Sex comparisons in the association of prediabetes and type 2 diabetes with cognitive function, depression, and quality of life: The Maastricht study
Abstract
Aims
There are sex differences in the excess risk of diabetes-associated cardiovascular disease. However, it is not clear whether these sex differences exist with regard to other complications like mental health aspects. Therefore, we investigated sex differences in the association of prediabetes and type 2 diabetes (T2D) with cognitive function, depression, and quality of life (QoL).
Materials and Methods
In a population-based cross-sectional cohort study (n = 7639; age 40–75 years, 50% women, 25% T2D), we estimated sex-specific associations, and differences therein, of prediabetes and T2D (reference: normal glucose metabolism) with measures of cognitive function, depression, and physical and mental QoL. Sex differences were analysed using multiple regression models with interaction terms.
Results
In general, T2D, but not prediabetes, was associated with higher odds of cognitive impairment, major depressive disorder, and poorer QoL. The odds ratio (OR) of cognitive impairment associated with T2D was 1.29 (95% CI: 0.96–1.72) for women and 1.39 (1.10–1.75) for men. The OR of major depressive disorder associated with T2D was 1.19 (0.69–2.04) for women and 1.68 (1.02–2.75) for men. The mean difference of the physical QoL score (ranging from 0 to 100, with 100 indicating the best possible QoL) associated with T2D was −2.09 (−2.92 to −1.25) for women and −1.81 (−2.48 to −1.13) for men. The mean difference of the mental QoL score associated with T2D was −0.90 (−1.79 to −0.02) for women and −0.52 (−1.23 to 0.20) for men. There was no clear pattern of sex differences in the associations of either prediabetes or T2D with measures of cognitive function, depression, or QoL.
Conclusions
In general, T2D was associated with worse cognitive function, depression, and poorer QoL. The strength of these associations was similar among women and men.
What is already known about this subject?
- Type 2 diabetes is associated with an excess risk of cardiovascular disease and other complications, such as worse cognitive function, depression, and poorer quality of life.
- Women with type 2 diabetes have a greater excess risk of cardiovascular disease than men with type 2 diabetes.
New findings
- There were no sex differences in the associations of prediabetes and type 2 diabetes with cognitive function, depression, and quality of life.
Clinical impact
- People with type 2 diabetes, independent of sex, have an excess risk of worse cognitive function, depression, and poorer quality of life.
1 INTRODUCTION
Diabetes mellitus is an established risk factor for several major cardiovascular diseases (CVD), including coronary heart disease (CHD) and stroke.1 However, not everyone with diabetes has the same degree of excess risk. Several studies have indicated that the excess risk of CVD conferred by diabetes is substantially greater for women than for men.1 There is emerging evidence that diabetes has also detrimental effects on mental health aspects, like cognitive function2 and depression,3 which might be partly vascular complications.4 Additionally, it has been shown that diabetes adversely affects an individual's quality of life.5 However, in contrast to CVD, it is less clear whether sex differences exist in mental health aspects and quality of life. Identifying these sex differences is important to allow guidelines to be more sex-sensitive and to allow clinicians and health professionals to better tailor care to individuals.6
Several,7, 8 but not all,9 previous studies among people with type 2 diabetes have shown that women are more likely to have cognitive impairment than men. Also, a generally higher prevalence of depression10, 11 and a poorer health-related quality of life12, 13 were reported among women with type 2 diabetes than among men with type 2 diabetes. Although these previous studies have shown sex differences in the prevalence of cognitive impairment, depression, and quality of life among people with type 2 diabetes, they did not include a reference group of people without type 2 diabetes. As such, it is unknown whether type 2 diabetes also is a stronger risk factor for the development of these outcomes in women than in men. A previous meta-analysis on sex differences in the association between type 2 diabetes and dementia showed that the diabetes-related risk of vascular dementia was substantially greater in women than in men.14 As the development of type 2 diabetes is a gradual process of increasing glucose intolerance, it is pertinent to include comparisons with individuals at different stages of the glucose intolerance spectrum, so as to understand where in the spectrum any sex difference might emerge. Subsequently, appropriate interventions, like sex-sensitive prevention for diabetes-associated cognitive decline, depression, and poorer quality of life, may be initiated.
In view of these considerations, we assessed whether the association of prediabetes and type 2 diabetes with cognitive function, depression, and quality of life differed between women and men.
2 MATERIALS AND METHODS
2.1 Study population
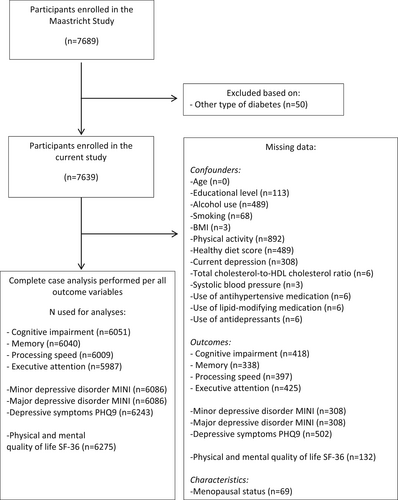
Data were used from The Maastricht Study, an observational prospective population-based cohort study. The rationale and methodology have been described previously.15 In brief, The Maastricht Study focuses on the aetiology, pathophysiology, complications, and comorbidities of type 2 diabetes and is characterised by an extensive phenotyping approach. Individuals aged between 40 and 75 years old at the study baseline, and living in the southern part of the Netherlands, were eligible to participate. Participants were recruited through mass media campaigns, and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known type 2 diabetes status, with an oversampling of individuals with type 2 diabetes, for reasons of efficiency. The present report includes cross-sectional data from the first 7689 participants, who completed the baseline survey between November 2010 and December 2017. The examinations of each participant were performed within a time window of 3 months. Participants with other types of diabetes than type 2 diabetes were excluded (n = 50; Figure 1). The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088–105234-PG). All participants gave written informed consent.
2.2 Assessment of glucose metabolism
To determine the glucose metabolism status, all participants underwent a standardised 2-h 75 g oral glucose tolerance test after fasting overnight. For safety reasons, participants using insulin or with a fasting glucose level above 11.0 mmol/L, as determined by a finger prick, did not undergo the oral glucose tolerance test. For these individuals (n = 64), fasting glucose levels and information about diabetes medication were used to determine glucose metabolism status. Glucose metabolism status was defined according to the World Health Organization 2006 criteria into normal glucose metabolism (NGM), impaired fasting glucose, impaired glucose tolerance (combined as prediabetes), and type 2 diabetes.15 Participants on blood glucose-lowering medication were classified as having type 2 diabetes. Laboratory assessments of Haemoglobin A1c (HbA1c), fasting glucose, and of 2-h post-load glucose were described elsewhere.15
2.3 Assessment of cognitive function and cognitive impairment
A concise battery (30 min) of neuropsychological tests was administered to assess cognitive function. For conceptual clarity, and to reduce the number of cognitive outcomes, test results were divided into three cognitive domains (i.e., verbal memory, processing speed, and executive function and attention), as specified elsewhere.16 In short, verbal memory was evaluated using the Verbal Learning Test by averaging total immediate recall and delayed recall scores. The composite score for information processing speed was derived from the Stroop Colour Word Test Part I and II, the Concept Shifting Test Part A and B, and the Letter-Digit Substitution Test. Executive function and attention was assessed by the Stroop Colour Word Test Part III and the Concept Shifting Test Part C. Where necessary, individual test scores were inverted so that higher scores indicated better cognitive performance. In addition, participants were categorised as cognitively impaired (yes/no) based on a regression-based normalisation procedure per test that predicted expected scores for each individual given their age, sex, and level of education from a published normative sample, as described elsewhere.17-20 The difference between observed and expected scores and their standard deviation were used to calculate norm-corrected z-scores, which were then averaged per domain. Individuals performing <−1.5 SD below their norm-based expected score in one or more domains were categorised as having a significant cognitive impairment.
2.4 Assessment of depressive disorder and depressive symptoms
Depressive symptoms (dichotomous variables) were assessed using the Mini-International Neuropsychiatric Interview (MINI)21 and a validated Dutch version of the 9-item Patient Health Questionnaire (PHQ-9).22
Depressive symptoms measured by the MINI were used to assess prevalent (the preceding 2 weeks) and/or lifetime history of (minimally 2 weeks over lifetime) minor or major depressive disorder (dichotomous variables) according to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV).23 Minor depressive disorder was diagnosed if participants had at least 1 core symptom (depressed mood or anhedonia) and 1–3 other symptoms of depression (weight change, change in appetite, insomnia/hypersomnia, psychomotor agitation/retardation, fatigue/loss of energy, feelings of guilt/worthlessness, diminished ability to think/concentrate or indecisiveness and suicidal thoughts/plans). Major depressive disorder was diagnosed as the presence of at least 1 core symptom and at least 4 other symptoms of depression.
The Patient Health Questionnaire-9 (PHQ-9) is a self-administered questionnaire based on the DSM-IV23 criteria for major depressive disorder. It comprises each of the 9 DSM-IV criteria rated on a 4-point scale ranging from 0 to 3 (0 = not at all; 3 = nearly every day). Response options can generate a continuous score ranging from 0 (no symptoms) to 27 (all symptoms present nearly every day). A predefined cut-off score of ≥10 was used as a dichotomous scoring system for defining clinically relevant depressive symptoms.24 Both cognitive symptoms of depression, comprising thoughts about oneself and problems of the mind, and somatic symptoms of depression, comprising various bodily sensations perceived as unpleasant or worrisome, were measured with the PHQ-9.25
2.5 Assessment of health-related quality of life
The 36-Item Short Form Health Survey (SF-36) was used for the assessment of the generic health-related quality of life.26 The scores of the questionnaire were aggregated into two summary scores; a physical and mental health component summary score.27 In both these summary scores, four subdomain scores were covered. For the physical component summary score, these domains were physical functioning, role limitations due to physical health, bodily pain, and general health. For the mental health component summary score, these were mental health, role limitations due to emotional health, social functioning, and vitality. The responses to the questionnaire were transformed to get a score from 0 to 100, with 100 indicating the best possible quality of life.
2.6 Assessment of covariates and population characteristics
A questionnaire was used to assess age (years), sex (male/female), smoking status (never, current, former), alcohol use (g/day), adherence to the Dutch dietary guidelines, and the indication of diet quality (based on 14 out of 15 components of the Dutch Healthy Diet index 2015, as information on filtered coffee intake was not collected, calculated from a validated food frequency questionnaire28; the total score ranges between 0 (no adherence) and 140 (complete adherence)), educational level (low, intermediate, high), self-reported physical activity level (hours of moderate to vigorous physical activity per week) and post-menopausal status in women.15 The MINI, as described above, was used to assess current depression21 as a covariate for measures of cognitive function. Current depression was diagnosed as the presence of two core symptoms and at least three other symptoms, or one core symptom and at least four other symptoms. Medication use, for example, glucose-lowering, lipid-modifying, and antihypertensive medication use, as well as post-menopausal hormone replacement therapy, was determined during a medication interview where generic name, dose, and frequency were registered.15 Waist- and hip circumference, BMI, triglyceride levels, total cholesterol, high-density lipoprotein (HDL) cholesterol, and systolic and diastolic office blood pressure were determined as described elsewhere.15
2.7 Statistical analyses
Population characteristics by sex and glucose metabolism status were described as mean ± standard deviation and median (interquartile range) for normally and non-normally distributed variables, respectively, or n (%) for discrete variables.
Linear and logistic regression models were used to estimate sex-specific associations (and 95% CIs) of prediabetes and type 2 diabetes with cognitive function, depression, and quality of life. The reference category consisted of those with NGM (i.e., no prediabetes or type 2 diabetes). To test for sex differences in the associations, interaction terms of sex by dummy-coded prediabetes or type 2 diabetes were incorporated into the models. Several sets of adjustments were used in which we corrected for potential demographic, lifestyle, biological, and medication-related confounders. For cognitive function and depression, model 1 was adjusted for age and educational level. Model 2 was additionally adjusted for alcohol use, smoking, body mass index (BMI), physical activity, and healthy diet score (and for current depression in the models for measures of cognitive function). Model 3 (main model) was additionally adjusted for total cholesterol-to-HDL cholesterol ratio, systolic blood pressure, and the use of antihypertensive and lipid-modifying medication (and for antidepressants in the models for measures of depression). For quality of life, model 1 was adjusted for age and educational level. Model 2 (main model) was additionally adjusted for alcohol use, smoking, BMI, physical activity, and healthy diet score. For all outcomes, for each potential confounder included, an interaction term (sex × confounder) was also incorporated in the same model, otherwise, the adjustments made in the interaction models will not vary by sex as they do in the sex-specific models.29, 30 For the sex-specific results, and the interactions of sex with (pre)diabetes a p-interaction < 0.05 was considered statistically significant and results are presented with a 95% CI. Since missing data differ between the measures of cognitive function, depression, and quality of life complete-case analysis per outcome was performed (Figure 1). Analyses were exploratory and not controlled for multiple testing.
Statistical package for social sciences version 25.0 (IBM SPSS, IBM Corporation) was used for the statistical analyses.
3 RESULTS
The study population consisted of 7639 individuals (50% of women), with a mean age of 58.8 in women and 60.9 years in men. Of these, 4605 (57% of women) had NGM, 1141 (46% of women) had prediabetes, and 1893 (33% of women) had type 2 diabetes (Table 1). The overall prevalence of cognitive impairment was 22% in women and 27% in men. The prevalence of major depressive disorder was 5% in both sexes. For the quality of life, the physical health component summary score was identical in women and men, whereas the mental health component summary score was on average, two units lower in women than men (52 in women vs. 54 in men) (Table S1).
| Women | Men | |||||
|---|---|---|---|---|---|---|
| NGM n = 2632 | Prediabetes n = 525 | T2DM n = 631 | NGM n = 1973 | Prediabetes n = 616 | T2DM n = 1262 | |
| Demographics | ||||||
| Age (years) | 57.4 ± 8.5 | 61.6 ± 8.6 | 62.3 ± 8.2 | 58.7 ± 8.7 | 62.8 ± 7.9 | 63.2 ± 7.6 |
| Educational level | ||||||
| Low, N (%) | 842 (32.4) | 229 (44.6) | 364 (57.7) | 463 (23.6) | 200 (32.9) | 509 (41.3) |
| Middle, N (%) | 751 (28.9) | 136 (26.5) | 147 (23.3) | 532 (27.2) | 166 (27.3) | 346 (28.1) |
| High, N (%) | 1005 (38.7) | 149 (29.0) | 105 (17.0) | 964 (49.2) | 241 (39.7) | 377 (30.6) |
| Post-menopausal women | 1941 (74.9) | 432 (83.9) | 537 (87.9) | NA | NA | NA |
| Hormone replacement therapy | 55 (2.8) | 10 (2.3) | 14 (2.5) | NA | NA | NA |
| Clinical characteristics | ||||||
| Fasting glucose (mmol/L) | 5.0 [4.7–5.3] | 5.7 [5.2–6.2] | 7.5 [6.4–8.2] | 5.3 [5.0–5.5] | 6.0 [5.6–6.4] | 8.0 [6.8–8.7] |
| 2-h post-load glucose (mmol/L)a | 5.4 [4.6–6.2] | 8.6 [8.0–9.4] | 14.5 [11.9–17.3] | 5.3 [4.4–6.2] | 8.0 [6.8–9.3] | 14.2 [11.7–16.7] |
| HbA1c (mmol/mol) | 35 [33–38] | 38 [35–41] | 50 [43–54] | 35 [33–38] | 38 [35–41] | 51 [44–56] |
| HbA1c (%) | 5.4 [5.2–5.6] | 5.6 [5.4–5.9] | 6.7 [6.1–7.1] | 5.4 [5.2–5.6] | 5.6 [5.4–5.9] | 6.9 [6.2–7.2] |
| HOMA2-S (%) | 97.4 [67.8–122.5] | 73.6 [42.3–95.2] | 57.6 [32.3–72.4] | 85.7 [56.1–106.8] | 67.2 [39.3–82.1] | 60.0 [32.5–75.5] |
| Newly diagnosed T2DM | NA | NA | 129 (20.4) | NA | NA | 194 (15.4) |
| Diabetes duration | NA | NA | 5.54 ± 6.69 | NA | NA | 6.91 ± 7.58 |
| Glucose-lowering medication excl. insulin | 0 (0) | 0 (0) | 409 (64.8) | 0 (0) | 0 (0) | 897 (71.1) |
| Glucose-lowering medication insulin | 0 (0) | 0 (0) | 100 (15.8) | 0 (0) | 0 (0) | 259 (20.5) |
| Antihypertensive medication use | 520 (19.8) | 222 (42.4) | 437 (69.3) | 516 (26.2) | 304 (49.4) | 909 (72.1) |
| Lipid-modifying medication use | 321 (12.2) | 151 (28.8) | 425 (67.4) | 394 (20.0) | 240 (39.0) | 938 (74.4) |
| Antidepressants use | 220 (8.4) | 45 (8.6) | 86 (13.6) | 78 (4.0) | 31 (5.0) | 83 (6.6) |
| Cardiovascular risk factors | ||||||
| History of CVD | 320 (12.3) | 71 (13.6) | 130 (21.1) | 240 (12.3) | 135 (22.1) | 395 (32.0) |
| BMI (kg/m2) | 25.2 ± 4.0 | 27.7 ± 4.7 | 30.7 ± 5.6 | 26.1 ± 3.2 | 28.0 ± 3.7 | 29.6 ± 4.6 |
| Waist circumference (cm) | 85.9 ± 10.6 | 93.2 ± 12.2 | 102.0 ± 13.9 | 96.2 ± 9.7 | 102.5 ± 10.4 | 107.9 ± 12.4 |
| Office SBP (mm Hg) | 126.0 ± 16.7 | 134.0 ± 17.7 | 139.0 ± 18.2 | 134.6 ± 15.4 | 139.9 ± 17.3 | 142.7 ± 17.6 |
| Hypertension | 870 (33.1) | 305 (58.2) | 495 (78.6) | 941 (47.7) | 425 (69.3) | 1086 (84.7) |
| Total/HDL cholesterol ratio | 3.3 [2.6–3.8] | 3.6 [2.8–4.2] | 3.5 [2.7–4.0] | 3.9 [3.0–4.6] | 4.1 [3.2–4.8] | 3.8 [3.0–4.4] |
| Smoking | ||||||
| Never, N (%) | 1105 (42.3) | 195 (37.4) | 248 (39.9) | 781 (39.7) | 183 (29.9) | 309 (24.9) |
| Former, N (%) | 1203 (46.0) | 265 (50.9) | 278 (44.8) | 909 (46.2) | 351 (57.4) | 733 (59.1) |
| Current, N (%) | 307 (11.7) | 61 (11.7) | 95 (15.3) | 276 (14.0) | 78 (12.7) | 199 (16.0) |
| Alcohol use (g/day)b | 8.5 ± 9.3 | 8.3 ± 10.4 | 5.1 ± 8.2 | 16.3 ± 14.7 | 18.7 ± 19.4 | 13.8 ± 16.0 |
| Healthy diet score (minus alcohol)b | 81 ± 13 | 79 ± 13 | 77 ± 14 | 74 ± 15 | 73 ± 14 | 70 ± 14 |
| Physical activityb | ||||||
| Total self-reported physical activity (h/week) | 15.9 [10.0–20.3] | 15.3 [9.8–19.8] | 14.1 [8.5–18.7] | 13.0 [7.0–16.8] | 5.1 [6.5–16.3] | 11.4 [6.0–15.3] |
| Cognition | ||||||
| Cognitive impairmentb, N (%) | 517 (20.6) | 105 (21.2) | 183 (31.8) | 429 (22.7) | 163 (27.8) | 374 (32.2) |
| Memory score (Z-score) | 0.4 ± 0.8 | 0.3 ± 0.9 | 0.0 ± 0.9 | −0.1 ± 0.9 | −0.4 ± 0.9 | −0.5 ± 0.9 |
| Processing speed (Z-score)b | 0.2 ± 0.7 | 0.0 ± 0.8 | −0.3 ± 0.8 | 0.0 ± 0.7 | −0.2 ± 0.8 | −0.4 ± 0.8 |
| Executive attention (Z-score)b | 0.1 ± 0.8 | −0.1 ± 0.8 | −0.3 ± 0.9 | 0.1 ± 0.8 | −0.1 ± 0.8 | −0.3 ± 0.8 |
| Depression | ||||||
| Minor depressive disorder (MINI), N (%) | 31 (1.2) | 8 (1.6) | 17 (2.9) | 26 (1.4) | 9 (1.5) | 31 (2.6) |
| Major depression disorder (MINI), N (%) | 104 (4.1) | 23 (4.6) | 55 (9.4) | 71 (3.7) | 27 (4.5) | 87 (7.3) |
| Current depression (MINI), N (%) | 73 (2.9) | 16 (3.2) | 38 (6.5) | 45 (2.4) | 18 (3.0) | 46 (4.7) |
| Depressive symptoms (PHQ9)b, N (%) | 108 (4.3) | 25 (5.1) | 58 (10.5) | 58 (3.1) | 19 (3.3) | 60 (5.3) |
| Quality of life scores – SF36 | ||||||
| Physical component summary score | 51 ± 8 | 49 ± 9 | 45 ± 11 | 52 ± 7 | 51 ± 7 | 47 ± 9 |
| Physical functioning | 87 ± 16 | 82 ± 19 | 71 ± 24 | 91 ± 13 | 87 ± 16 | 79 ± 22 |
| Role limitations due to physical | 84 ± 33 | 83 ± 34 | 69 ± 42 | 89 ± 27 | 88 ± 29 | 78 ± 36 |
| Bodily pain | 83 ± 19 | 82 ± 19 | 74 ± 24 | 87 ± 17 | 86 ± 18 | 82 ± 21 |
| General Health | 70 ± 17 | 67 ± 17 | 58 ± 19 | 71 ± 15 | 68 ± 16 | 61 ± 18 |
| Mental component summary score | 52 ± 9 | 54 ± 8 | 51 ± 10 | 54 ± 8 | 54 ± 8 | 54 ± 9 |
| Mental health | 78 ± 14 | 78 ± 14 | 74 ± 17 | 81 ± 14 | 81 ± 14 | 80 ± 16 |
| Role limitations due to emotional health | 88 ± 29 | 91 ± 26 | 79 ± 37 | 91 ± 25 | 91 ± 26 | 87 ± 30 |
| Social functioning | 87 ± 17 | 87 ± 18 | 80 ± 21 | 90 ± 15 | 88 ± 17 | 85 ± 19 |
| Vitality | 69 ± 16 | 69 ± 17 | 63 ± 19 | 75 ± 15 | 74 ± 16 | 69 ± 18 |
- Note: Data are expressed as mean ± SD, median [interquartile range], or n (%), as appropriate.
- Abbreviations: BMI, body mass index; CVD, cardiovascular disease; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; HOMA-2S, Homeostasis Model Assessment; MINI, Mini-International Neuropsychiatric Interview; PHQ-9, Patient Health Questionnaire; SBP, systolic blood pressure; SF-36, 36-Item Short Form Health Survey; T2DM, type 2 diabetes.
- a Missing data in 25% of individuals with T2DM per protocol.
- b >5% missing data per variable.
3.1 Cognitive function and cognitive impairment
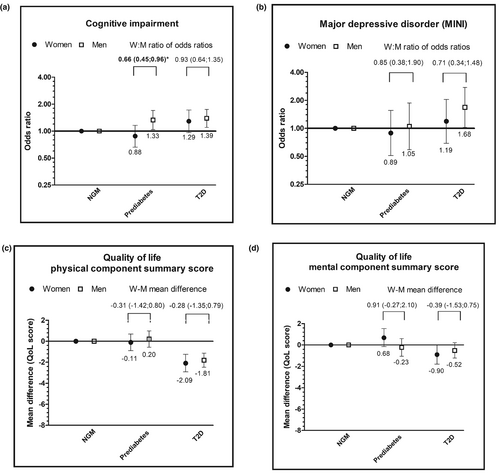
Prediabetes, as compared with NGM, was not significantly associated with performance in any of the three cognitive domains in either women or men (Table 2). Type 2 diabetes, as compared with NGM, was significantly associated with worse performance on each of the three cognitive domains in both women and men (Table 2). Prediabetes and type 2 diabetes were significantly associated with higher odds of cognitive impairment in men, but not in women (Figure 2a, Table 2). The sex difference was statistically significant in the association of prediabetes with cognitive impairment (women to men ratio of odds ratios (WM-OR) (95% CI) 0.66 (0.45–0.96)), but not in type 2 diabetes (WM-OR 0.93 (0.64–1.35)) (Figure 2a, Table 2).
| Prediabetes β or OR (95%-CI) | Type 2 diabetes β or OR (95% CI) | Sex difference WM-β or WM-OR (95% CI) | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Prediabetes | Type 2 diabetes | |
| Cognitive impairment (yes/no)* N = 6051 | ||||||
| Model 1 | 0.98 (0.75 to 1.29) | 1.36 (1.07 to 1.73) | 1.81 (1.43 to 2.31) | 1.61 (1.33 to 1.96) | 0.72 (0.50 to 1.03) | 1.12 (0.83 to 1.53) |
| Model 2 | 0.90 (0.69 to 1.19) | 1.35 (1.06 to 1.73) | 1.50 (1.15 to 1.95) | 1.53 (1.23 to 1.89) | 0.67 (0.46 to 0.97) ** | 0.98 (0.70 to 1.27) |
| Model 3 | 0.88 (0.66 to 1.16) | 1.33 (1.04 to 1.70) | 1.29 (0.96 to 1.72) | 1.39 (1.10 to 1.75) | 0.66 (0.45 to 0.96) ** | 0.93 (0.64 to 1.35) |
| Memory (SD) N = 6040 | ||||||
| Model 1 | −0.02 (−0.10 to 0.06) | −0.02 (−0.11 to 0.06) | −0.17 (−0.25 to −0.08) | −0.18 (−0.25 to −0.11) | 0.00 (−0.11 to 0.12) | 0.01 (−0.09 to 0.12) |
| Model 2 | −0.01 (−0.10 to 0.07) | −0.01 (−0.10 to 0.07) | −0.13(−0.22 to −0.04) | −0.14(−0.21 to −0.07) | 0.00 (−0.12 to 0.12) | 0.01 (−0.11 to 0.12) |
| Model 3 | −0.01 (−0.10 to 0.07) | 0.00 (−0.09 to 0.08) | −0.15 (−0.25 to −0.05) | −0.12 (−0.20 to −0.04) | −0.01 (−0.13 to 0.11) | −0.03 (−0.16 to 0.09) |
| Processing speed (SD) N = 6009 | ||||||
| Model 1 | −0.03 (−0.10 to 0.04) | −0.05 (−0.11 to 0.02) | −0.17 (−0.23 to −0.10) | −0.15 (−0.21 to −0.10) | 0.02 (−0.08 to 0.11) | −0.02 (−0.10 to 0.07) |
| Model 2 | −0.01 (−0.08 to 0.06) | −0.04 (−0.11 to 0.03) | −0.12 (−0.19 to −0.04) | −0.12 (−0.18 to −0.06) | 0.03 (−0.07 to 0.12) | 0.01 (−0.09 to 0.10) |
| Model 3 | −0.01 (−0.07 to 0.06) | −0.04 (−0.10 to 0.03) | −0.08 (−0.16 to 0.00) | −0.09 (−0.15 to −0.03) | 0.03 (−0.07 to 0.13) | 0.01 (−0.09 to 0.11) |
| Executive attention (SD) N = 5987 | ||||||
| Model 1 | −0.09 (−0.17 to −0.02) | 0.00 (−0.07 to 0.07) | −0.18 (−0.26 to −0.11) | −0.12 (−0.18 to −0.06) | −0.10 (−0.20 to 0.01) | −0.06 (−0.15 to 0.03) |
| Model 2 | −0.08 (−0.15 to 0.00) | 0.01 (−0.06 to 0.09) | −0.14 (−0.22 to −0.07) | −0.10 (−0.16 to −0.03) | −0.09 (−0.20 to 0.01) | −0.05(−0.15 to 0.05) |
| Model 3 | −0.07 (−0.15 to 0.00) | 0.02 (−0.06 to 0.09) | −0.12 (−0.21 to −0.04) | −0.09 (−0.16 to −0.02) | −0.09 (−0.20 to 0.02) | −0.03 (−0.15 to 0.08) |
| Minor depressive disorder (MINI) (yes/no)* N = 6086 | ||||||
| Model 1 | 1.40 (0.59 to 3.31) | 0.77 (0.26 to 2.31) | 1.91 (0.91 to 4.02) | 2.42 (1.27 to 4.61) | 1.82 (0.45 to 7.36) | 0.79 (0.30 to 2.11) |
| Model 2 | 1.21 (0.50 to 2.93) | 0.72 (0.24 to 2.20) | 1.54 (0.68 to 3.52) | 1.96 (0.96 to 3.99) | 1.68 (0.41 to 6.97) | 0.79 (0.27 to 2.34) |
| Model 3 | 0.87 (0.35 to 2.16) | 0.69 (0.23 to 2.14) | 0.81 (0.33 to 2.00) | 1.77 (0.80 to 3.91) | 1.26 (0.30 to 5.34) | 0.46 (0.14 to 1.52) |
| Major depressive disorder (MINI) (yes/no)* N = 6086 | ||||||
| Model 1 | 1.19 (0.70 to 2.04) | 1.33 (0.77 to 2.30) | 2.04 (1.32 to 3.15) | 2.37 (1.60 to 3.53) | 0.90 (0.42 to 1.94) | 0.86 (0.48 to 1.55) |
| Model 2 | 0.99 (0.57 to 1.71) | 1.15 (0.66 to 2.02) | 1.48 (0.91 to 2.40) | 1.72 (1.11 to 2.67) | 0.86 (0.39 to 1.87) | 0.86 (0.45 to 1.65) |
| Model 3 | 0.89 (0.51 to 1.56) | 1.05 (0.59 to 1.87) | 1.19 (0.69 to 2.04) | 1.68 (1.02 to 2.75) | 0.85 (0.38 to 1.90) | 0.71 (0.34 to 1.48) |
| Depressive symptoms PHQ9 (yes/no)* n = 6243 | ||||||
| Model 1 | 1.59 (0.97 to 2.59) | 1.40 (0.76 to 2.57) | 2.98 (1.98 to 4.48) | 2.21 (1.42 to 3.46) | 1.13 (0.52 to 2.47) | 1.35 (0.74 to 2.46) |
| Model 2 | 1.32 (0.80 to 2.18) | 1.25 (0.67 to 2.33) | 2.10 (1.33 to 3.32) | 1.66 (1.01 to 2.73) | 1.05 (0.47 to 2.34) | 1.27 (0.65 to 2.48) |
| Model 3 | 1.29 (0.76 to 2.16) | 1.21 (0.64 to 2.29) | 1.62 (0.96 to 2.73) | 1.64 (0.93 to 2.89) | 1.06 (0.47 to 2.43) | 0.99 (0.46 to 2.13) |
- Note: Sex-specific differences are expressed as linear regression coefficients (95% CI) of the dependent variables, which indicate mean differences (βs) or odds ratios (ORs) in measures of cognition and depression according to glucose metabolism status. The reference category for prediabetes and type 2 diabetes is normal glucose metabolism status. *OR. Differences between sexes are expressed as linear regression coefficients (95% CI) of the interaction terms sex × prediabetes and sex × type 2 diabetes, which indicates differences between women and men in mean differences (WM-βs) or women to men ratio of odds ratios (WM-OR) in measures of cognition and depression according to glucose metabolism status. Statistically significant differences between the sexes are typed in bold. Model 1: adjusted for age and educational level. Model 2: additionally adjusted for alcohol use, smoking, BMI, physical activity, and healthy diet score (and for current depression in the models for measures of cognition). Model 3 (main model): additionally adjusted for total cholesterol-to-HDL cholesterol ratio, systolic blood pressure, and the use of antihypertensive and lipid-modifying medication (and for antidepressants in the models for measures of depression). All models: for each potential confounder included, an interaction term (sex x potential confounder) was also incorporated in the same model.
- * WM-OR
- ** p < 0.05.
3.2 Depressive disorder and depressive symptoms
Prediabetes, as compared with NGM, was not significantly associated with higher odds of depressive disorder or depressive symptoms in either sex (Figure 2b, Table 2). Type 2 diabetes, as compared with NGM, was associated with both minor and major depressive disorder and clinically relevant depressive symptoms in the basic models in both sexes. After additional adjustments for cholesterol, blood pressure, and medications, type 2 diabetes was only significantly associated with major depressive disorder in men (Figure 2b, Table 2). There was no evidence for a sex difference in the associations of prediabetes or type 2 diabetes with minor or major depressive disorder or the presence of clinically relevant depressive symptoms (prediabetes: WM-OR 1.26 (0.30–5.34), 0.85 (0.38–1.90) and 1.06 (0.47–2.43), type 2 diabetes: WM-OR 0.46 (0.14–1.52), 0.71 (0.34–1.48) and 0.99 (0.46–2.13), respectively) (Figure 2, Table 2).
3.3 Health-related quality of life
Prediabetes, as compared with NGM, was not significantly associated with a lower score on the physical or mental component of quality of life in either women or men (Figures 1d and 2c, Table 3). Type 2 diabetes, as compared with NGM, was significantly associated with lower scores on the physical component of quality of life in both sexes (Figure 2c, Table 3). For the mental health component, the association with type 2 diabetes was only statistically significant in women, and directionally similar in men (Figure 2d, Table 3). There were no sex differences in the associations of prediabetes or type 2 diabetes with the physical and mental health score of quality of life (prediabetes: women minus men (W-M) mean difference: −0.31 (−1.42 to 0.80), 0.91 (−0.27 to 2.10), type 2 diabetes: W-M mean difference: −0.28 (−1.35 to 0.79), −0.39 (−1.53 to 0.75), respectively). Without adjustment for lifestyle factors, there were statistically significant sex differences to the disadvantage of women in the association of type 2 diabetes with two of the four subscores of physical quality of life (i.e., physical functioning: W-M mean difference: −2.21 (−4.37 to −0.05) and bodily pain: W-M mean difference: −2.65 (−5.10 to −0.21)) (Model 1, Table 3).
| Prediabetes β (95% CI) | Type 2 diabetes β (95% CI) | Sex difference WM-β (95% CI) | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Prediabetes | Type 2 diabetes | |
| Physical component summary score (QoL score) N = 6275 | ||||||
| Model 1 | −1.49 (−2.30 to −0.67) | −0.81 (−1.61 to −0.01) | −4.79 (−5.59 to −3.98) | −3.88 (−4.53 to −3.23) | −0.68 (−1.82 to 0.47) | −0.90 (−1.94 to 0.13) |
| Model 2 | −0.11 (−0.90 to 0.68) | 0.20 (−0.57 to 0.98) | −2.09 (−2.92 to −1.25) | −1.81 (−2.48 to −1.13) | −0.31 (−1.42 to 0.80) | −0.28 (−1.35 to 0.79) |
| Physical functioning (QoL score) N = 6275 | ||||||
| Model 1 | −3.60 (−5.30 to −1.89) | −2.31 (−3.97 to −0.64) | −11.36 (−13.04 to −9.68) | −9.15 (−10.50 to −7.80) | −1.29 (−3.67 to 1.09) | −2.21 (−4.37 to −0.05)* |
| Model 2 | −0.04 (−1.65 to 1.57) | 0.44 (−1.13 to 2.01) | −4.46 (−6.15 to −2.78) | −3.69 (−5.06 to −2.33) | −0.48 (−2.73 to 1.76) | −0.77 (−2.94 to 1.40) |
| Role limitations due to physical health (QoL score) N = 6275 | ||||||
| Model 1 | −1.14 (−4.42 to 2.13) | −0.83 (−4.03 to 2.37) | −10.99 (−14.22 to −7.76) | −8.70 (−11.30 to −6.09) | −0.31 (−4.89 to 4.27) | −2.29 (−6.44 to 1.85) |
| Model 2 | 2.03(−1.26 to 5.31) | 1.14(−2.06 to 4.35) | −4.68 (−8.12 to −1.24) | −4.44 (−7.22 to −1.65) | 0.88 (−3.71 to 5.47) | −0.24 (−4.67 to 4.19) |
| Bodily pain (QoL score) N = 6275 | ||||||
| Model 1 | −1.71 (−3.64 to 0.22) | −0.97 (−2.86 to 0.92) | −6.59 (−8.49 to −4.68) | −3.93 (−5.47 to −2.40) | −0.74 (−3.45 to 1.96) | −2.65 (−5.10 to −0.21)* |
| Model 2 | 0.33 (−1.60 to 2.26) | 0.57 (−1.32 to 2.46) | −2.67 (−4.70 to −0.64) | −0.88 (−2.52 to 0.76) | −0.24 (−2.94 to 2.47) | −1.79 (−4.40 to 0.82) |
| General health (QoL score) N = 6275 | ||||||
| Model 1 | −2.52 (−4.22 to −0.82) | −2.40 (−4.06 to −0.74) | −10.15 (−11.82 to −8.47) | −9.16 (−10.51 to −7.81) | −0.12 (−2.50 to 2.26) | −0.99 (−3.14 to 1.17) |
| Model 2 | −0.74 (−2.43 to 0.95) | −0.87 (−2.52 to 0.78) | −6.79 (−8.56 to −5.02) | −5.99 (−7.42 to −4.55) | 0.13 (−2.23 to 2.49) | −0.80 (−3.08 to 1.47) |
| Mental component summary score (QoL score) N = 6275 | ||||||
| Model 1 | 0.64 (−0.20 to 1.47) | −0.33 (−1.15 to 0.48) | −0.90 (−1.72 to −0.08) | −0.69 (−1.35 to −0.03) | 0.97 (−0.19 to 2.14) | −0.21 (−1.26 to 0.84) |
| Model 2 | 0.68 (−0.16 to 1.53) | −0.23 (−1.06 to 0.60) | −0.90 (−1.79 to −0.02) | −0.52 (−1.23 to 0.20) | 0.91 (−0.27 to 2.10) | −0.39 (−1.53 to 0.75) |
| Mental health (QoL score) N = 6275 | ||||||
| Model 1 | 0.20 (−1.23 to 1.63) | −0.45 (−1.85 to 0.95) | −2.49 (−3.90 to −1.08) | −1.87 (−3.01 to −0.73) | 0.65 (−1.36 to 2.65) | −0.62 (−2.43 to 1.19) |
| Model 2 | 0.47 (−0.98 to 1.92) | −0.06 (−1.47 to 1.36) | −2.27 (−3.79 to −0.75) | −1.09 (−2.32 to 0.14) | 0.52 (−1.50 to 2.55) | −1.17 (−3.13 to 0.78) |
| Role limitations due to emotional health N = 6275 | ||||||
| Model 1 | 1.96 (−0.83 to 4.75) | −1.45 (−4.18 to 1.28) | −6.23 (−8.99 to −3.48) | −3.63 (−5.85 to −1.41) | 3.41 (−0.50 to 7.31) | −2.60 (−6.14 to 0.93) |
| Model 2 | 3.54 (0.71 to 6.37) | −0.31 (−3.08 to 2.45) | −3.12 (−6.09 to −0.15) | −1.58 (−3.99 to 0.82) | 3.85 (−0.11 to 7.82) | −1.53 (−5.35 to 2.29) |
| Social functioning N = 6275 | ||||||
| Model 1 | 0.02 (−1.71 to 1.75) | −1.98 (−3.67 to −0.29) | −5.18 (−6.88 to −3.47) | −4.62 (−6.00 to −3.25) | 1.99 (−0.42 to 4.41) | −0.55 (−2.74 to 1.64) |
| Model 2 | 1.28 (−0.46 to 3.03) | −1.00 (−2.71 to 0.70) | −2.71 (−4.54 to −0.89) | −2.64 (−4.12 to −1.16) | 2.29 (−0.15 to 4.72) | −0.07 (−2.42 to 2.28) |
| Vitality N = 6275 | ||||||
| Model 1 | −1.50 (−3.14 to 0.13) | −0.94 (−2.54 to 0.66) | −6.04 (−7.65 to −4.42) | −5.54 (−6.84 to −4.24) | −0.57 (−2.86 to 1.72) | −0.50 (−2.57 to 1.57) |
| Model 2 | 0.14 (−1.48 to 1.76) | 0.43 (−1.15 to 2.02) | −3.01 (−4.71 to −1.31) | −2.76 (−4.14 to −1.39) | −0.29 (−2.56 to 1.97) | −0.25 (−2.43 to 1.94) |
- Note: Sex-specific differences are expressed as linear regression coefficients (95% CI) of the dependent variables, which indicate mean differences (βs) in measures of quality of life according to glucose metabolism status. The reference category for prediabetes and type 2 diabetes is normal glucose metabolism status. Differences between sexes are expressed as linear regression coefficients (95% CI) of the interaction terms sex × prediabetes and sex × type 2 diabetes, which indicates differences between women and men in mean differences (WM-βs) in measures of quality of life according to glucose metabolism status. Statistically significant differences between the sexes are typed in bold. Model 1: adjusted for age and educational level. Model 2 (main model): additionally adjusted for alcohol use, smoking, BMI, physical activity, and healthy diet score. All models: for each potential confounder included, an interaction term (sex x potential confounder) was also incorporated in the same model.
- Abbreviation: QoL, quality of life (QoL score ranges from 0 to 100, with 100 indicating the best possible quality of life).
- * p < 0.05.
4 DISCUSSION
This study showed that in general, type 2 diabetes was associated with worse cognitive function, depression, and poorer physical and mental health-related quality of life in both women and men. There was no clear pattern of sex differences in the associations of either prediabetes or type 2 diabetes with measures of cognitive function, depression, or quality of life.
Several previous studies have demonstrated that women with type 2 diabetes are more likely to have cognitive impairment than men with type 2 diabetes,7, 8 but, it has also been observed that women have better cognitive performance independent of the presence of type 2 diabetes.9 However, as most previous studies did not include a reference group of people without diabetes, it remains unclear whether diabetes confers a greater risk for cognitive impairment in women than in men. Studies that reported on sex differences in the diabetes-associated risk of cognitive impairment have reported conflicting results.9, 31, 32 In a population-based cohort study with 6892 participants and 4 years of follow-up, type 2 diabetes was associated with mild cognitive impairment in men but not in women,31 but the sex difference was not quantified. In two population-based cross-sectional studies on cognitive outcomes including 1237 and 1936 participants, respectively, no statistical sex-diabetes interactions were observed.9, 32 In the current study, we additionally accounted for the potential presence of sex-specific confounding, which has been shown to meaningfully alter the conclusions in previous work.30 Our findings suggest that the association between type 2 diabetes and cognitive impairment is similar in women and men. Our findings are at odds with a previous meta-analysis, which showed that the diabetes-related risk of vascular dementia, but not of non-vascular dementia, was substantially greater in women than in men.14 In the present study, we were not able to determine whether the cognitive impairment was vascular or non-vascular in nature. However, the discrepancy between our findings and those from the meta-analyses might suggest that the sex difference in the association between diabetes and vascular dementia occurs at an advanced stage of the cognitive impairment spectrum.
It should be noted that in contrast to type 2 diabetes, we did observe a sex difference in the association of prediabetes with cognitive impairment, to the disadvantage of men. However, we consider this as a chance finding as these sex differences were not observed in the association of type 2 diabetes or in the associations (of either prediabetes or type 2 diabetes) with the three subdomains (i.e., memory, processing speed, and executive attention). Further research is needed to investigate a possible sex difference in the association of prediabetes with cognitive impairment.
The association between type 2 diabetes and depression has previously been shown.3 Among people with diabetes, the prevalence of depression is generally higher among women than among men.10, 11 Additionally, in a cross-sectional study including 123,232 patients with diabetes mellitus and 1,933,218 controls, diabetes was more strongly associated with major depressive disorder in women than in men.33 On the contrary, a previous meta-analysis showed that when compared with NGM, prediabetes, undiagnosed diabetes, and previously diagnosed diabetes were significantly associated with the odds of depression in men but not in women.34 However, the interaction of diabetes status with sex was not tested. We also observed higher odds of depression associated with type 2 diabetes in men than women (only significant in the former), but the sex difference was not statistically significant. The discrepancy with the previously observed higher risk of diabetes on depression in women might be explained by the different age groups. They observed that the peak of the gender gap was around the age of 40–49 years, our study population is generally older. Additionally, as our population is relatively healthy, we cannot exclude sex differences in the association of type 2 diabetes with later stages of depression.
Studies on the association between type 2 diabetes and quality of life are sparse but generally show that people with diabetes have a poorer quality of life than people with no chronic illness.35 We add to these finding by showing that after adjustment for lifestyle factors, the associations of diabetes with physical and mental quality of life was similar in women than in men. However, without adjustment for lifestyle factors, sex differences in two of the subdomains of physical quality of life (i.e., physical functioning and bodily pain) were statistically significant to the disadvantage of women. This suggests that some of the effect of diabetes on physical quality of life may be driven by these lifestyle factors. Explanations for the sex differences in the basic models of physical quality of life may be related to the excess risk of comorbidities, such as CVD, and more adverse cardiometabolic profile associated with type 2 diabetes in women.1 Also, gender roles, for example, with regard to domestic labour and care tasks, and gender differences in health consciousness, health-seeking behaviour, and health perception may affect the management and control of diabetes and related health problems.36 As research on sex differences in diabetes-associated quality of life is sparse, further research is required to investigate whether type 2 diabetes is a stronger risk factor for poorer physical quality of life in women than in men and to investigate possible explanations.
4.1 Strengths of our study
Strengths of our study include its population-based design combined with oversampling of individuals with type 2 diabetes, which enables an accurate comparison of individuals with and without type 2 diabetes. Additionally, this study benefits from a large sample size and a detailed phenotypic assessment. The study also has some limitations. First, the data were cross-sectional. Hence, we cannot determine the causality and direction in the associations of prediabetes and type 2 diabetes with cognitive function, depression, and quality of life. However, we do not expect this to affect the investigated sex differences. Second, as our population is generally relatively healthy, most of the participants with cognitive impairment probably have early stages of cognitive impairment, and the number of participants with depression is relatively low. Due to lack of power, we can, therefore, not exclude sex differences in the associations of (pre)diabetes with later stages of cognitive decline and depression. Third, we conducted many analyses, which increases the risk of chance findings. Fourth, we have twice as many men than women with type 2 diabetes. If the underrepresentation of women with type 2 diabetes was due to health selection, this could influence the sex differences. At any rate, the recruitment strategy was the same for women as for men. Additionally, our study population exists of middle-aged Caucasian individuals. Our results are generalisable to individuals with similar characteristics, but it should be kept in mind that the associations and sex differences may differ in populations with a different distribution of determinants or in other ethnic groups. Finally, we did not have data on gender-related factors like health-seeking behaviour or distribution of domestic labour within families, which could also affect diabetes-associated outcomes differently for women and men.
5 CONCLUSION
In general, type 2 diabetes, but not prediabetes, was associated with worse cognitive function, depression, and poorer quality of life. However, the strength of these associations was similar among women and men. Different from what is found for CVD, no clear sex difference seems to be present in the association between type 2 diabetes and mental disorders and health-related quality of life.
AUTHOR CONTRIBUTIONS
RdR conducted the analyses and wrote this paper under the supervision of SP, SS, and CvdK. All authors reviewed the draft paper and provided critical intellectual content. All authors approved the final version of the manuscript and its submission to Diabetic Medicine. SP is taking responsibility for the contents of the article.
FUNDING INFORMATION
This study was supported by ZonMw (project no 849200001), the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), Stichting De Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), CARIM, School for Cardiovascular Diseases (Maastricht, the Netherlands), School CAPHRI, Care and Public Health Research Institute (Maastricht, the Netherlands), NUTRIM, School of Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), Stichting Annadal (Maastricht, the Netherlands), Health Foundation Limburg (Maastricht, the Netherlands) and by unrestricted grants from Janssen-Cilag B.V. (Tilburg, the Netherlands), Novo Nordisk Farma B.V. (Alphen aan den Rijn, the Netherlands) and Sanofi-Aventis Netherlands B.V. (Gouda, the Netherlands). SAEP is supported by a UK Medical Research Council Skills Development Fellowship (MR/P014550/1).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data of this study derive from The Maastricht Study, but restrictions apply to the availability of these data, which were used under license for the current study. Data are, however, available from the authors upon reasonable request and with permission of The Maastricht Study management team.




