The impact of diabetes on cancer detection during the prevalence round of a national screening program for colorectal cancer
Abstract
Aims
Diabetes is associated with a higher risk of colorectal cancer (CRC) and inferior survival after CRC. Screening may enable the early detection of CRC. We aimed to assess the impact of diabetes on cancer detection and disease stage during the prevalence round of a national CRC screening program.
Methods
We performed a register-based cohort study based on the randomized procedure for inviting Danish residents aged 50–74 years to the prevalence round of national CRC screening program in 2014–2017. By comparing the random half of the population who had been invited by 1 May 2016 with the not yet invited half, the effect of screening was assessed by the detection of CRC and disease stage among individuals with and without diabetes. Further, the impact of diabetes on the screening participation rate was calculated.
Results
By randomisation, 504,673 individuals had been invited to the CRC screening by 1 May 2016, and 549,359 individuals had not yet been invited. The diabetes prevalence was 10% in both groups. When comparing those not yet invited to those invited, the effect of screening on the number of detected cancers per 100,000 individuals was higher in those with diabetes (from 207 to 494 cancers) than in those without diabetes (from 147 to 364 cancers), and screening resulted in overall higher proportions of stage I cancer. Among those invited to screening, the participation rate was 9.1% lower (95% CI: 8.7%–9.5%) in individuals with versus without diabetes.
Conclusions
Despite a lower participation rate, the effect of CRC screening was higher in individuals with diabetes.
What's new?
- Diabetes is associated with a 20% higher risk of colorectal cancer (CRC) and inferior survival after CRC.
- CRC screening enables early detection of CRC.
- Conflicting evidence has been reported on CRC screening participation in populations with diabetes.
- In individuals with diabetes, screening increased the likelihood of being diagnosed with CRC at stage I.
- The effect of CRC screening on cancer detection was more pronounced in individuals with diabetes despite a lower screening participation rate.
- Faecal immunochemical testing may be an effective tool to detect CRC at early disease stages, especially in individuals with diabetes.
1 INTRODUCTION
Colorectal cancer (CRC) is one of the few cancer types with a higher incidence in individuals with diabetes.1-3 Additionally, inferior survival1, 4, 5 is seen in this group compared with individuals without diabetes. This may be caused by direct mechanisms (hyperglycemia, insulin resistance and hyperinsulinemia),6 and/or it could be due to shared risk factors (obesity, physical inactivity, diet, alcohol use and smoking).5
Regardless of the causal explanation, early detection of premalignant lesions and CRC is crucial to reduce adverse outcomes in individuals both with and without diabetes. Screening is a key step in early detection. Many healthcare systems have implemented organized population-based CRC screening programs, and older adults are recommended to have a faecal test or an endoscopy performed at regular intervals.7
A recent review by Bhatia et al8 aimed to quantify the association between diabetes and participation in screening for breast cancer, cervical cancer and CRC. However, the included studies were concluded to have poor methodological quality with mainly cross-sectional designs based on self-reported diabetes data in American healthcare settings. Therefore, the authors called for methodologically sound studies to evaluate the influence of diabetes on screening participation. Furthermore, no previous studies have analysed the effect of a national screening program on CRC detection and disease stage in individuals with diabetes.
The aim of this study was to explore the impact of diabetes on cancer detection and disease stage during the prevalence round of a national CRC screening using a faecal immunochemical test (FIT) and to compare the screening participation rate between individuals with and without diabetes.
2 METHODS
2.1 Setting
The Danish national CRC screening program was initiated on 3 March 2014, and the implementation was completed by 31 December 2017.9 During this prevalent round, all residents aged 50–74 years on 1 January 2014 were invited once to organize CRC screening with invitations sent out in March 2014. The order of invitation was randomly decided according to the birth month of the residents. However, residents who turned 50 years during the first screening round and residents nearing 75 years without having received an invitation yet, were invited in a nonrandom manner just before their 50th or 75th birthday. After the prevalent round, all Danish residents aged 50–74 years have been invited to CRC screening every two years.
The Danish CRC screening program is free of charge. This includes FIT, colonoscopy and cancer treatment. All residents receive a postal invitation to CRC screening along with a screening kit (immunochemical detection of blood in the self-administrated faecal sample; the FIT device is produced by OC Sensor System; Eiken Chemical Company), a guidance on faeces sampling and a prepaid (self-addressed) return envelope. After 45 days, a digital reminder is sent to those who have not returned a sample. Test results are sent to the resident within seven days. If the sample is considered positive (>100 ng/mL buffer), the resident receives a colonoscopy appointment within 14 days.
2.2 Study population and design
All Danish residents aged 50–74 years on 1 January 2014 who had no previous diagnosis of CRC were eligible for inclusion (Figure 1).
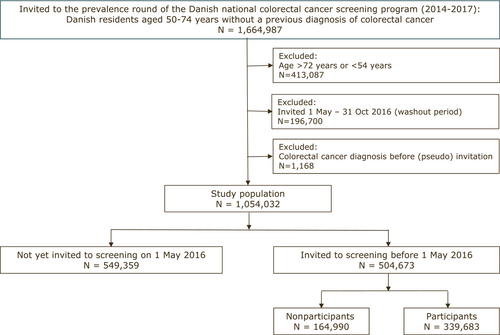
Due to the randomized procedure of invitation according to birth month during the prevalence round of CRC screening, the study was designed to estimate the effect of CRC screening on cancer detection and disease stage by dividing the study population into two groups on 1 May 2016, when a random half of the study population had received a screening invitation (invited group), and the other random half had not yet received an invitation (not yet invited group). The capacity of colonoscopy increased during the prevalence round, which is why a larger proportion was screened in the later stages of the study period. To ensure a follow-up period of at least six months after having received an invitation letter, those invited from 1 May 2016 to 31 October 2016 were excluded (washout period). The end of the follow-up was 31 October 2016.
Individuals in the not yet invited group were assigned a random pseudo date of invitation (with the same distribution of dates as in the invited group) to ensure uniform exclusion criteria for both groups due to previous CRC. Finally, to ensure the inclusion of randomly invited individuals only, those at risk of being invited in a nonrandomized manner (as described earlier) were excluded.
2.3 Data sources
The study was based on information from five different Danish nationwide health registers, as described below. All data are recorded with reference to a civil registration number, which is a unique personal identification number assigned to all Danish residents. This number permits accurate linkage of recorded information in the registers at the personal level.10
The study population was identified through the Danish Colorectal Cancer Screening database. This clinical quality database comprises information on invited residents, screening participation and results of faecal immunochemical tests.11 Individuals were considered as participants in the screening program if a faeces sample had been returned within three months after a reminder had been sent.
Information on CRC diagnosis was retrieved from the Danish Colorectal Cancer Group Database, which contains prospectively collected data on all patients with CRC since 2001, including data on diagnostics, surgical treatment, postoperative complications and histopathology data.12 The patient completeness is high (>95%),12 and the data are known to be valid and of high quality.13 Cancer stage was classified according to the 5th edition of the TNM (tumour, node, metastasis) staging system by the Union for International Cancer Control.14 Accordingly, the cancer stage at the time of diagnosis was defined as stage I/II for local tumour growth and stage III/IV for tumours with metastases.
The main exposure was pre-existing diabetes at the date (or pseudo date) of invitation. Diabetes was characterized as either type 1 or type 2 and further as either prevalent diabetes (duration ≥2 years) or incident diabetes (duration <2 years) to explore the effect of chronic (prevalent) versus newly diagnosed (incident) diabetes on screening participation. Individuals with diabetes were identified from data in the Danish National Patient Register (comprising information on diagnoses from all hospital contacts in Denmark), the Danish National Prescription Registry (comprising information on all prescriptions redeemed at Danish pharmacies) and the Danish National Health Service Register (comprising information on podiatrist services) as described by Carstensen et al.15 The classification of diabetes (type 1 and type 2) was based on the same data sources, and an algorithm developed at the Steno Diabetes Center Aarhus (unpublished manuscript) was used. In brief, individuals were classified as having type 1 diabetes if they had received prescriptions for insulin combined with a diagnosis of type 1 from a medical department. Otherwise, diabetes was classified as type 2.
2.4 Ethics approval
According to Danish law and the Committee on Health Research Ethics in the Central Denmark Region, the study required no ethical approval since it was based on registered data. This committee also waived patient consent for the use of registered data. In accordance with Danish law, the study was approved by the Central Region Denmark (file no. 1-16-02-304-19).
2.5 Statistical analyses
Descriptive data were presented as means with standard deviations (SD) for continuous variables and as proportions (n, %) for categorical variables.
We used the chi-square test to analyse differences between invited and not yet invited, individuals with and without diabetes and between participants and nonparticipants in the invited group.
To estimate the effect of screening on detecting CRC, we calculated the total number of diagnosed cancer cases per 100,000 invited and not yet invited individuals according to diabetes status from 3 March 2014 until 31 October 2016 (ie the study period). Risk differences and relative risks were estimated for those with and without diabetes and for those invited and not yet invited using Poisson regression with robust error variance.16 Furthermore, we computed a measure of the interaction between both screening and diabetes to estimate the excess effect of screening on detecting cancer in individuals with diabetes, which was not explained by the independent effects of screening or diabetes, respectively.
To estimate the impact of diabetes on screening participation, we assessed the participation difference between individuals with and without diabetes in the invited group using generalized linear models. Unadjusted analyses were performed for each independent variable (including diabetes diagnosis at the date of invitation, type of diabetes and duration of type 2 diabetes) along with a multivariate model adjusting for age (at the date of invitation) and sex.
Results were presented with 95% confidence intervals (CI) when relevant. The statistical significance level was set at 0.05. Stata, version 17, was used for all analyses (Stata Corp). The STROBE guideline for observational studies was followed.
3 RESULTS
A total of 1,664,987 individuals were assigned to receive an invitation to the first round of the CRC screening (Figure 1). We excluded individuals who were not randomly invited (N = 413,087), individuals receiving a diagnosis of CRC after 1 January 2014 but before the screening invitation (or pseudo invitation) (N = 1168) and individuals who were invited during the washout period (N = 196,700). Finally, 1,054,032 individuals were eligible for inclusion in the study cohort; 549,359 (52%) had not yet been invited to the CRC screening; and 504,673 (48%) had been invited. By chance, those not yet invited were slightly more often male and slightly older than the invited (Table 1).
| Not yet invited | Invited | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | No diabetes | Diabetes | All | p-value* | No diabetes | p-value* | Diabetes | p-value* | |||||||
| 549,359 | 492,349 | 89.6% | 57,010 | 10.4% | 504,673 | 454,480 | 90.1% | 50,193 | 9.9% | <0.001 | |||||
| Sex | |||||||||||||||
| Female | 278,318 | 50.7% | 254,840 | 51.8% | 23,478 | 41.2% | 257,377 | 51.0% | 0.001 | 236,472 | 52.0% | 0.008 | 20,905 | 41.6% | 0.121 |
| Male | 271,041 | 49.3% | 237,509 | 48.2% | 33,532 | 58.8% | 247,296 | 49.0% | 218,008 | 48.0% | 29,288 | 58.4% | |||
| Age, years (mean (SD)) | 63.8 | (6.2) | 63.6 | (6.2) | 65.4 | (6.0) | 63.6 | (6.0) | <0.001 | 63.4 | (6.0) | <0.001 | 65.1 | (5.8) | <0.001 |
| Diabetes | |||||||||||||||
| Type 1 | 2531 | 4.4% | 2231 | 4.4% | 0.967 | ||||||||||
| Type 2 | 54,479 | 95.6% | 47,962 | 95.6% | |||||||||||
| Type 2 diabetes duration | |||||||||||||||
| Years (mean (SD)) | 8.7 | (6.0) | 8.1 | (5.6) | |||||||||||
| <2 years (incident cases) | 7030 | 12.9% | 5695 | 11.9% | <0.001 | ||||||||||
| 2–5 years | 7709 | 14.2% | 10,367 | 21.6% | |||||||||||
| 5–10 years | 20,412 | 37.5% | 15,736 | 32.8% | |||||||||||
| ≥10 years | 19,328 | 35.5% | 16,164 | 33.7% | |||||||||||
| Cancer detected | 842 | 0.15% | 724 | 0.15% | 118 | 0.21% | 1904 | 0.38% | <0.001 | 1656 | 0.36% | <0.001 | 248 | 0.49% | <0.001 |
| Stage distribution | |||||||||||||||
| I | 121 | 14.4% | 101 | 14.0% | 20 | 16.9% | 456 | 23.9% | <0.001 | 396 | 23.9% | <0.001 | 60 | 24.2% | 0.001 |
| II | 204 | 24.2% | 170 | 23.5% | 34 | 28.8% | 388 | 20.4% | 347 | 21.0% | 41 | 16.5% | |||
| III | 209 | 24.8% | 189 | 26.1% | 20 | 16.9% | 379 | 19.9% | 329 | 19.9% | 50 | 20.2% | |||
| IV | 146 | 17.3% | 129 | 17.8% | 17 | 14.4% | 256 | 13.4% | 220 | 13.3% | 36 | 14.5% | |||
| Unknown | 162 | 19.2% | 135 | 18.6% | 27 | 22.9% | 425 | 22.3% | 364 | 22.0% | 61 | 24.6% | |||
- * p-value for differences between invited and not yet invited, both overall and among those without and with diabetes.
3.1 Characteristics of the study population
In total, 107,203 (10.2%) individuals had a diagnosis of diabetes at the date of invitation (Table S1). The prevalence of diabetes was slightly higher in the not yet invited group (10.4%) than in the invited group (9.9%) (Table 1). Compared with individuals without diabetes, individuals with diabetes were older (mean age 65 years versus 63 years), were more likely to be men (59% vs. 48%) and had more CRC detected (0.34% vs. 0.25%) (Table S1). Further, individuals with type 2 diabetes had a mean diabetes duration of 8.4 years (SD 5.8) at the time of (pseudo) invitation.
3.2 Impact of diabetes on the effect of screening on cancer detection
When comparing those invited to those not yet invited, the CRC screening increased the number of detected cancers throughout the study period from 147 to 364 cancers per 100,000 individuals without diabetes (risk difference (RD): 217 per 100,000, 95% CI: 197–238), whereas the number of detected cancers increased from 207 to 494 cancers per 100,000 individuals with diabetes (RD: 287 per 100,000, 95% CI: 215–359) (Table 2). The excess effect of screening on cancer detection in individuals with diabetes was an additional 70 cases per 100,000 (95% CI: 5–134 per 100,000); this was beyond what could be expected from the independent effect of being invited (217 per 100,000) and the independent effect of having a diagnosis of diabetes (60 per 100,000). Being invited to screening more than doubled the risk of being diagnosed with CRC, and the relative risk was similar for those without and with diabetes.
| No diabetes | Diabetes | Interaction contrast (95% CI) | |||
|---|---|---|---|---|---|
| Not yet invited | Invited (95% CI) | Not yet invited (95% CI) | Invited (95% CI) | ||
| CRC incidence per 100,000 individuals | 147 | 364 | 207 | 494 | |
| (a) Effect of screening on cancer detection | |||||
| Risk difference | ref | 217 (197–238) | ref | 287 (215–359) | — |
| Relative risk | ref | 2.48 (2.27–2.70) | ref | 2.39 (1.92–2.97) | — |
| (b) Effect of screening on cancer detection due to interaction | |||||
| Risk difference | ref | 217 (197–238) | 60 (16–104) | 347 (308–385) | 70 (5–134) |
| Relative risk | ref | 2.48 (2.27–2.70) | 1.41 (1.16–1.71) | 3.36 (2.91–3.88) | 0.96 (0.76–1.22) |
3.3 Stage of disease at the time of diagnosis
The highest proportion of stage I cancer at the time of diagnosis was seen in the invited group (24%) compared with the not yet invited group (14%) (Table 1). In the invited group, this effect of screening on identifying early stages of CRC was similar among those with and without diabetes. However, in the not yet invited group, a higher proportion of stage I/II cancers was seen in individuals with diabetes (46%) than in individuals without diabetes (37%). Among individuals with diabetes, more cancers were overall detected in the invited group (0.49%) than in the not yet invited group (0.21%); however, the proportion of stage I/II cancers was similar in the invited and the not yet invited group.
3.4 Impact of diabetes on screening participation in the invited group
In the invited group, 339,683 (67%) individuals participated in the CRC screening by returning a faeces sample (Figure 1, Table 3). Compared with participants, nonparticipants were more likely to be men (54% versus 47%) and more often had a diagnosis of diabetes (12% vs. 9%) (Table 3). The proportion of CRCs diagnosed in stage III/VI was higher among nonparticipants with diabetes (60%) than among participants with diabetes (28%), which yielded a risk difference of 32% (95% CI 17%–46%). Table 4 presents the impact of diabetes on screening participation. The participation rate was higher among those without diabetes (68.2%) than among those with diabetes (59.0%), which amounted to an RD of −9.1% (95% CI −9.5; −8.7) when adjusting for age and sex. The findings were not affected by the duration of type 2 diabetes (ie incident and prevalent diabetes); however, when stratifying the analyses on type of diabetes, individuals with type 1 diabetes tended to have a slightly higher participation rate than individuals with type 2 diabetes (RD −5.8% vs. −9.3%) (Table 4).
| Nonparticipants | Participants | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | No diabetes | Diabetes | Total | p-value* | No diabetes | p-value* | Diabetes | p-value* | |||||||
| 164,990 | 144,407 | 87.5% | 20,583 | 12.5% | 339,683 | 310,073 | 91.3% | 29,610 | 8.7% | <0.001 | |||||
| Sex | |||||||||||||||
| Female | 75,831 | 46.0% | 67,568 | 46.8% | 8263 | 40.1% | 181,546 | 53.4% | <0.001 | 168,904 | 54.5% | <0.001 | 12,642 | 42.7% | <0.001 |
| Male | 89,159 | 54.0% | 76,839 | 53.2% | 12,320 | 59.9% | 158,137 | 46.6% | 141,169 | 45.5% | 16,968 | 57.3% | |||
| Age, years (mean (SD)) | 63.3 | (6.1) | 63.1 | (6·1) | 64.9 | (6.0) | 63.8 | (5.9) | <0.001 | 63.6 | (5.9) | <0.001 | 65.3 | (5.7) | <0.001 |
| Diabetes | |||||||||||||||
| Type 1 | 858 | 4.2% | 1373 | 4.6% | 0.012 | ||||||||||
| Type 2 | 19,725 | 95.8% | 28,237 | 95.4% | |||||||||||
| Type 2 diabetes duration | |||||||||||||||
| Years (mean (SD)) | 8.3 | (5.7) | 8.0 | (5.6) | <0.001 | ||||||||||
| <2 years (incident cases) | 2408 | 12.2% | 3287 | 11.6% | <0.001 | ||||||||||
| 2–5 years | 3153 | 16.0% | 5039 | 17.8% | |||||||||||
| 5–10 years | 6409 | 32.5% | 9443 | 33.4% | |||||||||||
| ≥10 years | 7755 | 39.3% | 10,468 | 37.1% | |||||||||||
| Cancer detected | 397 | 0.24% | 345 | 0.24% | 52 | 0.25% | 1507 | 0.44% | <0.001 | 1311 | 0.42% | <0.001 | 196 | 0.66% | <0.001 |
| Stage | |||||||||||||||
| I/II | 121 | 30.5% | 109 | 31.6% | 12 | 23.1% | 723 | 48.0% | <0.001 | 634 | 48.4% | <0.001 | 89 | 45.4% | <0.001 |
| III/IV | 196 | 49.4% | 165 | 47.8% | 31 | 59.6% | 439 | 29.1% | 384 | 29.3% | 55 | 28.1% | |||
| Unknown | 80 | 20.2% | 71 | 20.6% | 9 | 17.3% | 345 | 22.9% | 293 | 22.3% | 52 | 26.5% | |||
- * p-value for differences between nonparticipants and participants, both overall and among those without and with diabetes.
| Participation (95% CI) | Adjusted RDa (95% CI) | |
|---|---|---|
| No diabetes | 68.2% (68.1;68.4) | ref |
| Diabetes | ||
| All | 59.0% (58.6;59.4) | −9.1% (−9.5;-8.7) |
| Type 1 | 61.5% (59.5;63.6) | −5.8% (−7.8;-3.9) |
| Type 2 | 58.9% (58.4;59.3) | −9.3% (−9.7;-8.8) |
| Type 2 Diabetes duration | ||
| <2 years (incident cases) | 57.7% (56.4;58.9) | −10.6% (−11;-8.8) |
| ≥2 years (prevalent cases) | 59.2% (58.7;59.6) | −9.0% (−9.4;-8.5) |
- Note: Screening participation = Returned faeces samples within 3 month/invitations sent out.
- Abbreviation: RD, risk difference.
- a Adjusted for age and sex.
4 DISCUSSION
This study evaluated the impact of diabetes on the effect of CRC screening in terms of the number of detected CRCs and stage of disease at the time of diagnosis in individuals randomly invited to participate in the prevalence round of the Danish national CRC screening program. The effect of screening was a higher number of detected cancers and more CRCs diagnosed at earlier stages, and the effect of detecting cancer was more pronounced in individuals with diabetes despite a lower participation rate.
The overall effect of the prevalence round of the Danish national CRC screening program based on FIT demonstrated that individuals in the invited group were more likely to be diagnosed with CRC and that their proportion of stage I cancers was higher compared with the not yet invited group. These findings on cancer detection and stage distribution are similar to the results reported after the implementation of CRC screening programs in the Netherlands and Canada.17, 18 Further, during a prevalence round of screening, higher detection of CRC in the invited vs the not yet invited group is expected due to detection of already prevalent and not yet clinically evident cancers. With longer periods of follow-up, removal of precancerous lesions would most likely result in reduced CRC incidence.19
The increased detection of CRC associated with diabetes among both invited and not yet invited individuals was expected since it has become evident during the past decades that individuals with diabetes have a 20% higher risk of developing CRC compared with those without diabetes.3 However, it was unexpected that we found an additive effect of screening in persons with diabetes, which could not be explained by the independent effects of diabetes or being invited to screening, respectively. No previous studies have reported on the impact of diabetes on the effect of CRC screening on cancer detection.
Among the invited individuals, the similar proportion of screen-detected early-stage cancers in individuals both with and without diabetes was expected, since FIT-based screening has proven to be a valid screening tool in public screening programs,17, 18 and a large proportion of the target population is expected to suffer from age-related chronic diseases.20 The most unexpected finding was the high proportion of early-stage CRCs in the not yet invited individuals with diabetes. It was similar to the proportion in the invited group, but it was higher than the proportion in the group of not yet invited individuals without diabetes. Two large-scale population-based studies of CRC found no impact of diabetes with respect to stage distribution.21, 22 One explanation for the current finding could be that a diagnosis of diabetes is associated with a higher number of clinical investigations in the context of a well-established universal healthcare system, and this could lead to the detection of CRC at an earlier stage.23 Furthermore, individuals with diabetes have more contact with healthcare providers due to diabetes follow-up. For example, gastrointestinal symptoms may be discussed in the context of side effects of oral antidiabetic medications, and abnormal symptoms may then trigger further investigation. The observed higher proportion of late-stage CRCs in nonparticipants with diabetes emphasizes the importance of focussing on health initiatives that aim to increase the screening participation rates of individuals with diabetes, as this may help reduce the adverse effects of diabetes on CRC outcomes.
Conflicting evidence has been reported on CRC screening participation in populations with diabetes.8 The current study was based on data from a publicly funded CRC screening program using free-of-charge screening kits mailed to all Danish residents (aged 50–74 years), which allowed equal access to screening. In a similar setting with universal health care, a Canadian study found that having a major chronic medical condition (such as diabetes) was associated with lower adherence to regular CRC screening.20
A major strength of the present study was the randomized invitation procedure in the prevalence round of the national CRC screening program, which involved the entire Danish population in the relevant age group. To our knowledge, this is the first study to explore the effect of FIT-based public CRC screening in individuals with diabetes. Nevertheless, as reported in a previous paper,24 the birth months of January and February were both (by chance) randomized to the last part of the prevalence round. This could explain the minor age difference seen between invited and not yet invited individuals, which, in turn, may explain the slightly higher prevalence of type 2 diabetes in the not yet invited group (10.4%) compared with the invited group (9.9%).
In designing the study, reporting the effect of being invited, not the effect of participating, reduced the risk of healthy screened bias, and the randomization should eliminate potential confounders between invited and not yet invited individuals. Another strength was the use of validated and comprehensive registries, which allowed accurate identification of individuals with diabetes and valid information on CRC screening and CRC diagnoses. Still, a risk of information bias might have been present due to the high number of missing data on the disease stage at the time of diagnosis. Another limitation was the potential misclassification of diabetes. Individuals with type 2 diabetes who are undiagnosed, treated with lifestyle change or are noncompliant to antidiabetic medications would be classified as not having diabetes. Furthermore, insulin-treated type 2 diabetes might have been misclassified as type 1 diabetes. However, we have no reason to believe that the potential misclassification of diabetes differed between the screening invited group and the not yet invited group. Therefore, the effect estimates are assumed to be reliable.
In summary, the study adds new knowledge regarding the impact of diabetes on the effect of public CRC screening, and the presented findings demonstrate that CRC screening based on FIT could be an effective tool to detect CRC at earlier disease stages, and that there is an additive effect of CRC screening among individuals with diabetes. This new evidence has the potential to increase the survival after CRC in individuals with diabetes by detecting CRC at earlier disease stages and by removing precancerous polyps before they develop into CRC. Furthermore, the study highlights the need for a more tailored approach to CRC screening to increase the participation among individuals with diabetes and thereby obtain better effects of the screening efforts.
AUTHOR CONTRIBUTIONS
Susanne Boel Graversen, Mette Bach Larsen, Berit Andersen and Tinne Laurberg conceptualized the study and contributed to the study design. Susanne Boel Graversen and Tinne Laurberg performed the analyses, and accessed and verified the data. Susanne Boel Graversen and Tinne Laurberg wrote the first draft of the manuscript. All authors contributed to reviewing the drafts of the manuscript and approved the final manuscript draft. All authors had final responsibility for the decision to submit for publication.
ACKNOWLEDGMENTS
This study was conducted using data provided by the Danish Health Data Authority. TL was supported by a grant from the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation (grant number NNF17SA0031406).
CONFLICT OF INTEREST STATEMENT
BA is the Head of cancer screening in the Central Denmark Region and had Received HPV test kits from Roche for other studies about cervical cancer screening. TL has received a research grant (covering part-time post.doc salary) from the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation, grant number NNF17SA0031406. The funder was not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report. All other authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Danish Health Data Authority's Research Services, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Access to data may be obtained with permission of the Danish Health Data Authority through a collaboration with a Danish research institution.




