Treatments in Aicardi–Goutières syndrome
Abstract
Comprehensive reviews of the clinical characteristics and pathogenesis of Aicardi–Goutières syndrome (AGS), particularly its contextualization within a putative type I interferonopathy framework, already exist. However, recent reports of attempts at treatment suggest that an assessment of the field from a therapeutic perspective is warranted at this time. Here, we briefly summarize the neurological phenotypes associated with mutations in the seven genes so far associated with AGS, rehearse current knowledge of the pathology as it relates to possible treatment approaches, critically appraise the potential utility of therapies, and discuss the challenges in assessing clinical efficacy.
What this paper adds
- Progress in understanding AGS disease pathogenesis has led to the first attempts at targeted treatment.
- Further rational therapies are expected to become available in the short- to medium-term.
What this paper adds
- Progress in understanding AGS disease pathogenesis has led to the first attempts at targeted treatment.
- Further rational therapies are expected to become available in the short- to medium-term.
Abbreviations
-
- AGS
-
- Aicardi–Goutières syndrome
-
- RTI
-
- Reverse transcriptase inhibitor
What this paper adds
- Progress in understanding AGS disease pathogenesis has led to the first attempts at targeted treatment.
- Further rational therapies are expected to become available in the short- to medium-term.
Aicardi–Goutières syndrome (AGS) was originally defined as an early onset, progressive encephalopathy characterized by intracranial calcification, white matter disease, and cerebrospinal fluid lymphocytosis, suggestive of an inflammatory process.1 Over time, other features were recognized as consistent associations, most frequently chilblain-like skin lesions, glaucoma, hypothyroidism, and lupus-like disease. After the identification, in 2006, of mutations in the TREX1 (AGS1), RNASEH2A (AGS4), RNASEH2B (AGS2), and RNASEH2C (AGS3) genes as causative of AGS, it became clear that significant clinical differences could exist between patients, both within families and across genotypes, and that none of the core signs as originally delineated were necessary for the diagnosis. The subsequent description of disease-causative mutations in SAMHD1 (AGS5), ADAR1 (AGS6), and IFIH1 (AGS7) has further emphasized this point.2
The AGS-associated genes encode proteins involved in either the metabolism or sensing of nucleic acid (DNA; RNA), and mutations in these genes result in the induction of the antiviral cytokine type I interferon, which has been hypothesized, although not proven, to be directly relevant to pathogenesis. Thus, although the label AGS has continued clinical utility in designating a characteristic phenotype and, thereby, prompting relevant diagnostic investigations and informing prognosis, we suggest that the term ‘type I interferonopathy’ is appropriate for the wider spectrum of disease that results from dysfunction of these genes, implying, as it does, the possibility of a common ‘anti-interferon’ approach to therapy.3
Phenotypes Associated with Mutations in AGS1-7
We recently summarized the range of relatively stereotyped clinical scenarios due to mutations in the seven AGS-related genes that can present to the paediatric neurologist4 (Fig. 1). These include: (1) ‘classic’ AGS with prenatal or infantile onset, representing a remarkable mimic of trans-placentally acquired infection (‘pseudo-TORCH’) with irritability, feeding difficulties, microcephaly, abnormal movements, and epileptic seizures, as well as haematological disturbances such as thrombocytopenia, anaemia, and liver dysfunction, associated with white matter disease and intracranial calcification on neuroimaging; (2) Disease presenting beyond the first year of life, with either the subacute onset of profound neurological regression, otherwise similar to classic AGS, or with a more slowly progressive, variable combination of spasticity and dystonia associated with normal neuroimaging or the presence of non-specific white matter changes and/or intracranial calcification; (3) Dystonia and neuroimaging features characteristic of bilateral striatal necrosis, manifest at a few months of age or in later childhood, almost exclusively due to ADAR1 mutations (leading us to recommend that ADAR1-related disease be considered in any child presenting with bilateral striatal necrosis or otherwise unexplained subacute onset dystonia);5 (4) Patients with slowly progressive (‘non-syndromic’) spastic paraparesis confined to the lower limbs due to mutations in ADAR1, IFIH1, and RNASEH2B, some with completely normal spinal and cranial imaging;6 and (5) Intracerebral, large vessel disease including moyamoya and aneurysms with intracerebral haemorrhage and infarcts, representing a particular feature of SAMHD1-related disease.7 We note that some affected individuals have completely normal psychomotor development, perhaps only demonstrating chilblain like lesions of the skin before presentation of a cerebrovasculopathy.
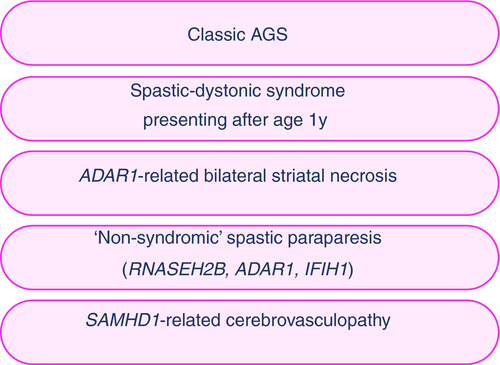
Such apparently distinct scenarios trigger different clinical trains of thought, investigative strategies, and prognostic considerations. At the same time, an overlap of core features, variably encompassing spasticity, dystonia, white matter disease, and intracranial calcification, sometimes with associated tell-tale non-neurological stigmata (e.g. chilblains, glaucoma, abnormal dentition, deforming arthropathy), can serve as important clues to the underlying diagnosis. In this review we emphasize the value of computed tomography over magnetic resonance imaging as a means of identifying cerebral calcification. Although susceptibility weighted and gradient echo magnetic resonance sequences are highly sensitive in detecting mineralization, subtle spot calcifications may be overlooked or thought to be blood vessels or microhaemorrhages. Computed tomography can easily resolve this.
Implications for Therapies Derived From Insights Into Pathogenesis
Most antiviral responses are mediated by cellular receptors that detect viral nucleic acid. Since the basic composition and structure of DNA and RNA are conserved across species, the existence of such receptors indicates a requirement for mechanisms to avoid the sensing of self-derived nucleic acids as viral. As mentioned above, all seven genes associated with an AGS phenotype are involved in the metabolism or sensing of nucleic acid, and mutations in any of these genes can result in enhanced type I interferon signalling, analogous to that after exposure to a virus; this may explain why the clinical features of AGS can resemble those of congenital infection. If AGS involves an abnormal response to self-derived nucleic acids, rather than an exogenous virus, the question arises as to the source of the endogenous nucleic acid which might trigger an inappropriate (‘antiviral’) response against self. Data derived through in vitro and animal experimentation suggest that the answer to this question varies according to genotype: self-derived DNA is relevant to disease consequent upon TREX1, RNase H2 complex, and SAMHD1 dysfunction; self-derived RNA drives interferon signalling in the context of ADAR1 and IFIH1 mutations.
Considering the likely inflammatory basis of AGS, a few reports have described the use of ‘broad spectrum’ immunosuppression in affected individuals.8, 9 Unfortunately, judging the efficacy of these interventions is difficult because of the small numbers involved, the different regimens employed, and the stage of the disease process at which treatment was started. We do not discount the possibility that such immunosuppression has a role in the treatment of AGS. However, if, as hypothesized above, self-derived nucleic acids cause pathology by inducing type I interferon, a more targeted approach to therapy might be envisaged through either: (1) limiting the production or enhancing the removal of putative self-nucleic acid stimuli; or (2) blocking signalling downstream of putative self-nucleic acid stimuli.
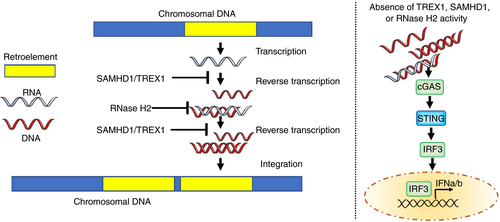
The conceptual distinction between self and non-self breaks down when considering endogenous retroviruses and retroelements. Retroelements are ‘remnants’ of ancient viruses that have become incorporated into the human genome, that now constitute up to 40% of our DNA. Such retroelements were first suggested as a driver of AGS in 2008,10 with recent papers adding weight to this hypothesis.11, 12 Salvaging of the Trex1 null mouse phenotype with combination reverse transcriptase inhibitors (RTIs) has been reported,13 premised on a reduction of immunostimulatory DNA derived through an essential (reverse transcription: RNA>DNA) step in the lifecycle of certain endogenous retroelements (Fig. 2). These results were not reproduced by another group,14 and the reason for this discrepancy is unclear. However, based on the former study,13 and supported by in vitro data generated using induced pluripotent stem cell technology,12 the results of the first clinical trial of RTIs in AGS have recently been published.15 In an open-label pilot study, patients with mutations in TREX1, RNASEH2A, RNASEH2B, or SAMHD1 were treated with a combination of three anti-human immunodeficiency virus-1 RTIs (abacavir, lamivudine, and zidovudine) over a period of 12 months, with interferon status assessed before, during, and after treatment. The results of this study indicated an effect of triple RTI therapy in reducing interferon signalling, with individuals harbouring mutations in components of the RNase H2 complex possibly being most responsive. This trial was not designed to capture meaningful clinical gains, as patients were already demonstrating significant neurological impairment at enrolment. However, increased cerebral blood flow, measured using arterial spin labelling sequences, indicated an effect of treatment on cerebral status. A follow-up study is now planned to better define the utility of RTIs in AGS and interrogate the mechanism by which such drugs might limit interferon signalling and neuroinflammation.
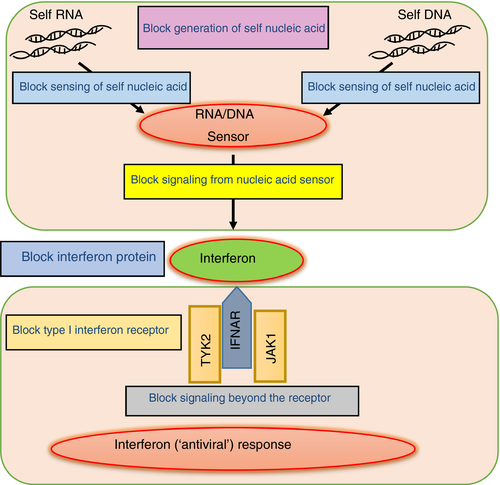
Anti-interferon antibodies, antibodies against the type I interferon receptor, and molecules targeting components of the pathways transducing a type I interferon response, represent examples of the strategy to block interferon signalling downstream of a nucleic acid stimulus (Fig. 3). Of note, a handful of recent reports suggest encouraging results with the use of Janus kinase (JAK) inhibition in several distinct type I interferonopathies,16, 17 with JAK1 comprising an essential component of the type I interferon receptor complex. We have seen definite improvement in TREX1-related skin disease18 and IFIH1-determined systemic inflammation19 with the JAK1/2 inhibitor, ruxolitinib. We also described a child with a gain-of-function mutation in IFIH1, apparently demonstrating significant developmental gains, against a background of previous regression and then stagnation, with ruxolitinib therapy.20 These early reports are important since they suggest that these drugs are addressing a relevant biological pathway. This may or may not relate to an inhibition of interferon signalling, given that they have effects beyond JAK1 inhibition at the type I interferon receptor. On the other hand, the number of patients treated so far is very small; no data derived in a clinical trial context are yet available and the effect of such drugs on the central nervous system disease is difficult to judge. Relating to this last point, in a child with biallelic mutations in RNASEH2B, we have seen the onset of disease at the age of 14 months, after completely typical development to that time, despite treatment with ruxolitinib from 4 months of age (unpublished data).
Other therapeutic approaches can also be envisaged, for example, compounds modulating components of cytosolic nucleic acid signalling pathways,21 with a recent paper describing a role for aspirin in the acetylation of cyclic GMP-AMP synthase and rescue of the Trex1-null mouse inflammatory phenotype after treatment with that drug.22 Other less developed areas of possible interest include the finding that cytosolic immunostimulatory nucleic acid in RNase H2 deficient mouse cells is removed by autophagy,23 perhaps implying that induction of autophagy by pharmacological mammalian target of rapamycin inhibition might be beneficial in this genotype.
The Potential Utility of Treatments
AGS is undoubtedly a severe disease; 19% of patients captured in the largest natural history study published to date24 had died, and 74% left profoundly disabled; therefore, treatments are urgently needed. We would not expect to reverse neurological damage already accrued at the time of treatment initiation, a fact of particular relevance for patients displaying the disease at birth. However, a possible window of therapeutic opportunity may be suggested the fact that close to 20% of patients in the natural history study mentioned above demonstrated apparently typical development until the time of symptom onset, with 8% of patients presenting after the age of 1 year. Thus, we consider that effective treatment administered in the earliest stages of disease might result in important clinical gains.
A question of interest here relates to whether AGS represents a progressive or static disease. In the abstract of their original 1984 report,1 Aicardi and Goutières described the phenotype that they observed as a ‘progressive disorder of the central nervous system with bilateral spasticity and dystonia, acquired microcephaly, and a rapid course toward profound deterioration and death’. Based on our own clinical experience and the testimony of many parents, in most cases the period of neurological damage appears to be confined to an initial encephalopathic phase of a few months, after which further disease progression is apparently unusual, with a number of patients demonstrating some gain of skills thereafter and stability into young adulthood (follow-up data is limited beyond that time). However, we are aware that some patients experience intermittent ‘decompensations’, and the identification of autosomal dominant genotypes, due to mutations in IFIH1 or the recurrent p.G1007R mutation in ADAR1 in particular, has been important in highlighting the possibility of progression (for an example see Video 1 at https://www.ed.ac.uk/centre-genomic-medicine/research-groups/crow-research-group). The risk of progression, together with situations where chilblains are a particular problem or where SAMHD1-related cerebrovascular disease exists, suggests that long-term treatment might be warranted.
Challenges in Assessing Therapeutic Efficacy
The difficulties of randomization and controlled studies in rare disorders with small populations apply to AGS, highlighting the need for the development of multicentre treatment networks and the collaborative involvement of families and patient support groups. Data from historical cohorts are important in this regard, so that a focus on natural history remains warranted. Radiological features and clinical indices are frequently difficult to measure quantitatively or objectively. Thus, the identification and characterization of biomarkers is of high priority. In terms of interferon status, such markers currently include a measure of interferon alpha protein using an ultra-sensitive enzyme-linked immunosorbent assay (digital ELISA) on a single molecule array platform,25 quantification of the expression of genes induced by interferon (so-called interferon stimulated genes),26, 27 and an assay of interferon activity based on antiviral cytopathic protection capacity. Evidence that interferon status might represent a reactive biomarker comes from the clinical trial of RTIs discussed above. In contrast, our experience of using ruxolitinib in patients with AGS has been that apparently quite significant clinical improvements can be observed in the face of little change in interferon signalling. Such might be explained by differential effects on local versus systemic interferon production, highlighting the potential importance of cerebrospinal fluid, rather than blood, monitoring. Not yet assessed in AGS, several groups have shown the potential utility of the aforementioned single molecule array digital ELISA technology to measure brain-specific proteins (e.g. glial fibrillary acidic protein, Tau, and neurofibromatosis type l) in cerebrospinal fluid and blood as markers of brain cellular health.28
Given the high rate of morbidity and mortality associated with mutations in AGS1-7, determining a positive effect of an effective therapy if large enough numbers of patients could be ascertained in the early stages of disease is probably possible. However, in view of the relative rarity of the disorder, well recognized, sometimes extreme, variation in disease expression between siblings is likely to present a major challenge to data interpretation in future trials. Indeed, even if sibling recurrences are well recognized, a hesitancy to test asymptomatic siblings for mutation status in childhood means that we do not actually know the true frequency of clinical non-penetrance (although the absence of homozygotes for recurrent mutations in TREX1 and RNASEH2B in control databases, such as the genome aggregation database [gnomAD], perhaps indicates that this is infrequent).
Conclusion
Progress in understanding the molecular and cellular pathogenesis of the type I inteferonopathies has informed recent novel approaches to the treatment of this devastating set of diseases. We believe that we are entering an exciting time in AGS clinical research, with molecules antagonizing cytosolic nucleic acid signalling pathways and the type I interferon receptor29 likely to become available in the short- to medium-term. Therapy will be of greatest benefit in the early stages of the disease, making rapid genetic diagnosis of the utmost importance. However, type I interferon signalling upregulation appears to be a life-long phenomenon, and the recognition of a progressive disease and later onset phenotypes in certain patients suggests that treatment might have beneficial effects at any age. Many unanswered questions remain; for example, whether blocking interferon signalling will prove effective for disease due to any genotype; or if therapies targeting the production and signalling of specific self-derived nucleic acid species, varying according to genotype, will be more appropriate. It might be that a combined strategy is necessary. The potential value of skin disease (present in up to 40% of patients with AGS), as an easy read-out of therapeutic efficacy should be taken advantage of. Challenges of note relate to treating brain disease, not least the need to achieve sufficiently high levels of drug activity in the central nervous system, difficulties in monitoring treatment efficacy, and the need to balance an effective interferon blockade with the risk of susceptibility to infection. We are unaware of any examples of bone marrow transplant or efforts at gene therapy as treatment strategies in AGS. Although both might be potentially interesting, the importance of accessing the central nervous system, current ignorance as to the precise brain cell types involved in the disease, and the absence of mouse models of AGS demonstrating a neurological phenotype are significant issues. This latter point highlights the potential value of induced pluripotent stem cell technology12, 30 in exploring the biological effects of different treatments, although it will only be through experimental medicine that the true value of different therapeutic approaches will become clear.
Acknowledgements
We sincerely thank colleagues and patients for helping to inform the arguments developed here, and we apologise for the omission of important publications not cited. The authors have stated that they had no interests that might be perceived as posing a conflict or bias.




