but there is an implementation study ongoing which is looking at the possible added value of Baby Steps. Other authors have no conflicts of interest to declare.




