Real-time continuous glucose monitoring immediately after severe hypoglycaemia requiring emergency medical services: A randomised controlled trial
Joint senior authors—Rachael Fothergill and Monika Reddy.
Material in this manuscript has previously been presented at the ATTD conference held online in 2021.
Abstract
Aims
Severe hypoglycaemia requiring emergency medical services remains prevalent despite advances in all aspects of diabetes self-management. Real-time continuous glucose monitoring (RTCGM) technologies can reduce the risk of severe hypoglycaemia for adults with type 1 diabetes, but the impact of these devices has not been assessed in the acute phase after an episode of severe hypoglycaemia.
Methods
We recruited and randomised 35 adults with type 1 diabetes in the acute period after an episode of severe hypoglycaemia requiring emergency medical services and randomised participants to RTCGM with alerts and alarms, or usual care with self-monitored blood glucose for 12 weeks with intermittent blinded CGM. The primary outcome was the difference between groups in percentage time spent in hypoglycaemia (≤3.0 mmol/L, 55 mg/dL).
Results
Thirty participants completed the study (median (IQR) age, duration of diabetes, and BMI was 43 (36–56) years, 26 (19–37) years, and 24.9 (21.9–29.0) kg/m2, respectively). Sufficient CGM data was available for 15 participants in RT-CGM group and 8 in SMBG group for the primary outcome analysis. The RTCGM group had a significantly larger reduction in exposure to glucose below 3.0 mmol/L (RTCGM −0.16 [−1.23 to 0.01] vs. SMBG 1.58 [0.41 to 3.48], p = 0.03) and episodes of nocturnal hypoglycaemia (RT-CGM −0.03 [−0.15 to 0.02] vs. SMBG 0.05 [−0.03 to 0.40], p = 0.02). Episodes of severe hypoglycaemia were significantly lower in the RTCGM group (RTCGM 0.0 vs. SMBG 4.0, p 0.04).
Conclusions
RTCGM implemented acutely after an episode of severe hypoglycaemia is feasible and clinically effective with important implications for hypoglycaemia management pathways and self-monitoring cost effectiveness.
What's new?
- It is well established that real-time CGM is associated with reduced time spent in hypoglycaemia in people at high risk of hypoglycaemia.
- The outcomes of this study shows potential clinical benefit of real-time CGM implementation immediately after an episode of severe hypoglycaemia
- These findings have important implications for hypoglycaemia management pathways and self-monitoring cost effectiveness.
1 INTRODUCTION
Severe hypoglycaemia requiring emergency medical services remains prevalent despite improvements in insulins, monitoring technologies, support, education, and care for people living with type 1 diabetes.1 Real-time continuous glucose monitoring (RTCGM) systems with alerts and alarms for impending and established hypo- and hyperglycaemia increase time in target while reducing the time spent in hypoglycaemia, compared with self-monitored blood glucose (SMBG) and flash monitoring.2, 3 Additionally, RTCGM reduces the incidence of severe hypoglycaemia in people at highest risk.4
For people with type 1 diabetes, structured education is associated with a reduction in paramedic attendance, nights spent in hospital, and admissions with hypoglycaemia.5 For people with impaired awareness of hypoglycaemia, education and psychological interventions can support restoration of awareness.6
It is not known if RTCGM implemented acutely after an episode of severe hypoglycaemia can reduce time below target or recurrent episodes of severe hypoglycaemia. The aim of this study is to assess the impact of RTCGM implementation immediately after severe hypoglycaemia requiring emergency medical services, supported remotely by a specialist healthcare team, on the frequency, duration, awareness, and severity of hypoglycaemia in adults with type 1 diabetes.
1.1 Research design and methods
The protocol has been described previously.7 In brief, participants with type 1 diabetes aged 18 years and older with an episode of severe hypoglycaemia requiring emergency medical services were recruited and screened within 2 weeks of the episode. Participants were excluded if they had used RTCGM or flash glucose monitoring within the last 6 months, were using a pre-mixed (biphasic) insulin preparation, were pregnant or planning a pregnancy, were breastfeeding, were enrolled in another clinical trial, or had no access to a computer or smartphone.
Following screening, participants were randomised 1:1 to receive RTCGM with the Dexcom G6 system (Dexcom, San Diego, CA; intervention) or continue SMBG as per standard care (control). Randomisation was performed on-line using sealedenvelope.com and stratified by insulin delivery modality. Participants were followed up for 12 weeks with telephone visits by the study team to support treatment adjustments informed by RTCGM or SMBG data. These visits were conducted twice in the first week, weekly for the next 3 weeks, and every 2 weeks thereafter. Participants in the control group wore blinded G6 systems at weeks 1 and 2, weeks 4–6, and weeks 9–12. The participants completed diabetes-specific questionnaires (DTSQ, PAID, HFS-II, CGM usability, and Gold) at baseline and at the end of the study. Ethical approval was obtained from the London Hampstead Research Ethics Committee (18/LO/1525) and the study was registered at ClinicalTrials.gov (NCT03748433). The primary outcome was the difference between groups in percentage time spent in hypoglycaemia (<3.0 mmol/L, 55 mg/dL) at the end of the study period with secondary outcomes including times in ranges, HbA1c, and questionnaire data. The primary and secondary outcomes were assessed by the non-parametric Mann–Whitney hypothesis tests. Fisher's exact test was used to compare protocol compliance between the RTCGM and control group. Results were considered statistically significant if p < 0.05, with statistical analyses performed using Stata version 13 (StataCorp).
2 RESULTS
The intention was to recruit 55 participants. However, the unforeseen challenges posed by the global pandemic led to suspension of recruitment in March 2020 and an interim analysis was done at this point. Thirty-five participants were recruited and randomised between January 2019 and March 2020, and of these, 30 completed the trial (16 RTCGM and 14 SMBG). One participant was withdrawn from the RTCGM group following a diagnosis of adrenal insufficiency detected at screening. In the control group, two participants withdrew immediately after randomisation to the SMBG arm, one participant was withdrawn due to hospitalization during the trial, and another withdrew after commencing NHS-funded RTCGM (Figure 1).
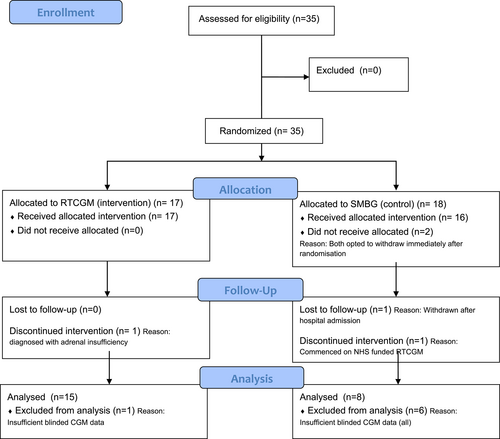
The baseline demographics including median (IQR) age, gender, duration of diabetes, ethnicity, HbA1c and BMI of the participants completing the study (n = 30) are summarised in Table 1. All the participants were on multiple daily injections (MDI) of insulin. The median (IQR) % of scheduled telephone consultations attended by participants was 61 (28–89) % in the RTCGM group and 44 (33–56) % in the SMBG group, p = 0.09. Insufficient CGM use (<70%, real-time and blinded CGM) resulting in exclusion from endpoint analysis was observed in 23% of participants, with a significantly higher rate among the SMBG participants using blinded CGM compared to RTCGM participants (43% vs. 6%, p = 0.03).
| Demographics | Combined (n = 30) | RT-CGM (n = 16) | SMBG (n = 14) |
|---|---|---|---|
| Age (years) | 43 (36–56) | 40 (32–54) | 47.5 (38–60) |
| Gender (male) | 17 (56.7%) | 8 (50%) | 9 (64.3%) |
| Duration of diabetes (years) | 26 (19–37) | 24 (22–36) | 26.5 (13.8–36) |
| Ethnicity | |||
| White European | 25 (83.3%) | 13 (81.25%) | 12 (85.7%) |
| Black British | 4 (13.3%) | 2 (12.5%) | 2 (14.3%) |
| Asian | 1 (3.3%) | 1 (6.25%) | 0 (0%) |
| HbA1c mmol/mol | 61 (54–72) | 55 (52–64) | 67 (57–77) |
| BMI (kg/m2) | 24.9 (21.9–29.0) | 25.2 (23.0–28.0) | 23.4 (21.3–29.0) |
| Insulin modality (MDI) | 30 (100%) | 16 (100%) | 14 (100%) |
Sufficient CGM data were available and analysed for 15 participants in the RTCGM group and 8 participants in the control group, after blinded periods with insufficient data were excluded (Table 2). A significant difference in change between groups was observed for both median (IQR) percentage time <3.0 mmol/L (RTCGM −0.16 [−1.23 to 0.01] vs. SMBG 1.58 [0.41 to 3.48], p = 0.03) and time <2.8 mmol/L (RTCGM −0.09 [−0.81 to 0.02] vs. SMBG 1.35 [−0.29 to 3.14], p < 0.01). A significant between-group difference in overnight hypoglycaemia events <3.0 mmol/L lasting more than 20 min was also seen (RT-CGM −0.03 [−0.15 to 0.02] vs. SMBG 0.05 [−0.03 to 0.40], p = 0.02). No significant difference was observed for time spent <3.9 mmol/L and other predefined thresholds of time in range and hyperglycaemia. Four episodes of severe hypoglycaemia requiring third party assistance were reported by four different participants in the SMBG group with none in the RTCGM group (p < 0.04); one participant sustained a fracture after a hypoglycaemia-related fall. In addition, one participant in the SMBG group was hospitalised with diabetic ketoacidosis (DKA). There was no significant change in HbA1c within or between groups.
| Metric | Control Baseline (median, IQR) | RTCGM Baseline (median, IQR) | Control change from baseline to endpoint (median, IQR) | RTCGM change from baseline to endpoint (median, IQR) | p |
|---|---|---|---|---|---|
| % time in hypoglycaemia | |||||
| <3.9 mmol/L (<70 mg/dL) | 8.06 (4.97 to 10.65) | 7.09 (3.27 to 9.00) | 2.69 (0.18 to 4.17) | −0.48 (−2.18 to 0.40) | 0.12 |
| <3.0 mmol/L (<54 mg/dL) | 2.20 (0.90 to 4.05) | 2.71 (0.93 to 3.54) | 1.58 (0.41 to 3.48) | −0.16 (−1.23 to 0.01) | 0.03 |
| <2.8 mmol/L (<50 mg/dL) | 1.22 (0.46 to 2.63) | 1.57 (0.50 to 2.61) | 1.35 (0.29 to 3.14) | −0.09 (−0.81 to 0.02) | <0.01 |
| % time in range | |||||
| 3.9-10 mmol/L (70-180 mg/dL) | 51.48 (46.23 to 57.50) | 57.32 (50.45 to 63.42) | 6.88 (−4.31 to 15.86) | 0.12 (−2.35 to 4.52) | 0.33 |
| % time in hyperglycaemia | |||||
| >10 mmol/L (>180 mg/dL) | 37.09 (32.31 to 42.59) | 33.64 (30.17 to 43.68) | −9.54 (−12.53 to −4.32) | −0.01 (−3.57 to 2.54) | 0.05 |
| <3.0 mmol/L events > 20mins | |||||
| 24 hours | 0.19 (0.07 to 0.39) | 0.16 (0.07 to 0.25) | 0.01 (−0.06 to 0.07) | −0.02 (−0.07 to 0.01) | 0.48 |
| Overnight (00:00 to 07:00) | 0 (0.00 to 0.06) | 0.17 (0.05 to 0.29) | 0.05 (−0.03 to 0.40) | −0.03 (−0.15 to 0.02) | 0.02 |
| Gold score | 3.3 (3.0 to 4.8) | 4.0 (3.0 to 5.3) | 0.0 (0 to 2.0) | 0.0 (0.0 to 0.75) | 0.67 |
| HbA1c mmol/mol | 66.5 (56.3 to 77.3) | 55.0 (51.8 to 63.8) | 0.0 (−0.5 to 2.0) | −3.0 (−9.0 to 2.0) | 0.35 |
| HFS-II behaviour | 18.0 (12.25 to 19.50) | 22.0 (14.0 to 29.6) | 2.5 (0.3 to 8.3) | −1.0 (−6.5 to 4.5) | 0.08 |
| HFS-II worry | 32.5 (23.5 to 44.0) | 36.5 (22.80 to 44.0) | 2.0 (−1.5 to 10.0) | 0.0 (−4.5 to 7.5) | 0.37 |
| HFS-II total | 49.0 (39.3 to 58.5) | 60.0 (35.5 to 69.5) | 4.5 (3.0 to 19.50) | −1.0 (−9.25 to 4.50) | 0.06 |
| PAID | 26.5 (13.5 to 41.5) | 27.0 (17.8 to 42.8) | 11.5 (−3.0 to 16.5) | −1.5 (−6.9 to 5.8) | 0.12 |
| DTSQ | 26.0 (22.0 to 32.5) | 29.5 (28.0 to 33.3) | 4.0 (−3.0 to 14.2) | 7.0 (4.5 to 10.5) | 0.32 |
- Note: The p-values refer to the SMBG (Control) change from baseline to endpoint vs. RTCGM (intervention) change from baseline to endpoint. Significant outcomes (p<0.05) are highlighted in bold.
Participants randomised to receive RTCGM reported significantly improved within-group median (IQR) DTSQ scores (29.5 [28–33.3] at baseline vs. 38 [37–39] at 12 weeks, p < 0.01), but no significant difference was observed between groups. No significant changes were seen within or between groups when comparing the participant-reported measures of hypoglycaemia awareness (Gold score) and diabetes-specific quality of life (PAID, and HFS-II scores).
Due to the significant benefit seen in the intervention (RTCGM arm) in terms of reduction of hypoglycaemia, it was agreed that it would be unethical to continue to offer SMBG in this high risk cohort.
3 DISCUSSION
The data presented are the first to suggest that rapid provision of RTCGM following severe hypoglycaemia significantly improves hypoglycaemia outcomes compared to self-monitoring of blood glucose. Critically, this includes prevention of further severe hypoglycaemia with important implications for morbidity, mortality, and cost effectiveness. The pre-existing risk of hypoglycaemia in the recruited participants was high and is illustrated by the median (IQR) baseline Gold score of 4 (3–5), indicating diminished hypoglycaemia awareness and reaffirming the association between impaired awareness and incident severe hypoglycaemia. This study supports data from larger studies showing a reduction in percentage time in hypoglycaemia <3.0 mmol/L and other hypoglycaemia outcomes in high-risk individuals using RTCGM.4, 8, 9 Importantly, this study was conducted, in part, during the COVID-19 pandemic and utilised telephone consultation and remote glucose data review to support treatment optimisation and hypoglycaemia risk mitigation. With this approach, the overall telephone consultation attendance rate was relatively low at 50 (33–75)% (including successful consultations made after multiple attempts to contact participants and delayed consultations of up to 1 week).
The effectiveness of RTCGM is dependent on its continued functional operation and is subject to intermittent technical failure. We did not observe a change in hypoglycaemia awareness with RTCGM compared to the control group, consistent with other studies,4, 8 emphasising the importance of a pathway approach to severe hypoglycaemia, in which the risk of incident severe hypoglycaemia is immediately and effectively mitigated by RTCGM, allowing time for interventions to address impaired awareness which may include scrupulous hypoglycaemia avoidance,10 psychological approaches6 and education.5
Recruitment to the study was pre-maturely suspended due to the challenges and restrictions posed by the global COVID pandemic. Randomising high risk individuals to SMBG was deemed unethical after the interim analysis showed significant benefit of RTCGM in terms of reducing hypoglycaemia and therefore further recruitment was not undertaken.
Limitations to the study include the small enrolment numbers and challenges maintaining participants in the control group as access to CGM technologies became more widespread. The mainstay of glucose monitoring in type 1 diabetes was SMBG when this study was designed; however, this has changed over time and the latest NICE guidance for management of type 1 diabetes recommends a choice of CGM or intermittently scanned CGM (isCGM) to all individuals with type 1 diabetes based on the individual's needs and preferences.11 The disproportionate dropout and insufficient use of blinded CGM in the control group suggest the prospect of immediate RT-CGM access as a motivation for participation in this study. However, despite its limitations, this study is the first to demonstrate the feasibility of immediate RTCGM utilization after an episode of severe hypoglycaemia and its efficacy in reducing exposure to biochemically serious,12 nocturnal, and severe recurrent hypoglycaemia. These clinically important findings may have a significant impact on cost of care, especially for those who recurrently access emergency medical services. We conclude that RTCGM implemented acutely after an episode of severe hypoglycaemia is feasible and clinically effective with important implications for hypoglycaemia management pathways compared to SMBG. Further data supporting an integrated multimodal pathway for managing severe hypoglycaemia are urgently required.
ACKNOWLEDGMENTS
Infrastructure support was provided by the NIHR Imperial Biomedical Research Centre and the NIHR Imperial Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. Dexcom is a trademark of Dexcom, Inc.
FUNDING INFORMATION
This is an investigator-initiated study funded by Dexcom. The funding body had no role in the collection, analysis, and interpretation of data, or in writing the manuscript.
CONFLICT OF INTEREST STATEMENT
NO has received research funding from Roche Diabetes, Medtronic Diabetes, and Dexcom and has participated in advisory groups for Dexcom, Medtronic, and Roche Diabetes. MR has received research funding from Dexcom and has participated in advisory groups for Dexcom and Roche Diabetes. CU, VP, NJ, and RF have no conflict of interests.




