From cryogenic to on-scalp magnetoencephalography for the evaluation of paediatric epilepsy
Abstract
Magnetoencephalography (MEG) is a neurophysiological technique based on the detection of brain magnetic fields. Whole-head MEG systems typically house a few hundred sensors requiring cryogenic cooling in a rigid one-size-fits-all (commonly adult-sized) helmet to keep a thermal insulation space. This leads to an increased brain-to-sensor distance in children, because of their smaller head circumference, and decreased signal-to-noise ratio. MEG allows detection and localization of interictal and ictal epileptiform discharges, and pathological high frequency oscillations, as a part of the presurgical assessment of children with refractory focal epilepsy, where electroencephalography is not contributive. MEG can also map the eloquent cortex before surgical resection. MEG also provides insights into the physiopathology of both generalized and focal epilepsy. On-scalp recordings based on cryogenic-free sensors have demonstrated their use in the field of childhood focal epilepsy and should become a reference technique for diagnosing epilepsy in the paediatric population.
What this paper adds
- Magnetoencephalography (MEG) contributes to the diagnosis and understanding of paediatric epilepsy.
- On-scalp MEG recordings demonstrate some advantages over cryogenic MEG.
What this paper adds
- Magnetoencephalography (MEG) contributes to the diagnosis and understanding of paediatric epilepsy.
- On-scalp MEG recordings demonstrate some advantages over cryogenic MEG.
Abbreviations
-
- ETLE
-
- extratemporal lobe epilepsy
-
- He-OPM
-
- helium-based optically pumped magnetometer
-
- HFO
-
- high-frequency oscillation
-
- IED
-
- interictal epileptiform discharge
-
- MEG
-
- magnetoencephalography
-
- MSR
-
- magnetically shielded room
-
- OPM
-
- optically pumped magnetometer
-
- RFE
-
- refractory focal epilepsy
-
- SNR
-
- signal-to-noise ratio
-
- SQUID
-
- superconducting quantum interference device
-
- TLE
-
- temporal lobe epilepsy
WHAT IS MAGNETOENCEPHALOGRAPHY?
Magnetoencephalography (MEG) is a non-invasive neurophysiological technique based on the detection of the magnetic fields generated by neural electrical activity.1 It is akin to electroencephalography (EEG), which detects brain electrical activity.2 Both techniques are time sensitive as they record brain activity with a temporal resolution of the order of milliseconds. MEG is much less available than EEG, notably because of its much higher cost.
MEG systems are typically composed of about 200 to 300 sensors,1 called superconducting quantum interference devices (SQUIDs), that are able to detect the magnetic fields with a high sensitivity.3 SQUIDs require cryogenic cooling in liquid helium (−269°C) to be sensitive to the tiny (in the range of pico- to femtotesla) neuromagnetic fields. They are thus placed in a rigid whole-scalp-covering, one-size-fits-all, typically adult-sized helmet to keep a thermal insulation space (~2–4 cm) between the liquid helium and the scalp4 (Figure 1).

The neuromagnetic fields detected by MEG are mostly generated by the postsynaptic potentials of cortical pyramidal neurons oriented tangentially to the scalp (i.e. sources within the sulcal cortex).5 As opposed to EEG, MEG is almost blind to radial sources (i.e. at the convexity of the gyral cortex).5 Compared with the brain electrical activity, magnetic fields are unaffected by changes in conductivity and suffer minimal distortion owing to the different tissues they have to pass through to reach the scalp surface.6 As magnetic field amplitude decreases with the square/cube of the source-to-sensor distance depending on SQUID type, MEG is more sensitive to superficial neocortical sources than deep sources,4 notwithstanding its ability to record activities originating from deep brain structures (e.g. amygdala and hippocampus).7 MEG, compared with EEG, also provides a higher signal-to-noise ratio (SNR) for most focal neocortial sources, and a lower SNR for extended sources.8 Studies have shown that MEG and EEG are complementary techniques for investigating the whole activity of the human brain as MEG can detect neural activity not detected by EEG, and vice versa.2
MEG recordings usually take place inside an expensive, heavy, and bulky magnetically shielded room (MSR) to reduce the ambient magnetic noise (in the range of micro- to picoteslas). Lightweight MSRs, with active shielding-based electromagnetic coils to dynamically attenuate fleeting ambient noise, can also be used to reduce the size, weight, and cost of the MSR and facilitate the siting of MEG systems in clinical environments.9 Further noise reduction with advanced spatiotemporal filters is also used to dampen biological (e.g. heart beat) and noise (e.g. vagal nerve stimulator) artefacts.10, 11 Head movements can also be corrected with the help of movement compensation algorithms based on continuous head position tracking within the MEG helmet.12
Combined with brain magnetic resonance imaging (MRI), MEG signals can be used to perform source reconstruction aimed at localizing the neural sources at the origin of the recorded neuromagnetic signals.6 Various subtypes of the source reconstruction method exist, such as single equivalent current dipole modelling, beamformer, or distributed source modelling.3 All these methods aim to solve the inverse problem (i.e. modelling the current and locating its sources on the basis of the recorded signals5). To summarize their working, (1) single equivalent current dipole modelling looks for the best solution to locate presumed isolated activations by assuming that a single source is activated for a certain duration at the same location; (2) a beamformer scans all source locations of the brain, one after the other, and tests the contribution of a single dipole at this specific location to the recorded data; (3) distributed source modelling does not restrict a priori the number of sources and, rather, estimates the whole source distribution at once, under a priori constraints on the smoothness of source distribution.3 Owing to the intrinsic properties of magnetic fields and the higher number of magnetic field sensors, source reconstruction based on MEG benefits from a higher accuracy and precision compared with EEG, with a spatial resolution of about 5 mm.13
MEG currently has two validated and internationally recognized clinical applications: assessment of refractory focal epilepsy (RFE), by localization of epileptiform discharges, and presurgical mapping of functionally eloquent cortices.14
WHAT ARE THE CHALLENGES OF MEG IN PAEDIATRIC USE?
MEG helmets are typically based on adult head circumference, leading to increased brain-to-sensor distance in patients with small head circumference (e.g. children) and to increased head movements within the MEG helmet.4, 12 Still, paediatric recordings can be performed successfully using adult-sized MEG.15 Nevertheless, small head circumference leads to a decrease in SNR and a lower localization accuracy, unless the brain source of interest is known and placed as close as possible to the helmet.16 During infancy or the neonatal period, whole-brain investigations require repositioning or the use of a foam halo to maintain the head at the centre of the MSR.17 Few infant and paediatric MEG helmets have been designed on the basis of child head circumference to reduce the brain-to-sensor distance and increase SNR.4 These child-sized MEG devices are restricted to a specific age-range, which limits their use.17 In addition, the limitation of head movements in child-sized MEG can be uncomfortable, reducing stillness during awake recordings.17
The absence of head movement compensation might impede the interpretation of recordings from young children.18 Notwithstanding head movement tracking, relative stillness remains a requirement as the head needs to stay within the MEG helmet to allow proper head movement tracking/compensation, and movement artefacts after compensation may mimic brain signals.18
The MEG environment needs to be adapted (e.g. with toys or illustrations) to make children more cooperative and prevent crying or laughing, which induce movement and muscular artefacts.17
The youngest children and those with disability require sedation to benefit from MEG recordings longer than a few minutes with optimal SNR.6 Some sedative drugs can impact the brain signals of interest (e.g. reduction in the occurrence of epileptiform discharges with propofol, midazolam, or fentanyl, contrary to dexmedetomidine).6 Sedation can also be required to acquire good quality brain MRI scans, which is mandatory for performing source reconstruction.4
WHY USE MEG IN THE CLINICAL MANAGEMENT OF CHILDHOOD EPILEPSY?
Epilepsy is the most common chronic neurological disease of childhood, defined by the occurrence of unprovoked epileptic seizures, and affecting around 0.5% of children.19 Around 30% of children do not reach seizure-freedom despite antiseizure medication. They thus have refractory epilepsy and may benefit from epilepsy surgery.19
The American Clinical Magnetoencephalography Society was the first to endorse clinical practical guidelines for the use of MEG in the presurgical evaluation of RFE20 and presurgical functional brain mapping,21 both of them being used in childhood epilepsy. Clinical practical guidelines were subsequently established by the International Federation of Clinical Neurophysiology.22
RFE is the main recognized clinical application of MEG. MEG can indeed be integrated as a part of the presurgical assessment of children with RFE, mostly to enrich hypotheses about the localization of the epileptogenic zone.23 There are fewer MEG studies that have focused on the clinical added value of MEG in paediatric epilepsy (and usually with a low number of included patients) than in adults only or in both children and adults. We therefore focus here on paediatric or mixed (i.e. children and adults) studies.
MEG commonly allows detection and localization of interictal (i.e. between seizure) epileptiform discharges (IEDs),23 and rarely ictal (i.e. during seizure) discharges,24 or pathological high frequency (i.e. from 80–500 Hz) oscillations (HFOs).19 Although such epileptiform activities are commonly detected by EEG,13 MEG gives non-redundant information that can help to better localize the epileptogenic zone and lead to freedom from seizure.23 Indeed, epileptiform activities can be captured by MEG and missed by EEG, or vice versa, depending on their localization and source orientation.2 MEG should therefore be done simultaneously with EEG whenever possible to obtain a complete picture of patients' epileptic disorders.2
IEDs (e.g. spikes) are the main type of investigated epileptiform activity in MEG signals.14 They are typically visually detected, and then localized, mostly with single equivalent current dipole modelling25 (Figure 2). MEG changes patients' surgical management in about 20% to 30% of cases by (1) detecting IEDs not captured by EEG, helping to identify subtle brain lesions that are difficult to see on structural brain MRI, (2) guiding intracranial EEG electrode implantation, or (3) contributing to a better delineation of the anatomical relationship between IED sources, structural brain lesions, and functionally eloquent cortices.23 In patients for whom MEG does not change surgical plans, it may still be clinically relevant as an extra non-invasive technique to confirm the absence of an additional irritative zone not captured by EEG, and thus increase the level of confidence about the decision-making process.23 The yield of MEG is higher in the case of presumed extratemporal lobe epilepsy (ETLE) or epilepsy with unclear localization.23, 25 It also extends to some childhood epileptic syndromes (e.g. allowing resective surgery in 20% of patients with Landau–Kleffner syndrome with a unilateral perisylvian pacemaker26). Moreover, the spatial distribution (i.e. clustered vs scattered) of equivalent current dipoles obtained for each localized IED may also have clinical significance as clustered equivalent current dipoles have been associated with a higher positive predictive value of being seizure-free after resective surgery than scattered dipoles.27 Furthermore, surgical resection extended to the whole IED cluster is usually correlated with a better surgical outcome (sensitivity for Engel class 1A at 1 year 84% in ETLE, 56% in temporal lobe epilepsy [TLE]; specificity for Engel class 1A at 1 year 89% in ETLE, 77% in TLE; in a study that included 1000 patients, of whom 11% were younger than 18 years).25 The higher yield of MEG in ETLE compared with TLE can be easily understood. First, as neuromagnetic fields are not influenced by the layers they pass through and MEG has a heightened sensitivity to tangential sources, MEG is superior to EEG in detecting IEDs originating from the perisylvian/insular cortices,5, 28 and from bottom-of-sulcus focal cortical dysplasia.5 The localization value of temporal IEDs requires more interpretation to distinguish mesiotemporal from neocortical sources and to establish their pathological nature.29 As children more frequently have ETLE than TLE, all this argues for the use of MEG in paediatric RFE.6
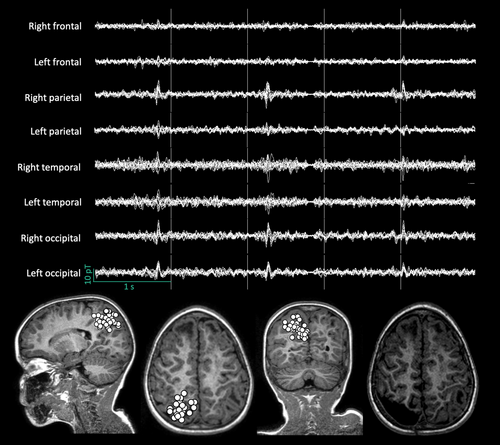
Pathological HFOs are not systematically investigated during MEG recordings,14 although they seem to be a marker of epileptogenicity, improving the presurgical diagnosis and the surgical outcome (as shown in a case series of two children with epilepsy),19 and can be simultaneous or not with spikes (in a study that included 25 patients, of whom 21 were younger than 18 years).30 MEG has some theoretical advantages over EEG for detecting and localizing HFOs owing to the lower influence of muscular activity on magnetic fields, the absence of signal distortion when passing through biological tissues, its higher sensor density (up to 306 sensors for MEG recordings, up to 256 electrodes for high-density EEG recordings, about 20 electrodes for conventional EEG recordings13), and the SNR for focal sources.19 Moreover, MEG-detected HFOs are less frequent but more specific and sensitive for the presumed epileptogenic zone than EEG-detected HFOs (in a study that included 30 patients, of whom nine were younger than 18 years).31
Ictal MEG recordings remain rare owing to the unexpected occurrence of seizures.2 Therefore, most ictal recordings are performed in patients with frequent and predictable seizures,32 or require prolonged MEG recordings.24 These ictal MEG recordings have a high sensitivity (96% at lobar level and 70% at infra-lobar level) and specificity (90% at lobar level and 73% at infra-lobar level) to localize the seizure-onset zone (in a study that included 23 patients, of whom 17 were younger than 18 years),24 with additional localization value compared with simultaneous EEG in about one-third of cases (in a study that included 44 patients, of whom 28 were younger than 18 years).33 Nonetheless, seizure-related head movements and muscle artefacts can impede the localization of ictal activity.2
MEG may also be used to map functionally eloquent cortices before surgical resection and to assess their localization compared with epileptiform discharges.6 Somatosensory and active/passive movement-related responses can be localized in awake or sedated children with epilepsy.6 Language can be lateralized and localized with MEG as well, even in children under sedation.6 Presurgical functional brain mapping with MEG is more child-friendly than functional MRI, which is noisier, less tolerant to subtle head movements, and whose results rely on neurovascular coupling, which changes markedly during development and can be altered by brain lesions/disorders.6
WHY USE MEG IN EPILEPSY RESEARCH?
MEG helps to better understand the physiopathology of paediatric epilepsies and to assess treatment/prognosis markers using source localization and connectivity analyses (e.g. in childhood absence epilepsy as an example of generalized epilepsy,34 or in epileptic encephalopathies with continuous spike–waves during sleep as an example of focal epilepsy35).
As illustrative examples, MEG studies have demonstrated that the pre-ictal state activity of childhood absence epilepsy (i.e. 50 ms before seizure onset) is focal,34 highlighting the continuum between focal and generalized epilepsies, which start from a local brain area with rapid subsequent generalization. MEG studies have also contributed to a better understanding of the pathophysiology of epileptic encephalopathies with continuous spike–waves during sleep by demonstrating the spatiotemporal dynamics of epileptiform discharges,26, 35 and their relationship with metabolic neural changes.35
Connectivity analyses may also explain the neural substrates underlying behavioural or neuropsychological disturbances (e.g. they may explain attention disorders and memory impairments in patients with childhood absence epilepsy or the maintenance of awareness despite generalized spike-wave discharges34) or predict the response to antiseizure medication (e.g. they may differentiate responders and non-responders to ethosuximide among patients with childhood absence epilepsy34).
Similar connectivity analyses can be performed in children with focal epilepsy, refractory (e.g. in TLE)36 or not (e.g. in self-limited epilepsy with centrotemporal spikes).37 They might help to accurately diagnose TLE, by comparison with children in whom a temporal lobe epileptogenic zone extends to extratemporal brain structures.36 Moreover, connectivity analyses might be helpful to better understand neural mechanisms underlying neurocognitive deficits in children with focal epilepsy, refractory or not (e.g. motor skill deficits in children with self-limited epilepsy with centrotemporal spikes37), and to better predict postoperative neurocognitive outcomes (e.g. verbal memory, visual memory, and working memory impairments in children with refractory TLE38).
With the continuing research efforts in the field, some of these approaches might become recognized clinical applications.
WHAT IS THE FUTURE OF MEG IN PAEDIATRIC EPILEPSY?
The main hopes for the future of MEG in children consist of (1) improved and automatized epileptiform discharge detection, (2) increased adaptability and comfort of paediatric MEG recordings, (3) increased compliance to spontaneous head movement, and (4) reduced cost or increased reimbursement to increase accessibility to MEG recordings.
On-scalp MEG based on optically pumped magnetometers (OPMs, OPM-MEG) should fulfil almost all these hopes. OPMs are novel, cryogenic-free MEG sensors that should revolutionize paediatric MEG by improving its feasibility, increasing the sensitivity and broadening the scope of MEG investigations performed in children at a reduced cost compared with cryogenic MEG. OPMs combine optical pumping with magnetic resonance and magnetic field-nulling techniques to non-invasively record brain magnetic fields in a free head-movement setting.39 Alkali OPMs work with photodetectors to record the light intensity of a laser beam passing through a cell of gaseous rubidium-87 heated at approximately 150°C (rubidium-based-OPMs).39 In short, optical pumping by the laser renders the gas completely transparent to the beam, whereas the presence of a magnetic field orthogonal to the laser axis opacifies the gas and proportionally decreases its intensity detected at the photodiode.40 Alkali OPMs have a similar noise level to MEG (~10–20fT/√Hz), a bandwidth limited to 0 to 130 Hz, a heat dissipation power of approximately 0.7 W per sensor, a reduced size (12.4 mm × 16.6 mm × 24.4 mm), and a very low weight (4.5–4.7 g).39 Their reduced size and low weight allow them to be placed directly on the scalp, or in close contact with it, in multichannel setups using EEG-like cap configurations or 3D-printed helmets adapted or not to each individual head (Figure 1). EEG-like caps have a high degree of comfort and adaptability to head circumference/morphology, ensure a placement of OPMs as close to the scalp as possible, and offer a large number of possible locations for OPMs. They have a non-rigid mounting that does not fully cover the OPMs and their cables. This can put OPMs at risk during high motor seizures and makes recording in the supine or sleeping position difficult. By contrast, helmets offer a rigid configuration that is well suited to rapid and repeated recordings on different participants of similar age and may represent the best solution for supine/sleep recordings. They may have rather heterogeneous brain-to-sensor distance owing to their non-adaptation to the head morphology. EEG-like caps and helmets therefore seem complementary, and users should choose one or the other according to their objectives. Studies are needed to determine which of them provides the best SNR and signals for accurate source reconstruction.
On-scalp OPM-MEG recordings (Figure 1) demonstrated their ability to record IEDs that were visually detected and localized in five school-aged children41 (Figure 3). The IED amplitude and SNR was higher compared with cryogenic MEG, and their localization was similar.41 SNR was not increased but similar in one child owing to substantial head and body movements inducing high-amplitude movement artefacts.41 OPM-MEG was also able to record and localize IEDs in a non-sedated 5-month-old infant with right centro-parietal epilepsy (Feys et al., forthcoming). The infant was free-to-move, lying on her mother's knee (Feys et al., forthcoming).
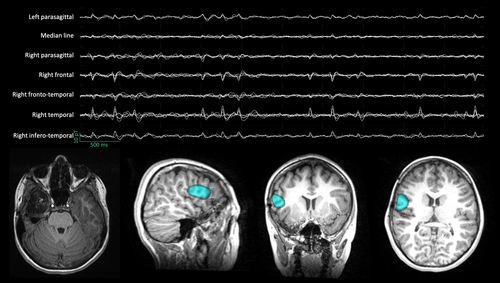
Owing to their on-scalp placement, increased SNR, and free head movements, OPMs are well suited for prolonged recordings, opening the door to easier ictal MEG recordings. The ability of OPM-MEG to record focal ictal discharges (at rest and during hyperventilation [i.e. activation procedure]) was demonstrated in one school-age child with RFE despite spontaneous and seizure-related movements, with a localization of the seizure-onset zones in agreement with intracranial EEG.42 This successful ictal recording, despite movements, was obtained thanks to the use of additional field-nulling coils that reduce the remnant magnetic field within the MSR to render OPM-MEG movement-compliant.43 Such reduction is mandatory when OPMs are used in an open-loop setup, as large magnetic deviations induced by movements lead to magnetic signal deformation and even saturation.40 The development of closed-loop OPMs should further improve the movement compliance of OPMs: by continuously zeroing the magnetic field around the sensor, closed-loop OPMs never undergo large magnetic deviations and avoid signal deformation and saturation, thereby increasing the dynamic range of OPM-MEG.44 Additional techniques could further help reduce head-movement-related noise/artefacts (e.g. head position tracking45). Their improvement is important for developing a powerful clinical OPM-MEG dedicated to children with epilepsy, including those with low collaboration (e.g. epileptic encephalopathy46). Ideally, OPMs should be able to withstand the occurrence of a seizure with secondary bilateralization or hypermotor semiology and maintain sufficient SNR to detect and localize ictal activity.
The heat dissipated by alkali OPMs might be uncomfortable in certain circumstances such as baldness in newborn infants (Feys et al., forthcoming). This can be avoided by adding insulating materials or a space of a few millimetres between the scalp and the OPMs (Feys et al., forthcoming). Other types of OPM, for example working with helium (He-OPMs) at ambient temperature, also represent an elegant solution to this issue (Feys et al., forthcoming). These He-OPMs also benefit from a larger bandwidth (0–2000 Hz) that could theoretically allow the recording of HFOs with OPM-MEG. Nevertheless, He-OPMs should benefit from technical improvements to reduce their intrinsic noise level (<50fT/√Hz over a frequency range of 1–1500 Hz) that is higher than rubidium-based-OPMs or SQUIDs, as well as their size/weight (current dimensions 40 g, 1.9 cm × 1.9 cm × 5 cm) before whole-scalp recording.
Regardless of the heat or size/weight of OPMs, the minimal number of sensors required for accurate clinical use remains to be determined. Theoretical studies suggest that the spatial resolution continues to improve while adding large numbers of sensors.47 The advent of triaxial Rb- and He-OPMs that allow recording simultaneously at a single position of the radial and tangential components of the brain magnetic field will maximize spatial brain sampling with a limited number of sensors (Feys et al., forthcoming).
Currently, OPM-MEG lacks commercial software that can be easily used by clinical magnetoencephalographers to identify and localize the source of epileptiform discharges. After adequate preprocessing, the OPM-MEG signal is akin to that acquired with cryogenic MEG. Still, the impact of on-scalp recording on the patterns of normal brain activity (e.g. normal MEG variants48) is currently not known. It is therefore unclear whether specific OPM-MEG training and clinical practice guidelines are needed.
The pioneering on-scalp OPM-MEG data available in the paediatric population should pave the way for future widespread clinical use of OPM-MEG for routine diagnostic assessment of epilepsy in this population, as well as interictal/ictal recordings performed in the context of the presurgical evaluation of RFE. Still, this novel technology is in its infancy and major steps are needed before its clinical acceptance and routine use in paediatric epilepsy. This will indeed require further advances in OPM technology per se (e.g. increased stability, electronics), whole-scalp OPM-MEG recordings, lightweight MSRs,43 noise reduction methods, developments and cost reductions in clinical software, as well as official approval from regulatory agencies for medical/clinical use. Large prospective studies are also mandatory to confirm the initial results obtained in the paediatric setting and to validate the accuracy of OPM-MEG by comparing its localization value of the irritative and seizure-onset zones with recognized ‘criterion standards’ such as intracranial recordings. Before we reach that point, cryogenic MEG remains the technology of reference for clinical MEG investigations.
High critical temperature SQUIDs are potential alternatives to low critical temperature (those cooled by liquid helium) SQUID-based MEG as they allow on-scalp recordings (sensor-to-scalp distance 1–3 mm) despite cooling within liquid nitrogen (−203°C).49 When guided by simultaneous EEG data, IEDs can be detected by high critical temperature SQUIDs with typical morphology and similar duration when compared with cryogenic MEG, despite a higher noise level than MEG (50–130fT/√Hz).49, 50 Still, high critical temperature SQUID setups have a large size and are not yet compatible for MEG recordings with sensors worn directly on the scalp such as OPM-MEG.
Improvements in the automatization of MEG data preprocessing and analyses are also expected by clinical magnetoencephalographers to automatically detect and localize IEDs. Data preprocessing and analysis of one single MEG investigation in patients with RFE is time-consuming, requiring on average about 8 hours.14 Some computational tools have been developed to automatize, ease, and shorten these analyses, for example independent component analysis,51 or virtual sensors based on spatial filtering (i.e. beamforming).30 They are nevertheless not yet validated for clinical routine and belong more to the field of research. All these tools ease and optimize the detection of visual epileptiform discharges and may also be combined with automatic IED detection algorithms (e.g. hidden Markov modelling,52 kurtosis53), which reduce the time and the subjective part of visual detection.51, 53 These computational tools could, in the future, move into the realm of clinical routine to reduce the time spent analysing clinical MEG recordings.
CONCLUSION
MEG contributes to the characterization of RFE in children, as an additional non-invasive tool that gives non-redundant information about the spatio-temporal dynamics of IEDs. It also provides unprecedented insights about the neural mechanisms at play in several types of paediatric epilepsy. The emergence of on-scalp recordings resulting from new cryogenic-free sensors is promising for the future of MEG. From a clinical point of view, on-scalp MEG might become a routine diagnostic evaluation of paediatric epilepsy because of its easiness for patients, its lifespan compliance, its tolerance to movements, and its increased SNR. From an experimental point of view, on-scalp MEG allows investigation of brain mechanisms with a high sensitivity and precision, and it paves the way towards longitudinal studies across the whole lifespan. Overall, on-scalp OPM-MEG might indeed represent the future of evaluation in paediatric epilepsy.
FUNDING INFORMATION
OF is supported by a research grant from the Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture (FRIA, Fonds de la Recherche Scientifique (FRS-FNRS), Brussels, Belgium). XDT is Clinical Researcher at the FRS-FNRS. The cryogenic MEG and on-scalp OPM-MEG projects at the CUB Hôpital Erasme and Hôpital Universitaire de Bruxelles are financially supported by the Fonds Erasme (Brussels, Belgium; research conventions ‘Les voies du savoir’ and ‘Les voies du savoir II’, clinical research project) and by the FRS-FNRS (research credit: J.0043.20F, equipment credit: U.N013.21F).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




