Clinical and genetic spectrum of hereditary spastic paraplegia in Chinese children
*Members of the Paediatric Neurology Study Group are listed in the Acknowledgements
This original article is commented on by Blackstone on pages 307–308 of this issue.
Abstract
Aim
To explore the clinical and genetic spectrum of hereditary spastic paraplegia (HSP) in Chinese children.
Method
This retrospective study was conducted between January 2014 and October 2021 in children clinically diagnosed with either pure HSP (pHSP) or complex HSP (cHSP).
Results
We investigated 45 children (32 males, 13 females; mean age [SD] at symptom onset 4 years [7 months]). clinically diagnosed with HSP and identified genetic causes in 35 patients. Most patients with autosomal dominant HSP had pHSP (16/18), whereas most patients with autosomal recessive HSP tended to have cHSP (14/16). SPG11 was the most common autosomal recessive subtype, followed by FA2H/SPG35, whereas SPAST/SPG4 was the most frequent cause of autosomal dominant HSP. Two patients with CPT1C mutations presented with a complex phenotype. Meanwhile, 10 patients were found to have likely pathogenic variants/variants of uncertain clinical significance in six genes related to HSP.
Interpretation
SPG11 and SPG4 were the most frequent subtypes in Chinese children with autosomal recessive HSP and autosomal dominant HSP. However, the prevalence of SPG4 was much lower than that in adults, which might be explained by the late onset of the disease. On the other hand, FA2H/SPG35 was common in our cohort, while it contributed to only a small proportion of adult cases, which might be explained by its rapid progression and early death in some patients. We also expanded the genetic and clinical spectra of SPG73.
Graphical Abstract
Chinese translation of this Original Article is available in the online issue.
Abbreviations
-
- ACMG
-
- American College of Medical Genetics and Genomics
-
- cHSP
-
- complex hereditary spastic paraplegia
-
- HSP
-
- hereditary spastic paraplegia
-
- pHSP
-
- pure hereditary spastic paraplegia
What this paper adds
- SPG11 and SPG4 were respectively the most frequent subtypes in autosomal recessive and autosomal dominant hereditary spastic paraplegia.
- The prevalence of SPG4 in children was much lower than in adults.
- FA2H/SPG35 was more common in paediatric series than adults.
- Genetic heterogeneity exists among different ethnicities and different age groups.
Hereditary spastic paraplegias (HSPs) are a group of genetically and clinically diverse inherited neurological disorders affecting around 2 to 8 in 10 0000 individuals across different populations.1-3 They are characterized by progressive lower limb weakness and muscle stiffness (spasticity) due to pyramidal tract dysfunction. This lower limb weakness and spasticity can be associated with other neurological or non-neurological symptoms (complex forms, cHSP) or in relative isolation (pure forms, pHSP).4 To date, more than 80 genetic subtypes of HSPs have been identified. The age at onset is spread across a wide range, from infancy to old age; however, HSP is usually reported in adults, and paediatric-onset forms have rarely been investigated in isolation. Here, we report our retrospective experience in diagnosing paediatric-onset HSP through clinical, laboratory, neuroimaging, and genetic testing results.
METHOD
Participants
A total of 45 individuals were recruited from the Neurological Department of Beijing Children's Hospital and the Child Neurology Unit of Peking University First Hospital between January 2014 to October 2021. Selected patients were clinically suggestively diagnosed with HSP, including children with developmental delay, gait disorders, and motor disabilities, with signs of pyramidal tract involvement. The patient data were acquired from the FUTang Updating Medical Records Database.5 Each individual underwent a detailed neurological examination by an experienced neuropaediatric specialist. Acquired spasticity and other genetic disorders were ruled out in all individuals. Exclusion criteria included: (1) children with static encephalopathies (cerebral palsy) related to pre- or perinatal complications; (2) those with confirmed diagnosis of other diseases affecting the grey/white matter of the brain (e.g. gangliosidoses, leukodystrophies); and (3) the presence of basal ganglia disorders, hereditary neuropathies, vitamin B12 deficiencies, spinal cord trauma, or demyelinating diseases.
The study protocol was approved by the Ethics Committees of Beijing Children's Hospital and Peking University First Hospital. Written informed consent for routine and investigative studies was obtained from the parents of all studied patients and families. Clinical data, including the age at onset, clinical manifestations, magnetic resonance imaging (MRI) findings, electromyography (EMG) laboratory examination, family history, treatment, outcomes, and genetic results, were collected.
Molecular analysis
Genomic DNA was extracted from the peripheral blood leukocytes of the patients and their parents according to a standard protocol. A minimum of 3 μg of DNA was used to create indexed Illumina libraries according to the manufacturer's protocol (MyGenostics, Beijing, China). The amplified DNA library was captured using a nuclear gene enrichment kit. The enrichment libraries were sequenced on Illumina HiSeq 2000 platform (Illumina, San Diego, CA, USA) with 150-bp paired-end reads.
Raw reads were aligned to UCSC hg19 and the mitochondria genome (NC_012920) with BWA software (Microsoft, Redmond, WA, USA). Aligned reads were processed using SAM tools (Microsoft, Redmond, WA, USA) and Picard (Broad Institute, Cambridge, MA, USA), following the best practice guidelines of the Genome Analysis Tool kit (Broad Institute, Cambridge, MA, USA). Single-nucleotide variants and small insertion–deletion were detected using the Genome Analysis Toolkit Haplotype Caller (Broad Institute, Cambridge, MA, USA). The variants were annotated using ANNOVAR (http://annovar.openbioinformatics.org/en/latest/). Common sites with population allele frequencies above 5% according to the dbSNP 138, 1000 Genome Project, esp6500si, and ExAC databases were excluded. The pathogenicity of the variants was interpreted according to the 2015 American College of Medical Genetics and Genomics (ACMG) standards and guidelines.6
RESULTS
Clinical profile
A total of 45 individuals (32 males, 13 females) were enrolled in our study. Only one patient had a consanguineous background (ID16; the parents were first cousins). Positive family history was recorded in three cases. pHSP and cHSP were diagnosed in 26 and 19 cases respectively. Symptoms occurred during the paediatric period in all cases. The mean age (SD) at symptom onset was 4 years (7 months). Approximately two-thirds (26/45) of the patients experienced symptoms initiation during early childhood (1–3 years).
Progressive spastic gait, weakness of the lower limbs, and pyramidal signs were observed in all cases. The upper limbs were involved in five patients at last follow-up. Twelve patients had a history of motor developmental delay before symptom onset. Pes cavus was observed in seven patients, and urinary dysfunction was found in two patients. In patients with cHSP, associated symptoms consisted of cognitive impairment/regression (n = 10), ataxia (n = 4), ocular impairment (n = 3), hearing impairment (n = 1), and seizures (n = 1).
Brain MRI was performed on 39 patients, 28 of whom had normal MRI. Dysplasia of the corpus callosum was observed in eight patients, seven of whom presented with cHSP. Other anomalies included abnormal bilateral paraventricular signals and enlargement of the lateral ventricles (Figure 1). Ten patients received EMG, and four displayed neurogenic injuries.
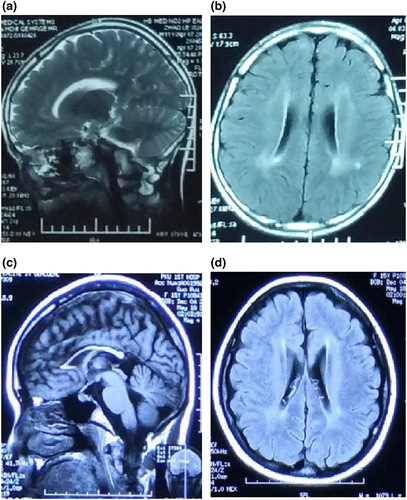
The time between onset and last follow-up ranged from several months to 14 years 6 months, and was at a mean age (SD) of 3 years 5 months (4 months). The level of motility at the last follow-up for the entire cohort was as follows: 36 patients could walk independently or with assistance with abnormal gaits, six were wheelchair bound, one could not raise their head, two patients were lost to follow-up, and the upper limbs were affected in five patients.
The clinical manifestations of the 45 patients are summarized in Table 1, and detailed clinical information is presented in Table S1.
| Characteristics | n | % |
|---|---|---|
| Sex | ||
| Male | 32 | 71.1 |
| Female | 13 | 28.9 |
| Onset age (years) | ||
| <1 | 3 | 6.7 |
| 1–3 | 26 | 57.8 |
| 3–7 | 5 | 11.1 |
| 7–12 | 9 | 20.0 |
| >12 | 2 | 4.4 |
| Type | ||
| Pure form | 26 | 57.8 |
| Complicated form | 19 | 42.2 |
| Manifestations | ||
| Spastic gait/weakness of lower limbs | 45 | 100.0 |
| Pyramidal signs | 45 | 100.0 |
| Motor development delay before onset | 12 | 26.7 |
| Weakness of upper limbs | 5 | 11.1 |
| Pes cavus | 7 | 15.6 |
| Urinary dysfunction | 2 | 4.4 |
| MRI anomalies (n = 39) | 11 | 28.2 |
| Complications in cHSP (n = 19) | ||
| Cognition impairment/regression | 10 | 52.6 |
| Ataxia | 4 | 21.1 |
| Ocular impairment | 3 | 15.8 |
| Hearing loss | 1 | 5.3 |
| Seizures | 1 | 5.3 |
- Abbreviations: cHSP, complicated hereditary spastic paraplegia; HSP, hereditary spastic paraplegia; MRI, magnetic resonance imaging.
Molecular spectrum
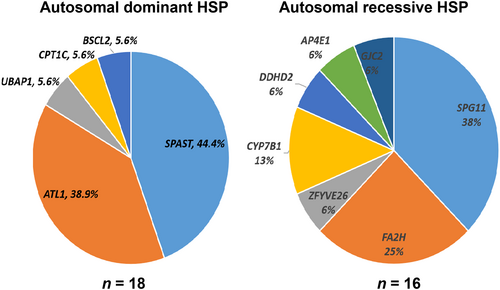
A total of 54 variants were identified in 14 genes. According to the 2015 ACMG standards and guidelines, 47 genetic variants were pathogenic in 35 patients, of which 25 were novel and 22 were previously reported. The genetic diagnosis was made in 35 patients in the whole cohort: 16 in the cHSP group and 19 in the pHSP group. Of the 35 patients, 18 patients had autosomal dominant HSP, 16 had autosomal recessive HSP, and one had X-linked dominant HSP. Variants that were likely pathogenic or variants of uncertain clinical significance were found in 10 patients (Table S2).
Clinical spectrum of HSP caused by different genes
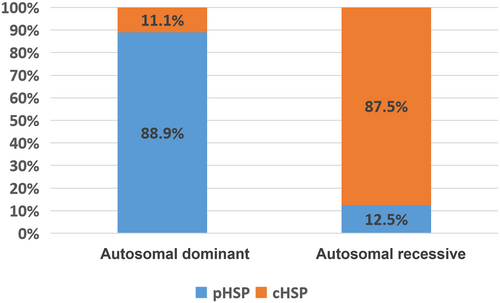
Five genes (ATL1, SPAST, BSCL2, CPT1C, UBAP1) were associated with autosomal dominant HSP forms; seven genes (SPG11, FA2H, CYP7B1, AP4E1, GJC2, ZFYVE26, DDHD2) were associated with autosomal recessive HSP forms (Figure 2); and one gene (PLP1) was associated with X-linked dominant HSP forms. Of the 18 patients with autosomal dominant-inherited mutations 16 presented with pHSP, whereas 14 of the 16 patients with autosomal recessive-inherited mutations presented with cHSP (Figure 3). We describe them in the following sections.
Autosomal recessive HSP
SPG11
The most common causative gene related to autosomal recessive HSP in our cohort was SPG11, identified in six patients. All cases presented with cHSP and had a very mild clinical course. Psychomotor development before disease onset was typical in all patients. The symptoms appeared relatively late, usually in the second decade of life (13–18 years), and presented with lower limb spasticity. Four patients showed varying degrees of cognitive impairment, two had ataxia, and the upper limbs were affected in only one patient. Dysplasia of the corpus callosum on MRI was seen in four out of six patients.
FA2H/SPG35
Four patients were identified with FA2H mutations in compound heterozygous condition. They presented with complex forms of HSP. Early psychomotor development was uneventful in these patients. Spastic gait and weakness of the lower limb occurred between the ages of 3 years 6 months and 10 years. There was relatively rapid progression from disease onset. Three patients presented with dysarthria and cognitive regression. Two of them gradually lost their walking ability and developed upper limb spasticity. Ataxia was observed in two cases. Scoliosis and bladder dysfunction were recorded separately for a single patient.
CYP7B1/SPG5A
Two sporadic patients (ID30 and ID31) with pHSP harboured homozygous mutations in CYP7B1. Slow progressed lower limb spasticity started at approximately 2 years of age in both patients.
AP4E1/SPG51
Compound heterozygous mutations of AP4E1 were found in a male (ID21) who presented with global developmental delay since birth. The neurological examination revealed spastic paraplegia of the lower limbs. At the time of the last follow-up, the 2-year-old male still could not walk with assistance or speak.
GJC2/SPG44
One patient (ID11) had GJC2 compound heterozygous mutations. This 6-year-old female showed mild cognitive delay and spastic gait during the second year of life, also had amblyopia and nystagmus. Therefore, a diagnosis of cHSP was made. Neuroimaging revealed delayed myelination. EMG revealed neurogenic injury.
ZFYVE26/SPG15
One patient (ID22) had ZFYVE26/SPG15 compound heterozygous mutations and slightly delayed early development. Lower limb hyperreflexia, spasticity, and weakness were noticed since the patient could walk at 15 months of age. Cognitive decline and bladder dysfunction was also present. Ocular involvement was not observed. Brain MRI showed dysplasia of the corpus callosum.
DDHD2/SPG54
One 7-year-old male (ID38) was found to have compound heterozygous mutations in the DDHD2 gene. Since birth, the patient showed profound delay in gross motor function and cognition. Lower limb spasticity was noticed at 2 years of age, and at that time the patient could stand with assistance. There was no significant improvement or regression in the following years. The patient remained wheelchair bound with poor fine motor ability of the upper limbs. Brain MRI revealed delayed myelination.
Autosomal dominant HSP
SPAST/SPG4
Mutations of the SPAST gene were found in eight patients. Five arose de novo and three were inherited from their parents, of whom two are asymptomatic. All patients presented with pHSP.
ATL1/SPG3A
Seven patients had pathogenic changes in the ATL1 gene. Five arose de novo, whereas the other two were inherited from the affected parent. Six patients had a pure phenotype, and one showed cHSP. Two affected parents (ID10 and ID17) showed isolated lower limb weakness since childhood, without significant progress during their life.
BSCL2/SPG17
A male (ID5) with BSCL2/SPG17 heterozygous mutation presented with gait abnormalities and lower limb weakness at about 5 years 6 months old. The upper limbs were unaffected, and neuroimaging findings were unremarkable.
CPT1C/SPG73
Motor developmental milestones were severely delayed in this patient (ID26) with CPT1C/SPG73 heterozygous mutation, but cognition was basically typical. At 1 year old, the patient still could not raise their head during last follow-up. Hypertonia and spasticity of the limbs were also evident. The patient carried a reported mutation in the CPT1C gene, inherited from their father; however, the father was asymptomatic.
UBAP1/SPG80
A patient (ID15) presented with pHSP. The patient carried a de novo frameshift mutation in the UBAP1 gene, which was predicted to be pathogenic according to the ACMG guidelines.
X-linked dominant HSP
PLP1/SPG2
A male (ID34) with PLP1/SPG2 mutation showed typical development in the first year of life. The patient's inability to walk unassisted at 2 years of age and the presence of hypertonia in his lower limbs drew attention to his condition. Motor development progressed slowly over the following years. When 5 years old, the patient remained wheelchair bound with poor fine motor ability of the upper limbs at the time of last follow-up.
Genetic variants likely pathogenic and variants of uncertain clinical significance
Eleven variants in six genes (SPAST, BSCL2, UBAP1, ATL1, CPT1C, REEP1) could not be defined as pathogenic according to the ACMG guidelines, including six as likely pathogenic, and five as variants of uncertain clinical significance. All six genes are known to be associated with HSP.
Among the 10 patients, seven presented with pHSP, of whom four had variants in SPAST, and the other three had variants in UBAP1, BSCL2, and REEP1. Three patients presented with cHSP. Two patients carried variants in BSCL2 and CPT1C. One patient had both variants in the ATL1 and SPAST genes.
DISCUSSION
We describe a retrospective cohort of 45 Chinese children with clinical diagnosis of HSP. This is the first study focusing on a Chinese paediatric cohort with HSP. Overall, pHSP seemed to have a higher prevalence (57.8%) than cHSP, which is close to previous studies focusing on paediatric HSP.7, 8 The peak age at onset in our cohort was 1 to 3 years. In the cHSP cohort, cognitive impairment was the most frequent clinical finding, similar to other series.7-9 The brain neuroimaging findings were normal in most patients. Dysplasia of the corpus callosum was common in patients with cHSP.
We identified the genetic cause in 35 patients. A large set of mutations detected in this study were novel, which further corroborated the high genetic heterogeneity of the HSP population. Regarding the inheritance patterns, a greater number of patients presented with pHSP in autosomal dominant forms, whereas it is on the contrary in autosomal recessive forms (Figure 3), consistent with previous studies.10
SPG11 was the most frequent cause of autosomal recessive HSP in our cohort, which is in line with several studies on adult HSP.10-12 The age at onset is usually in the second decade of life, most frequently presenting with walking problems due to progressive lower limb spasticity. Notably, two out of three patients with SPG11-related HSP displayed dysplasia of the corpus callosum on MRI. According to Dong et al.’s study in the adult HSP cohort, more than 30 mutations have been described in Chinese patients with SPG11-related HSP so far.10 In this study, we contributed eight novel mutations. However, there was no evident clustering of these mutations in the functional domain of the spatacsin protein, encoded by the SPG11 gene.
FA2H/SPG35 is more common than other paediatric series and was the secondary cause of autosomal recessive HSP in our cohort.7, 13 This finding highlights the heterogeneity of the genetic spectrum among different ethnic backgrounds. Autosomal recessive mutations in FA2H have been associated with several clinically defined disease entities with overlapping phenotypic spectra, including leukodystrophy with spasticity and dystonia, fatty acid hydroxylase-associated neurodegeneration, and HSP type SPG35. The disease manifests with early childhood onset of predominantly lower limb spastic tetraparesis. It is rapidly progressive with loss of ambulation and involvement of the upper extremities within 10 years after disease onset.14 Other common features include limb ataxia, dysarthria, anarthria, and dysphagia. Most patients in our study had cognitive deficits of varying degrees. Less common features included optic atrophy, rare seizures, and mild sensory involvement.14, 15 In our cohort, three out of four patients with FA2H mutations presented with dysarthria and cognitive regression. Ataxia has been described in two cases. Scoliosis and bladder dysfunction were recorded separately for a single patient. Common neuroimaging modalities include white matter changes, cerebellar atrophy, pons atrophy, and hypointensity of globus pallidia.14, 16 However, none of these changes were observed in our patients. An absence of MRI changes has also been reported in a small number of patients.17 FA2H/SPG35 accounted for a very small proportion, and was absent in adults.9-11 This might be explained by its rapid progression, with some patients dying at a young age.18, 19 This hypothesis needs further confirmation through research on the natural history of the disease.
Spastic paraplegia type 5 is caused by mutations in the CYP7B1 gene, and approximately half of the patients presented with complex forms.20 In our cohort, we found the same homozygous mutation (p.R112X) of CYP7B1 in two patients with pure forms, born from non-consanguineous parents. This mutation has been shown to be a founder mutation in the Chinese population.10
In Chinese adult-HSP, SPAST/SPG4 was the most frequent subtype (79%), and more than 90% of cases presented with the pure form.10 This percentage was similar in other adult studies of different ethnic populations.21-24 In our paediatric series, SPAST/SPG4 was the most common subtype (44%). However, its prevalence was much lower than that in the adult cohort. This proportion ranges from 27% to 50% in other paediatric cohorts.13, 25 The incidence of SPG4 in children is significantly lower than in adults. This might be explained by the fact that the onset of clinical signs in SPG4 is insidious and relatively late.26 The mean age at onset is 31 years 8 months, but cases have been reported with an onset age of up to 70 years.25 SPG4 is characterized by a clinically pure phenotype that is rarely associated with additional neurological signs. Consistent with this, all patients in our cohort presented with isolated lower limb spasticity. Both the age at onset and severity of the disorder are extremely variable, and such variability is both intra- and interfamilial and may suggest incomplete penetrance, with some patients carrying mutations remaining asymptomatic for their entire life.27 This phenomenon may explain why the two parents carrying the mutations in our study were asymptomatic.
The ATL1/SPG3A subtype ranked second in both our child-onset and adult cohort of Chinese patients with autosomal dominant HSP. However, the frequency (38.9%) was higher in the former than in the latter (11%).10 SPG3A is the most common subtype of paediatric-onset autosomal dominant HSP in Italian patients.7 According to a meta-analysis of 13 570 patients with HSP worldwide, Asian patients showed a lower frequency of mutations in the ATL1 gene in comparison to White patients with HSP.24 This reveals the variable genetic spectrum among different ethnicities as well as among different age groups.
Two CPT1C mutations were identified in our cohort. A novel mutation (p.P177Rfs*59) was found in a male presenting with cHSP (Tables S1 and S2; ID18), and the mutation was inherited from the patient's asymptomatic mother. According to the ACMG guidelines, the mutation was predicted to be likely pathogenic. Another CPT1C mutation (p.Q76X) was identified in a patient (ID26) with cHSP, which was inherited from their asymptomatic father. The same mutation has been described in two patients with pHSP.28 In vitro functional analysis confirmed its pathogenicity and messenger RNA decay was presumed responsible for this phenotype. Interestingly, the heterozygous mother in this study was asymptomatic as well. However, the mechanism underlying this phenomenon remain unclear. It is probable that a combination of unknown genetic and environmental factors were jointly involved in the genetic interaction network that sustains the disease phenotype. Before our study, there were only two reports on CPT1C/SPG73, and all patients presented with pHSP.28, 29 However, both patients in our study had complex forms. The clinical and genetic features, as well as the pathological mechanisms of this disease, are ambiguous. Our study expands the clinical and genetic spectrum.
Interestingly, a female (ID13) with pHSP had a missense variant (c.1349G > A, p.R450K) of SPAST, inherited from their asymptomatic father. A de novo missense ATL1 gene variant (c.482C > T, p.A161V) was also found. Both variants are predicated to be variants of uncertain clinical significance. Both genes may be involved in disease-causing processes. Further functional studies are required to confirm this hypothesis.
Mutations in other known HSP-related genes including AP4E1, DDHD2, GJC2, ZFYVE26, UBAP1, REEP1, PLP1, and BSCL2 were also found in a single case, confirming the wide genetic heterogeneity of paediatric-onset HSP.
It is worth noting that, in the autosomal dominant HSP group, some carrier parents were unaffected, including SPAST/SPG4, ATL1/SPG3A, and CPT1C/SPG73. These unaffected parents were in their late 20s or early 30s, which is relatively young. However, the onset age is quite variable (as late as 70s).25, 30 Hence, a long-term follow-up of these unaffected parents is necessary, as they might develop symptoms later.
Conclusion
In conclusion, this is the first description of paediatric HSP in a Chinese population which expands the clinical and genetic spectrum. Furthermore, our study revealed the different genetic landscapes of paediatric HSP from that of adults and highlighted the heterogeneity of HSP among different ethnicities.
ACKNOWLEDGMENTS
Members of the Paediatric Neurology Study Group are as follows: Jianbo Zhao and Hongmei Wang (Department of Neurology, Beijing Children's Hospital, Capital Medical University, National Centre for Children's Health, Beijing, China).
We thank the patients and their families who participated in this study.
The authors have stated that they had no interests which might be perceived as posing a conflict or bias.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.





