Mortality in children with cerebral palsy in rural Bangladesh: a population-based surveillance study
Abstract
Aim
To determine the mortality rate, immediate cause of death (CoD), and predictors of death in children with cerebral palsy (CP) in rural Bangladesh.
Method
We carried out a prospective population-based surveillance study of children with CP aged 0 to 18 years registered with the Bangladesh Cerebral Palsy Register (BCPR) between January 2015 and December 2016, with subsequent follow-up until December 2017. Verbal autopsy was applied to assign immediate CoD. Crude mortality rates, hazard ratios of death, and survival probabilities were estimated.
Results
Twenty-nine of the 678 children in the BCPR died during the study period, resulting in a crude mortality rate of 19.5 per 1000 person-years of observation (total follow-up duration 1486.8 person-years; mean 2y [standard deviation 6mo]). The leading immediate CoD was meningitis (n=9) and pneumonia (n=8). Survival probability and hazard ratio of death was significantly associated with age, Gross Motor Functional Classification System level, and associated impairments. Severe underweight and/or severe stunting was significantly overrepresented among deceased children than others in the cohort (p<0.05) when compared with the World Health Organization reference population.
Interpretation
The majority of deaths were due to potentially preventable causes. The life expectancy of these children could have been extended by ensuring primary healthcare and nutritional supplementation.
What this paper adds
- Mortality rate in children with cerebral palsy (CP) in rural Bangladesh is 19.5 per 1000 person-years.
- The majority of children with CP died from potentially preventable and treatable conditions.
- Motor severity, associated impairments, and malnutrition make children with CP vulnerable to premature death in rural Bangladesh.
What this paper adds
- Mortality rate in children with cerebral palsy (CP) in rural Bangladesh is 19.5 per 1000 person-years.
- The majority of children with CP died from potentially preventable and treatable conditions.
- Motor severity, associated impairments, and malnutrition make children with CP vulnerable to premature death in rural Bangladesh.
Abbreviations
-
- BCPR
-
- Bangladesh Cerebral Palsy Register
-
- CoD
-
- Cause of death
-
- HIC
-
- High-income country
-
- LMIC
-
- Low- and middle-income country
Cerebral palsy (CP) is a group of non-progressive disorders that affect movement and/or posture, and are due to an insult or injury of the developing brain.1, 2 CP is an important global public health issue and has an impact on health outcome, quality of life, and life expectancy.3-8 Globally, the estimated prevalence of CP is 2.1 per 1000 live births.9 However, the burden is higher in low- and middle-income countries (LMICs).10, 11 A recent study from Bangladesh found that the prevalence of CP in rural communities could be as high as 3.4 (95% confidence interval [CI] 3.2–3.7) per 1000 live births.11
The majority of studies on mortality and causes of death in children with CP are from high-income countries (HICs).5-8, 12, 13 It is documented that childhood CP mortality is higher than in the general child population; however, in HICs the majority of children with CP survive to adulthood.5-8 Research from HICs has shown that children with more severe motor types of CP are at greater risk of premature death.5-8 Studies also showed that children with CP who have associated impairments (i.e. intellectual, epilepsy, vision, hearing, speech), respiratory infections, and other chronic diseases are at increased risk of mortality.12-14 There is a lack of population-based data on CP mortality in children from LMICs. Currently, there are only few studies from LMICs conducted among highly selective institutional samples (e.g. hospital, rehabilitation centre attendees).14, 15
In HICs the availability of administrative health databases, including registers of birth, deaths, and CP registers, play a major role in generating such evidence, as well as precisely identifying the cause of death (CoD) and risk factors for mortality in children with CP.7, 8, 12, 13 In contrast, many low-income settings lack these provisions (e.g. death registers). In the absence of such systems, in LMICs, verbal autopsies are commonly used to ascertain the underlying and immediate CoD among specific populations of both children and adults.16, 17 However, population level mortality and/or verbal autopsy data are not routinely available for children with CP in LMICs such as Bangladesh.
Determining CoD is essential to identify preventable and treatable risk factors and to implement strategies to improve survival among children with CP. Although several studies have reported common causes of mortality among children with CP, the existing evidence almost exclusively represents the HICs. In this study, we aimed to estimate the mortality rate, immediate CoD, and determine the predictors of death in children with CP using a population-based register of children with CP in rural Bangladesh.
Method
This study used data from the Bangladesh Cerebral Palsy Register (BCPR); the first population-based CP register operating in a LMIC to date.11 The BCPR was established in 2015 in Shahjadpur, a northern sub-district of the Rajshahi division in Bangladesh. The BCPR surveillance area covers 324km2, incorporating 296 villages, 123 576 households, and 561 076 people (of whom about 232 037 are children aged <18y).18 Considering the sociodemographic characteristics (i.e. type of house, drinking water source, sanitation practices), household size, and population characteristics (i.e. age distribution and sex ratio), Shahjadpur is an excellent representation of rural Bangladesh.18
Study participants and data collection
The study population were children aged 0 to 18 years with a clinical diagnosis of CP who had been registered in the BCPR between 18th January 2015 and 28th December 2016. Children were identified from the community using the key informant's method.19 Diagnosis of CP was then confirmed by a multidisciplinary assessment team, which included a paediatrician and a physiotherapist. Children with confirmed CP were registered into the BCPR following the definition of CP used in the Surveillance of CP in Europe2 and Australian CP Register.20 The definition includes five key elements: ‘Cerebral Palsy: (1) is an umbrella term for a group of disorders; (2) is a condition that is permanent but not unchanging; (3) involves a disorder of movement and/or posture and of motor function; (4) is due to a non-progressive interference, lesion, or abnormality; and (5) the interference, lesion, or abnormality originates in the immature brain’.21
Once registered in the BCPR, follow-up (by telephone and/or home visit) was carried out prospectively every 6 months until 31st December 2017. Any death reported during this period was recorded. Verbal autopsies were started only after receiving informed written consent from the caregivers of the deceased children. One trained physician conducted the verbal autopsies by interviewing the primary caregivers (present before death) of the deceased children with CP at their home. To record the verbal autopsy, we adopted the standard age-specific, semi-structured form, developed, and validated by the Registrar General Office–Centre for Global Health Research team in their prospective study of ‘One Million Deaths in India’.22 The verbal autopsy form included detailed address and background characteristics of the respondents and their households, the child's age at death, specific sections on signs, symptoms, injury, and any treatment that the deceased child had received before death, and an open-ended section for additional narrative information in the end.
In addition to the verbal autopsy data, information from the BCPR (i.e. sociodemographic factors, birth details, anthropometric measurements, and clinical details) were used to analyse the factors contributing to the death of children with CP in our cohort. Assessment of motor severity (i.e. Gross Motor Function Classification System [GMFCS], Manual Ability Classification System) was carried out following standard guidelines during multidisciplinary assessment before BCPR registration.23, 24 The nature and typology of motor abnormalities (henceforth referred to as ‘predominant type of CP’) was assessed following the classification used in the Surveillance of CP in Europe and Australian CP Register.2, 20 In addition to three prominent type (i.e. spastic, dyskinetic, and ataxic), children presenting with diminished muscle tone in the absence of any other signs of motor impairment were categorized as ‘hypotonic CP’.20 Parent-reported feeding difficulties (i.e. difficulties in swallowing foods of regular texture) and presence of associated impairments (i.e. epilepsy, intellectual, visual, hearing, and speech) were also recorded as part of the BCPR registration based on detailed clinical history, available medical records, and clinical assessment by the medical assessment team. The working definitions of the associated impairments have been discussed previously.11, 21
Assigning immediate CoD
Two trained physicians reviewed each of the verbal autopsy forms and coded them independently to assign immediate CoD following the International Classification of Diseases-10 guidelines. Immediate CoDs were determined when both physicians assigned similar codes. Where multiple immediate CoDs could be determined, we used the term ‘primary’ and ‘secondary’ CoD. In the event of any disagreement a third expert physician reviewed the verbal autopsy forms anonymously and assigned the immediate CoD.
Statistical analysis
Descriptive and bivariate analyses were used to describe baseline characteristics, immediate CoD, and factors contributing to the death of children with CP in our cohort. The nutritional status of children was assessed at the time of recruitment following World Health Organization guidelines, using World Health Organization Anthro and World Health Organization AnthroPlus software.
The crude mortality rate per 1000 person-years of observation was calculated by dividing the total number of deaths by the total person-years at risk (sum of the person-years contributed by each of the children) during the follow-up period.
We assessed the time-to-event of the children with CP using Kaplan–Meier analysis. The children who were lost to follow-up before the last follow-up date of the study, were excluded from analyses. Cox proportional hazards regression was carried out to identify factors associated with the event of death in children with CP in our cohort. Factors that were found to be significantly associated in an unadjusted Cox proportional hazard regression model were included in an adjusted model following the forward-selection procedure. Statistical analyses were performed using SPSS Statistics software version 23 (IBM, Armonk, NY, USA).
Ethical considerations
Ethical approval was obtained from the Cerebral Palsy Alliance Human Research Ethics Committee (Ref no. 2015-03-02) in Australia, the Asian Institute of Disability and Development Human Research Ethics Committee (southasia-irb-2014-l-01), and the Bangladesh Medical Research Council Human Research Ethics Committee (BMRC/NREC/2013-2016/1267) in the Bangladesh. Informed written consent (after explaining the objectives of the study, methods, confidential handling of personal information, voluntary nature of participation, and rights of withdrawal) was given by all respondents. Verbal autopsies were conducted carefully by a trained physician, showing all respect and preference to the respondent's time, duration, and place of interview, with emotional support and condolences. Our project staff and a trained psychologist were involved throughout the process to mitigate any psychological distress. Moreover, as an ongoing population-based surveillance, we had regular contact with the family of the deceased.
Results
Between 18th January 2015 and 28th December 2016, 726 children with CP were registered in the BCPR. As of December 2017 (median follow-up duration 2y, interquartile range 11mo), we were able to follow-up 680 children (93.7% follow-up rate) of whom 31 had died. The primary caregivers of 29 deceased children provided consent for verbal autopsy (the family of one deceased child moved out of the surveillance area after the death of their child and another family did not give consent). The mean (standard deviation [SD]) age at registration in the BCPR and mean (SD) age at death of the deceased children was 6 years 4 months (5y) and 7 years 7 months (5y 2mo) respectively. Eleven were female.
Sociodemographic and clinical characteristics
The deceased and surviving children registered with the BCPR had similar sociodemographic characteristics. The majority of children who had died (93%; n=27) and who had survived (97.2%; n=631) were from families living below the poverty line (p=0.21). There were no significant differences between the group of children who had survived and those who had died in relation to housing (p=0.94), access to safe drinking water (i.e. tube-wells [p=0.77]), and access to sanitary latrines (p=0.92; Table SI, online supporting information).
We also compared CP-specific factors among the children who had died and those who survived. Deceased children with triplegia/quadriplegia (45%; n=13) and hypotonia (34%; n=10) were significantly overrepresented versus the children who had survived in this cohort (p=0.001). Similarly, children in GMFCS level III to V and/or with swallowing difficulties were also significantly overrepresented among the deceased children versus those who had survived (90% [n=26] vs 68.7% [n=446] [p=0.02]; 45% [n=13] vs 24.1% [n=156] [p=0.01], respectively; Table SII, online supporting information).
The deceased children had more associated impairments than those who had survived (mean 2.4 [SD 1.4] vs mean 1.6 [SD 1.3]; p=0.002). Intellectual impairment, visual impairment, and hearing impairment were significantly overrepresented in children who had died compared with those who had survived (Table SII).
In terms of nutritional status, the mean weight-for-age z score, height-for-age z score, and weight-for-height z score were in the severe range in deceased children versus those who survived in our cohort (−4.0 vs −2.8 [p=0.001]; −4.1 vs −3.0 [p=0.01] and −2.7 vs −1.1 [p=0.01] respectively). Severe underweight, severe stunting, and/or severe wasting were also significantly overrepresented in the deceased children (Table SII).
Mortality rate in children with CP
The estimated crude mortality rate was 19.5 per 1000 person-years of observation (total observation period 1486.8 person-years). The crude mortality rate was substantially high among young children registered in the BCPR, children in GMFCS level V, children with triplegia/quadriplegia or hypotonia, children with associated impairments (i.e. visual, hearing, speech, intellectual, and epilepsy), children who had difficulties in swallowing foods, and severely malnourished children (i.e. severely underweight, severely stunted, severely wasted) (Tables SI and SII).
Immediate CoD
The leading immediate CoD of children with CP was meningitis (n=9) and pneumonia (n=8). The other immediate CoDs were related to different infectious causes including sepsis (n=4), unspecified diarrhoeal disease, including gastroenteritis accompanied with unspecified protein-calorie malnutrition (n=2), pneumonia as primary CoD and meningitis as secondary CoD (n=1), and unspecified infectious disease (n=1). Two of the children with CP died from accidental drowning and one died from congestive heart failure. The immediate CoD was unknown for one child (Table 1).
| ICD-10 codes | Condition | n (%) |
|---|---|---|
| G00–G03 | Meningitis | 9 (31) |
| J12–J18 | Pneumonia | 8 (28) |
| A40–A41 | Sepsis | 4 (14) |
| A02–A09 and E46 | Other and unspecified diarrhoeal disease, including gastroenteritis (primary CoD) and unspecified protein-calorie malnutrition (secondary CoD)b | 2 (7) |
| J12–J18 and G00–G03 | Pneumonia (primary CoD) and meningitis (secondary CoD)b | 1 (3) |
| B99.9 | Unspecified infectious disease | 1 (3) |
| 150.21–150.23 | Congestive heart failure | 1 (3) |
| W69 | Accidental drowning | 2 (7) |
| R99 | Unknown | 1 (3) |
- aImmediate CoD remained undetermined for two children owing to unavailability of consent for verbal autopsy. bWhere multiple immediate CoD could be determined, we used the term ‘primary’ and ‘secondary’ CoD. ICD-10, International Classification of Diseases, Tenth Edition.
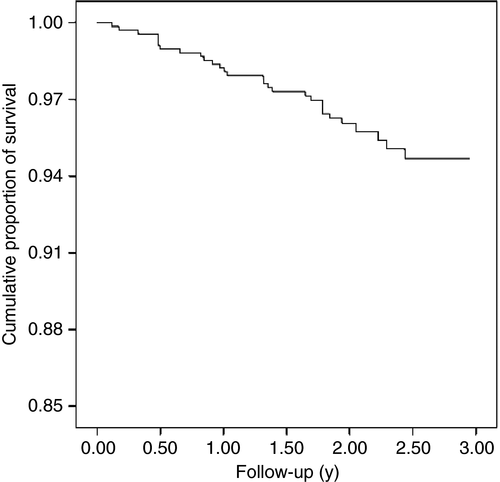
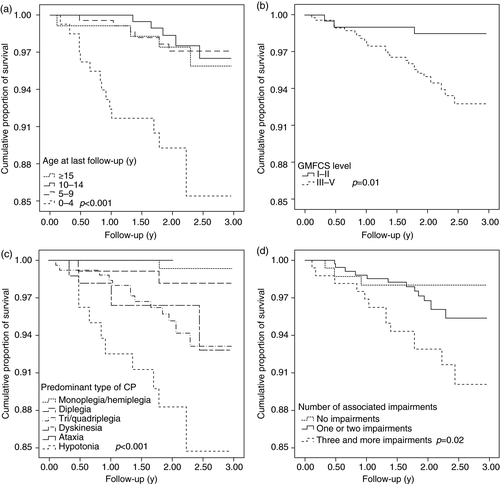
The cumulative survival of children with CP over the follow-up period and predictors of death in children with CP were examined. At the end of the 3-year follow-up period, 95% of the children registered with BCPR survived and the most deaths occurred within the first 2 years of follow-up (Fig. 1). Children aged younger than 5 years had the lowest probability of survival (86%) at the end of the 3-year follow-up period. The cumulative survival proportion was highest in children aged 5 to 9 years (98%) and then gradually decreased with increasing age (97% and 96% for children aged 10–14y and ≥15y respectively). This difference in survival across different age groups was statistically significant (p<0.001; Fig. 2). Furthermore, the cumulative proportion of survival at the end of 3-year follow-up was lower in children in GMFCS levels III to V than children in GMFCS levels I to II (93% vs 99%; p=0.01), in children with hypotonia or triplegia/quadriplegia than in children with monoplegia/hemiplegia (85% vs 93% vs 99%; p<0.001), and in children with associated impairments than in those without any associated impairment (survival probability was 90%, 96%, and 98% in children with ≥3 impairments, in children with ≤2 impairments, and in children without any impairment respectively; p=0.02; Fig. 2).
Tables 2 and SIII (online supporting information) summarize findings from unadjusted and adjusted survival analysis using a Cox proportional hazard model. The findings suggest that young children (aged <2y; hazard ratio [HR] 5.0, 95% CI 1.9–12.9), children in GMFCS levels III to V (HR 2.4, 95% CI 0.7–8.4), children with hearing impairment (HR 2.9, 95% CI 1.2–6.7), and children with swallowing difficulties (HR 2.3, 95% CI 1.0–4.9) were at significantly higher risk of death than others in the cohort when adjusted for covariates (age, GMFCS level, hearing impairment, intellectual impairment, and swallowing difficulties).
| Clinical characteristics and comorbidities | Adjusted analysisa | |
|---|---|---|
| HR adjusted (95% CI) | p | |
| Age (y) at assessment (n=678) | ||
| <2 | 5.0 (1.9–12.9) | 0.001 |
| 2–4 | 1.8 (0.7–4.2) | 0.21 |
| ≥5 | Ref. | |
| GMFCS level (n=678) | ||
| I–II | Ref. | |
| III–V | 2.4 (0.7–8.4) | 0.16 |
| Hearing impairment (n=675) | ||
| Absent | Ref. | |
| Present | 2.9 (1.2–6.7) | 0.01 |
| Intellectual impairment (n=672) | ||
| Absent | Ref. | |
| Present | 1.4 (0.5–3.7) | 0.46 |
| Swallowing difficulties (n=676) | ||
| Absent | Ref. | |
| Present | 2.3 (1.0–4.9) | 0.04 |
- a Covariates that were found to be significantly associated in unadjusted analysis (i.e. age at assessment, type of CP, Gross Motor Function Classification System [GMFCS] level, presence of hearing impairment, presence of visual impairment, presence of intellectual impairment, presence of swallowing difficulties, and presence of stunting) were included in the adjusted analysis following forward selection procedure. CI, confidence interval; HR, hazard ratio.
We also found that, among deceased children with hypotonic CP (n=10), four were aged younger than 2 years and almost all (n=9) were younger than 5 years of age at the time of their initial assessment/diagnosis of CP and recruitment into the BCPR (Table SIV, online supporting information). Moreover, the mean age at death of the children with hypotonic CP was significantly lower than children with another predominant type of CP (mean 4y 1mo [SD 2y 7mo] and 9y 5mo [SD 5y 5mo] respectively [p=0.002]) and the majority of them (n=8) probably had died from infectious diseases (Table SIV).
Discussion
In this study we investigated the deaths of children with CP in rural Bangladesh. The crude mortality rate demonstrated a high mortality rate among children with CP in rural Bangladesh. In a recent study conducted by Alam et al.,25 the mortality rate was reported as 4.03 per 1000 person-years in children younger than 15 years of age living in rural Bangladesh, whereas the mortality rate we observed in the BCPR cohort was nearly five times higher in children of the same age group.25 This lower life expectancy in children with CP might be due to the complex nature of clinical severity and neurodevelopmental impairment. Furthermore, the overall mortality rate was also higher in the BCPR cohort than in children with CP in HICs (19.5 per 1000 person-years vs 9.0 per 1000 person-years reported for children with CP in California).8 In LMICs like Bangladesh, the poor socio-economic status and inadequate management of preventable and treatable conditions associated with CP makes these children vulnerable to premature death.
Our study findings also demonstrate that younger children with CP, particularly those aged younger than 5 years, are at the highest risk of death. This finding is consistent with another study conducted in Bangladesh,14 and clearly differs from the findings from HICs.8 Moreover, in our study, younger age remained a significant predictor for death among children with CP when adjusted for motor severity, associated impairments, and swallowing difficulties.
As reported in our previous publication, diagnosis of CP is substantially delayed in children in the surveillance area; the mean age of diagnosis of CP in the BCPR cohort is 5 years 2 months.11 Hence, we speculate that the observed mortality rate in our study is an underestimate of the true burden as more children with CP in the surveillance area may have died before being diagnosed and registered in the BCPR.11
In addition to the information gained through verbal autopsy, the BCPR data set allowed us to explore the role of different sociodemographic, clinical, and environmental factors to identify possible contributing factors to early mortality. Our findings suggest that all children with CP in our cohort were highly exposed to poverty-linked risk factors for mortality versus the general population. In the BCPR cohort 97% of families were living below the poverty line, whereas this proportion is 19% among the general population of Bangladesh.26
The children who died had more severe forms of motor dysfunction than those who had survived. Thirteen of the 29 deceased children had multiple (≥3) associated impairments and the proportion of visual, hearing, and intellectual impairments among them were also significantly higher (p<0.05) than in those who survived. Moreover, children in our cohort with triplegic/quadriplegic spasticity, dyskinesia, and hypotonia had a significantly lower probability of survival than children with monoplegia/hemiplegia and diplegia. One study conducted in Australia also reported an increased risk of death in children with hypotonia (HR 2.3) and children with quadriplegia (HR 14.8) versus children with unknown motor type and unknown topography respectively.12 Nearly half of the children who had died also had swallowing difficulties and this proportion was significantly higher than in the children who had survived in our cohort. These deceased children who had severe motor severity and feeding difficulties were more likely to depend on caregivers for daily activities and feeding. Even in affluent HICs, children with such severe forms of CP, who cannot stand unaided or need to be fed by others, are at increased risk of a lower life expectancy than children with CP of a less clinical severity.6, 8
In our study, we relied on cautiously analysed and interpreted data from verbal autopsies to ascertain immediate CoD among children with CP in the surveillance area. This was necessary as the majority of the children (n=27) had died outside of the health system and there was an absence of medical records.
Of the 29 deceased children, 25 died from infectious causes and eight of them probably died from respiratory infection. Respiratory infection has been reported to be the major CoD in children with CP in previous studies conducted in both LMICs and HICs.13-15 These infections may have occurred as a result of environmental factors such as microbial contamination or exposure to communicable diseases. The risk of infection is likely to be related to the living conditions and the context of their poor socio-economic status. The other important overarching factor was malnutrition. Of the nine children whose immediate CoD was pneumonia, six were both severely underweight and had severe stunting and four also had parent-reported swallowing difficulties (Table SIV). The role of malnutrition in compromising the immune system of individuals, especially children, through mechanisms of immunological alterations has long been recognized.27, 28 This finding is found even in affluent HICs.29 Of those children who we believe died from infectious causes, three had monoplegia/diplegia and were severely malnourished (Table SIV). This indicates that despite the better motor function, severe malnutrition and infection likely aggravated their risk of death. In our cohort, nine children who had died had epilepsy and one died from accidental drowning. Drowning is considered one of the indirect causes of premature mortality in children with epilepsy in LMICs.30
Many of these immediate CoDs could be prevented or treated through availability of healthcare services, appropriate nutrition intervention, and timely management of infections. For instance, of the 25 children who died from infectious causes, only seven were on antibiotics preceding their death, which indicates poor health-seeking behaviour and/or poor availability of health services. The degree of malnutrition and exposure to other environmental factors could also be minimized through training of primary caregivers regarding feeding practices, safe preparation of nutrient-dense food, or arrangement of tube feeding for proper management of malnutrition. However, such arrangements, training, and awareness raising require carefully designed intervention programs.
Despite considerable effort, this study has several limitations. First, we could not report the status of the children who were lost to follow-up during the study period and have been excluded from the analysis; therefore, the findings presented here might vary slightly from the true findings. Second, we had to rely solely on the caregiver's response to ascertain the immediate CoD. There is a possibility of information and recall bias. Moreover, we could only assign immediate CoD at the broad category level based on verbal autopsy and not the underlying CoD. In some cases we were unable to assign the CoD more precisely owing to unavailability and/or inaccessibility of diagnostic tests and medical records. Although all the children included in our cohort were assessed by a trained paediatrician and a multidisciplinary medical assessment team, scope for diagnostic investigations, genetic testing, or brain imaging to ascertain any contributory structural lesions causing death were almost absent. So, it is likely that there could be a few children who might have a genetic condition (either in isolation or in association with CP) that we could not report. Third, even though it would have been ideal to conduct an observer-blinded analysis, the investigators in our study were not blinded. Fourth, the findings presented in this study are based on small frequencies within a short period of time versus the findings from HICs. However, the mortality rate reported within this short period of follow-up remains alarming.
Our findings indicate that the majority of children with CP died as a result of potentially preventable causes. With adequate support and proper healthcare services these children may have had longer survival times. As our surveillance is ongoing, we will continue to follow-up the registered children and our major concern is for those surviving children who have similar sociodemographic backgrounds, living environments, and clinical profiles, which increases the likelihood of more premature deaths in this cohort in the future. To minimize premature deaths in children with CP in LMICs like Bangladesh there is an urgent need to scale up low-cost early interventions, rehabilitation, and nutritional supplementation, and mobilize available resources to build the capacity of health professionals and caregivers in these settings.
Acknowledgements
We would like to express our heartfelt condolences to all family members of the children with CP who died. Our deepest gratitude is to the primary caregivers of these young children for their kind consent and participation in the interviews despite the agony of revisiting these difficult and painful memories. We would like to thank Mr Alamgir Kabir, Research Fellow (Biostatistics), Asian Institute of Disability and Development, for his expert suggestions on statistical analyses and result interpretation. We would also like to acknowledge the CSF Global team in Bangladesh for their support in the BCPR project implementation and for supporting the children with CP and their families with information and in accessing services along with a strong referral system. This study has been conducted as part of the BCPR study. The BCPR is funded by the Cerebral Palsy Alliance Research Foundation (PG4314 – Bangladesh CP Register) and also through internal funding from CSF Global, Bangladesh. TK is supported by the Cerebral Palsy Alliance Research Foundation Career Development Grant (CDG 04617). HS-S received support via a National Health and Medical Research Council Early Career Fellowship 1144566. The authors have no interests which might be perceived as posing a conflict or bias.




