Visual field defects after vigabatrin treatment during infancy: retrospective population-based study
Abstract
Aim
To investigate the prevalence of vigabatrin-attributed visual field defect (VAVFD) in infantile spasms and the utility of optical coherence tomography (OCT) in detecting vigabatrin-related damage.
Method
We examined visual fields by Goldmann or Octopus perimetry and the thickness of peripapillary retinal nerve fiber layer (RNFL) with spectral-domain OCT at school age or adolescence.
Results
Out of 88 patients (38 females, mean age at study 15y, SD 4y 3mo, range 6y 4mo–23y 3mo [n=65] or deceased [n=21] or moved abroad [n=2]) exposed to vigabatrin in infancy, 28 were able to perform formal visual field testing. Two had visual field defect from structural causes. We found mild VAVFD in four patients and severe VAVFD in one patient. Median vigabatrin treatment duration for those with normal visual field was 11 months compared to 19 months for those with VAVFD (p=0.04). OCT showed concomitant attenuated RNFL in three children with VAVFD, and was normal in one. The temporal half of the peripapillary RNFL was significantly thinner in the VAVFD group compared to the normal visual field group.
Interpretation
The overall prevalence of VAVFD is lower after exposure in infancy compared to 52% which has been reported after exposure in adulthood. The risk increases with longer treatment duration. Further studies should identify infants particularly susceptible to VAVFD and clarify the role of OCT.
Abbreviations
-
- OCT
-
- Optical coherence tomography
-
- RNFL
-
- Retinal nerve fiber layer
-
- TSC
-
- Tuberous sclerosis complex
-
- VAVFD
-
- Vigabatrin-attributed visual field defect
What this paper adds
- One-third of former patients with infantile spasms were able to perform formal visual field testing.
- Vigabatrin-attributed visual field defect was less common after exposure during infancy compared to older age.
- The risk of visual field defect was associated with duration of treatment.
Infantile spasms (West syndrome) is a severe epileptic encephalopathy that requires prompt and effective treatment to ensure positive outcome in regard to both future epilepsy and cognitive development.1, 2 Vigabatrin remains one of the first-line treatments, in particular for infantile spasms caused by tuberous sclerosis complex (TSC),3 but also for other aetiologies either in combination or sequentially with hormonal treatment. The addition of vigabatrin to hormonal therapy may lead to more rapid cessation of infantile spasms than hormonal therapy alone.4 Long-term use of vigabatrin carries the risk of permanent visual field constriction. A systematic review estimated that the risk of vigabatrin-attributed visual field defect (VAVFD) is higher in adults (52%, 95% confidence interval [CI] 46–59) than in school-age children (34%, 95% CI 25–42).5 The risk was associated with larger cumulative dose and advancing age.
The first studies on 16 patients6 and 15 patients7 exposed to vigabatrin in infancy suggested that the risk of VAVFD may be much lower (6–7%) than in older age groups. A higher risk estimate (34%) was obtained by a study including 32 children from six different centres.8 The results of this study showed a trend that longer vigabatrin treatment increases the risk of VAVFD, although the marked variation in VAVFD between the centres (e.g. 83% in Moscow compared with 0% in Zurich and Montreal) cannot be totally explained by different exposure times.
Mild or even moderate VAVFD may remain clinically asymptomatic, which underlines the importance of formal visual field testing.9 These tests require good cooperation and therefore are challenging in young children. Repeated testing is often necessary to obtain trustworthy results. In addition, all reported studies should be viewed with the understanding that only a minority of children with a history of infantile spasms show sufficient cooperation skills later in life for formal visual field testing.10
The pathophysiology of vigabatrin-related retinal toxicity is not fully understood. VAVFD seems to exhibit a high degree of symmetry between the eyes. It is first detectable at the extreme temporal periphery and increases later towards the centre along all meridians.11 The standard method for detecting peripheral visual field defect is visual field testing by kinetic Goldmann or semi-automated kinetic Octopus perimetry both requiring good cooperation. Electroretinogram provides an electrophysiological indirect measure of the retinal function. Abnormal electroretinogram amplitudes are measured in up to 21% of infants and toddlers treated with vigabatrin,12, 13 but also in almost 19% of vigabatrin-naive patients with infantile spasms as shown in a recent study.14 Optical coherence tomography (OCT) offers a direct measure of the retinal nerve fibers and requires less cooperation than visual field examination. After successful fixation, spectral-domain OCT scans the optic nerve head within seconds. OCT has shown thinning of the peripapillary retinal nerve fiber layer (RNFL) in adults exposed to vigabatrin.15-17 OCT studies in children have been inconclusive.18, 19
For future optimal use of vigabatrin in infantile spasms, it is crucial to define the risk of VAVFD and to identify methods for assessing retinal toxicity as well as infants especially vulnerable to visual field defect. To this end, we studied a population-based cohort of patients with a history of infantile spasms to determine the presence of VAVFD, and to assess the usefulness of OCT in detecting vigabatrin-related retinal damage.
METHOD
Data collection
From a population-based cohort comprising all patients with infantile epilepsy from the Uusimaa region of Finland, we identified patients with infantile spasms with epilepsy onset during the first year of life in 1997 through 2008. Patients were diagnosed and treated at the Helsinki University Hospital where all patients with infantile spasms in this region are cared for.
Hospital charts provided data on the aetiology, seizure history, spasm duration, vigabatrin treatment details, and concomitant antiseizure medicines. We calculated the cumulative vigabatrin dose and treatment duration from the patient charts. We also collected details on epilepsy as well as social and developmental outcome. A semi-structured telephone interview with the patient or a parent provided added information for those without recent medical follow-up to determine if the vision and developmental level were sufficient for formal visual field testing (questions provided in Table S1, online supporting information).
Participants
The cohort included 90 patients with infantile spasms of whom 88 patients (38 females, mean age at study 15y, SD 4y 3mo, range 6y 4mo–23y 3mo [n=65] or deceased [n=21] or moved abroad [n=2]) received vigabatrin either as a monotherapy or in combination with adrenocorticotropic hormone and corticosteroids, or rarely with other antiseizure medicines. Only two patients had hormonal treatment alone. Aetiology was identified in 62 (69%) patients of the original cohort. For follow-up, we were able to contact 65 patients, of whom 36 (55%) had significant developmental delay often accompanied with cortical visual impairment making visual field testing impossible. We invited all 29 patients with a normal or near-normal cognitive level to formal visual field testing (Fig. S1, online supporting information). One patient declined; in a telephone interview with the parent, no symptoms of visual field constriction were reported in daily life.
Etiologies for infantile spasms in those examined were structural in seven, structural-genetic (TSC) in three patients, and unknown in the remainder (n=18). There were no infantile spasms relapses after tapering off vigabatrin. At the time of visual field evaluation, 19 patients were seizure free without antiseizure medicine and nine had ongoing antiseizure medicine treatment for focal epilepsy. Nine patients were included in an earlier report from our centre.6 For the present study, we retested five of them at age 16 to 20 years with Octopus perimetry and performed OCT (Fig. S1).
Visual field testing and peripapillary optic nerve evaluation
Visual field testing with Goldmann perimetry using V4e and I2e stimuli for six children took place in an experienced private ophthalmological laboratory, which earlier performed all visual field testing for our pediatric patients with epilepsy. One experienced optometrist examined the visual fields of the remaining 22 patients using Goldmann (stimuli III4e and I2e) or Octopus 900 (Haag-Streit Diagnostics, Koeniz, Switzerland) kinetic perimetry at the Eye and Ear Hospital of Helsinki University Hospital. Among these 22, we also performed spectral-domain OCT with SPECTRALIS (Heidelberg Engineering, Heidelberg, Germany), and a neuro-ophthalmologist (ML) examined these patients and evaluated all the visual field perimetries as well as the OCT results. The clinical evaluation consisted of visual acuity, eye pressure, pupillary reflexes, eye motility, slit lamp examination, and fundoscopic examination. We verified all abnormal findings by repeated visual field testing.
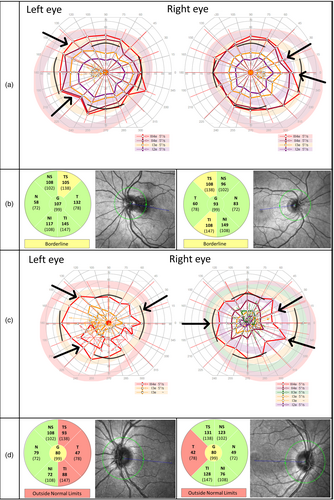
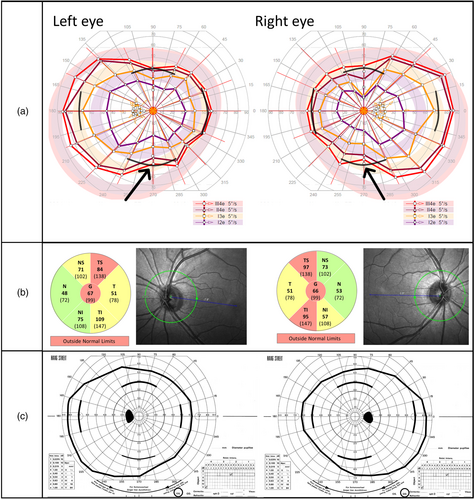
Vigabatrin-attributed visual field defect was defined as binocular concentric narrowing with meridian limits for normality as applied in former studies: normal field with temporal meridian extending over 70 degrees, nasal over 50 degrees, superior over 40 degrees, and inferior over 50 degrees. We classified visual field constriction mild if the temporal field was 50 to 70 degrees and severe if less than 50 degrees. Using OCT, we evaluated the retinal nerve fibers around the optic nerve: SPECTRALIS OCT provides automatically measured mean thickness of the peripapillary RNFL segmented into six sectors plus a global average score in the centre, and a build-in analysis compares the values to an age-adjusted adult reference database (Heidelberg Engineering, SPECTRALIS Product Family User Manual, Article no. 230131-006). We regarded values greater than the fifth centile as normal (green-coloured sectors in the pie charts in Figs 1 and 2), values between the first and fifth centile as borderline abnormal (yellow-coloured sectors in the pie charts in Figs 1 and 2), and values below the first centile as abnormal (red-coloured sectors in the pie charts in Figs 1 and 2). We also compared the values of those younger than 18 years to a previously reported normative pediatric cohort with 87 children aged 5 to 15 years20 as they and others have reported thicker peripapillary RNFL in children compared with adults. For the statistical analyses, mean value of both eyes for each sector of the RNFL was counted, except for the two patients with TSC for whom only one eye was analysed as both had a peripapillary hamartoma in the other eye distorting the values.
Statistical analysis and ethical approval
For group comparisons, we used non-parametric testing with two-tailed Mann–Whitney U and Fisher's exact test. Correlation was tested with Spearman and Kendall's Tau coefficient for the connection between vigabatrin treatment details, age, and peripapillary RNFL thickness. We computed the Wilson method for the 95% CI of the proportion of VAVFD. Significance was set at p<0.05. Because of multiple comparisons, we adjusted the p-values with the Benjamini-Hochberg procedure with a false discovery rate of 0.05 when investigating RNFL thickness in different sectors. Statistical analyses were performed with IBM, SPSS Statistics 25 software (IBM Corp., Armonk, NY, USA). This study was approved by the ethics committee at the Helsinki University Hospital and adhered to the tenets of the Declaration of Helsinki. We obtained written informed consent from the patients or the parents of those evaluated within the research project.
RESULTS
Participant and vigabatrin treatment characteristics
For the examined 28 patients (12 females), vigabatrin was initiated at a mean age of 6 months (range 2–13mo) with the treatment lasting on average 12 months (range 1–28mo). The mean cumulative dose reached 418g (range 21–1109g) and the mean highest daily dose amounted to 149mg/kg (range 100–200mg/kg). Visual field examinations took place at a mean age of 13 years 7 months (range 6y 5mo–20y 10mo). Two patients with structural visual field defect were excluded from further analyses. One had traumatic brain injury and the other had undergone occipital lobe resection for cortical dysplasia (focal cortical dysgenesia type IIA), both resulting in homonymous hemianopia.
Prevalence of VAVFD, comparison between groups with normal visual field and visual field defect
Of the remaining 26 patients, five (95% CI 9–38%) showed VAVFD, which was mild in four patients and severe in one. VAVFD was present in none of the six children treated for 6 months or less, in one of the seven children treated for 6 to 12 months, and in four of the 13 children treated for more than 1 year. Longer duration of treatment significantly increased the probability of VAVFD (p=0.04, Mann–Whitney U test; Table 1). Cumulative vigabatrin dose showed a similar trend but did not reach statistical significance (Table 1). The proportion of those with unknown aetiology was higher in the normal visual field group compared with the VAVFD group (p=0.02, Fisher's exact test; Table 1). In the unknown aetiology group (n=18), the median duration of vigabatrin therapy was 13 months (interquartile range [IQR] 6–18mo) and the median cumulative dose amounted to 338g (IQR 151–602g). In the identified aetiology group (n=8), the median duration was 14 months (IQR 9–24mo) and the median cumulative dose was 497g (IQR 310–860g). These group differences were not statistically significant.
| Normal visual field | VAVFD | |
|---|---|---|
| n=21 | n=5 | |
| Unknown etiology, n (%) | 17 (81)a | 1 (20)a |
| Age at vigabatrin onset | ||
| Mean (SD) [range], mo | 6.5 (1.8) [3.7–12.5] | 6.1 (1.9) [3.2–8.2] |
| Vigabatrin duration | ||
| Median | 10.9b | 19.0b |
| Mean (SD) [range], mo | 11.5 (6.7) [0.9–23.7] | 19.6 (7.1) [9.2–27.8] |
| Cumulative dose | ||
| Median | 295 | 569 |
| Mean (SD) [range], g | 399 (303) [21–1109] | 618 (193) [476–956] |
| Age at visual testing | ||
| Mean (SD) [range], y:mo | 13:7 (4:0) [6:5–20:10] | 14:8 (3:8) [10:6–18:0] |
- Comparison between the groups: aproportion of unknown etiology: p=0.02 (Fisher's exact test); bduration of vigabatrin: p=0.04 (Mann–Whitney U test). All other group comparisons were nonsignificant.
Among the patients with VAVFD (Table 2), TSC was the underlying aetiology for patients 1 and 2 (visual field of patient 1 shown in Fig. 1a). Structural aetiology was verified for patients 3 and 5 (visual field of patient 5 shown in Fig. 1c). Cause of epilepsy was unknown for patient 4 who relapsed at the age of 8 years with focal epilepsy after initial good response to infantile spasms treatment. Neither the individuals with VAVFD nor their parents reported visual symptoms indicative of visual field constriction in the telephone interviews. Based on the hospital charts, parents of patient 3 had observed the child being prone to bump into objects at age 3 years.
| Patient ID | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex | F | F | M | M | M |
| Etiology | TSC | TSC | FCD | Unknown | MCA infarction |
| Age at vigabatrin onset (mo) | 6.0 | 5.7 | 7.6 | 3.2 | 8.2 |
| Vigabatrin duration (mo) | 28 | 24 | 19 | 18 | 9 |
| Cumulative vigabatrin dose (g) | 956 | 518 | 572 | 569 | 476 |
| Other ASMs | – | OXC+ LEV | OXC+ LEV+ | OXC+ LEV+ VPA+ | ACTH VPA |
| Age at visual field testing (y) | 18 | 12 | 10 | 19 | 14 |
| Perimetry method | Octopus | Octopus and Goldmann | Goldmann | Octopus and Goldmann | Octopus and Goldmann |
| VAVFD severitya | Mild | Mild | Mild | Mild | Severe |
| Peripapillary RNFL | Borderline attenuated | Normal | ND | Attenuated | Attenuated |
- + = antiseizure medicine (ASM) treatment still ongoing at the time of visual field testing. aMild: temporal field 50–70 degrees, severe: temporal field <50 degrees. VAVFD, vigabatrin-attributed visual field defect; TSC, tuberous sclerosis complex; FCD, focal cortical dysgenesia; MCA, median cerebral artery; OXC, oxcarbazepine; LEV, levetiracetam; ACTH, adrenocorticotropic hormone; VPA, valproic acid; RNFL, retinal nerve fiber layer; ND, no data.
Retesting five of the previously reported patients changed the visual field result in one: Octopus perimetry showed mild constriction in inferior fields not seen in the earlier Goldmann fields (patient 4, both visual fields presented in Fig. 2a,c); the other four had normal fields in both perimetries.
Results of OCT
Attenuated peripapillary RNFL in one or more of the six sectors was observed in 6 of 20 patients. Among the patients with visual field defect (Table 2), patients 1, 4, and 5 showed concomitant thinning in the nerve fiber layers around the optic nerve head (OCT of patient 1 shown in Fig. 1b, of patient 4 in Fig. 2b, and of patient 5 in Fig. 1d). OCT was normal in patient 2. Patient 3 underwent OCT for clinical indications. Macula was normal, but for reasons unknown to us the RNFL was not examined. Three patients with normal visual fields showed thinning of the peripapillary RNFL. In two patients, the attenuation appeared because of myopic conus, and in one patient, the cause remained unidentified. Neither the duration of vigabatrin treatment nor the cumulative vigabatrin dose showed statistically significant correlation with the thickness of the different sectors or global average of the peripapillary RNFL. We found no correlation between age at examination and thickness of peripapillary RNFL. At group level, temporal, superior temporal, and inferior temporal sectors were significantly thinner in those with VAVFD compared to those with normal visual field (Mann–Whitney U test, Benjamini-Hochberg adjusted p=0.04 for all) (Fig. S2, online supporting information).
DISCUSSION
We present visual field results of adolescents with vigabatrin exposure during infancy obtained by a systematic follow-up of a population-based cohort with ocular examinations performed by experienced staff in a single centre. Almost three-quarters of the original cohort were screened, but only a third of the exposed were able to cooperate in formal visual field testing. We detected VAVFD in 19% of the examined participants, and only in those with treatment exceeding 6 months. VAVFDs were mild in four out of five patients. In three patients, the constriction was mild enough to allow driving a motor vehicle according to the regulations by the European Union. No subjective symptoms were noticed in daily life.
Our data support that VAVFD seems to be less common in children exposed during infancy compared to exposure later in life. The risk of visual field constriction may be low if vigabatrin treatment continues for a maximum of 6 months. Our findings affirm the previously observed tendency that the risk of VAVFD increases with longer vigabatrin exposure, rising to 31% in those whose vigabatrin treatment exceeded 12 months.
In addition to duration of therapy, the underlying cause or an interaction between these two may also affect the risk of developing VAVFD. Despite similar length of treatment, the proportion of VAVFD was markedly lower in the unknown aetiology group (1/18 with unknown aetiology vs 4/8 with defined aetiology displayed VAVFD). Given that the majority (64%) of patients who were able to cooperate in visual field testing had unknown aetiology, our results are best representative of the unknown aetiology group and allow only limited comparison between the different defined causes.
Concerning specific aetiologies, two of the three patients with TSC in this cohort exhibited VAVFD; all three had treatment periods around 2 years. We suggest that the duration of their treatment was an important factor, and the role of aetiology remains uncertain. On the other hand, for patients with TSC the advantages may justify the risk as adequate vigabatrin therapy often shows good efficacy against infantile spasms and diminishes the risk of relapse,21 and successful treatment of early epilepsy may improve developmental and long-term epilepsy outcome.22, 23
We aimed to assess the feasibility of OCT in detecting vigabatrin-related retinal damage. To our knowledge, this is the biggest reported group of patients with vigabatrin exposure in infancy or childhood examined with OCT, but still the small number of measurements limits the interpretation of our results. OCT showed attenuation in the nerve layers around the papilla in the majority of those with VAVFD (3/4), but also in three of 16 individuals with normal visual fields. In previous studies, vigabatrin-attributed thinning around the optic nerve head has mostly been located superiorly, inferiorly, or nasally15, 16, 18, 24 but sparing the temporal sector, whereas our group showed reduced thickness mostly temporally, as well as in the temporal side of the superior and inferior sectors. In a previous study with 19 children, thinning of the peripapillary RNFL was detected only in those with a cumulative dose exceeding 1500g which is higher than the highest cumulative dose in our group (1109g).18 Future studies should clarify the role of OCT in the follow-up of vigabatrin-related side effects in children.
Further research is also needed to study if there are risk factors, for instance certain aetiologies, which make patients especially vulnerable to vigabatrin adverse effects. Among our patients with VAVFD, the patient with the shortest exposure time (9mo) and severe visual field constriction had perinatal stroke as the underlying aetiology. Perhaps ischemic brain injury could cause retinal dysfunction predisposing the infant to vigabatrin induced harm. McFarlane et al.'s findings show some support for this: the highest prevalence of electroretinogram change (almost 25%) of over 300 vigabatrin-naive infants with infantile spasms was in the aetiology group with perinatal stroke, hypoxic-ischemic encephalopathy, intraventricular haemorrhage, and periventricular leukomalacia.14 Furthermore, experimental data from an animal model of neonatal hypoxic-ischemic encephalopathy revealed functional and structural impairment of the retina.25 As infantile spasms caused by stroke has shown good outcome in the latest trials with both hormonal and combination treatment, hormonal therapy may be considered the first choice and vigabatrin added only in treatment-resistant cases.26, 27
To prevent vigabatrin-related ocular adverse effects, we would need methods to detect the first signs of retinal damage already in infancy while the treatment is on-going. Electroretinogram can be performed on an infant, but it requires sedation, and very little evidence exists on the correlation of electroretinogram change with clinically relevant visual field defect.19 Another option investigated is handheld OCT suitable for infant imaging in supine position even without sedation,28 which showed preliminary feasibility to evaluate peripapillary RNFL in a small group of infants and young children exposed to vigabatrin.29 Improved OCT techniques might also help to examine individuals with insufficient cooperation for formal visual field testing. Future research is needed to investigate if any of these methods could be used to identify the infants who are most vulnerable for vigabatrin adverse effects.
In conclusion, our data aid the clinician in giving an estimation of the risk of VAVFD when commencing vigabatrin treatment for infantile spasms. Although the optimal duration of vigabatrin therapy is unknown, current infantile spasm treatment recommendations30 suggesting vigabatrin therapy for a maximum of 6 months may be relatively safe. As suboptimal treatment of infantile spasm carries severe developmental and medical consequences, choosing the treatment is always a matter of balancing the benefits against the risks. It is important to bear in mind that if vigabatrin is not effective, the treatment should be tapered off without undue delay.
Acknowledgements
We would like to thank Associate Professor Jaana Lähdetie for providing help with patient identification. The Foundation for Pediatric Research supported this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




