Plasma bilirubin binding and bilirubin neurotoxicity
Abstract
This commentary is on the original article by Amin et al. on pages 297–303 of this issue.
The study of Amin et al.1 reminds us that we should not ignore the role of plasma bilirubin binding in the pathogenesis of acute bilirubin neurotoxicity (auditory toxicity in this study) in jaundiced newborn infants. Plasma bilirubin binding dictates how much bilirubin must accumulate in order to reach any total bilirubin concentration (TSB). For example, an infant with poor bilirubin binding must produce far more bilirubin to reach a TSB of 30mg/dL, compared to an infant with good binding, since poor binding allows more of the accumulating bilirubin to ‘leak’ into the extravascular space as the TSB increases to 30mg/dL. Consequently, the infant with poor bilirubin binding has more extravascular (brain) bilirubin and a greater risk of bilirubin neurotoxicity than the infant with good bilirubin binding.
This is not new information and it seems extraordinary that Japan is the only country in the world where clinical laboratories routinely provide clinicians with bilirubin binding measurements.2 The absence of clinical bilirubin binding measurements stems largely from a misunderstanding of what constitutes (quantifies) bilirubin binding.3 This is illustrated in the design of the Amin et al. study which evaluates TSB, the plasma unbound bilirubin concentration, and the bilirubin:albumin molar ratio (BAMR) as independent measures of the risk of bilirubin neurotoxicity. This design is based on the long held and well entrenched misconception that before ‘bilirubin binding tests’ (in this study unbound bilirubin or BAMR) can be used clinically, prospective proof is needed that they have better sensitivity and specificity than TSB in predicting bilirubin neurotoxicity.1 The truth is that TSB, unbound bilirubin, and BAMR are not independent determinants of bilirubin neurotoxicity but are mathematically related variables quantifying bilirubin binding.3
Bilirubin binding is quantified by measuring (1) how much and (2) how strongly plasma can bind bilirubin. How much is generally quantified by measuring the plasma bilirubin binding capacity.4 Since bilirubin binds primarily to albumin, Amin et al. use the albumin concentration to estimate the bilirubin binding capacity. This assumes each albumin molecule binds a single bilirubin, which unfortunately is not the case. The albumin concentration increasingly underestimates the bilirubin binding capacity as the BAMR approaches or exceeds 1,3 as occurred often in this study.1 How strongly plasma binds bilirubin is quantified by the mass action equilibrium association binding constant (KA) or dissociation constant (1/KA).
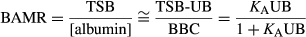
The considerable in vivo variation in bilirubin binding capacity and KA means quantifying bilirubin binding requires measuring bilirubin binding capacity, TSB, and unbound bilirubin, and then calculating KA (or KD). KA bilirubin binding capacity provides a robust, continuous indicator of how well plasma binds bilirubin. The failure to recognize that measuring ‘bilirubin binding’ requires all the binding components may be depriving us of a valuable clinical tool.
For example, the likelihood of bilirubin neurotoxicity is about 10% in term newborn infants with hazardous TSB greater than or equal to 30mg/dL. Measuring bilirubin binding might help further determine whether to treat with phototherapy alone or with exchange transfusion as well.5 Amin et al. report that unbound bilirubin or BAMR were no better than TSB in predicting bilirubin neurotoxicity at TSB greater than or equal to 25mg/dL, which some might take as evidence against the usefulness of measuring bilirubin binding in these infants. However, unbound bilirubin or BAMR alone do not accurately quantify bilirubin binding. Furthermore, at BAMR greater than or equal to 1, which occurred in many study samples, KA cannot even be calculated. In short, the study data1 do not provide KA and bilirubin binding capacity which is needed to evaluate the relationship between bilirubin binding and bilirubin neurotoxicity, especially at TSB greater than or equal to 25mg/dL.
The clinical use of bilirubin binding requires (1) reliable methods for measuring all the bilirubin binding components,3 and (2) the KA bilirubin binding capacity population parameters (mean, SD, range, etc.) for the various newborn infant populations (term, premature, etc.). Comparing KA bilirubin binding capacity in a jaundiced newborn infant with the appropriate KA bilirubin binding capacity population parameters may provide important additional information regarding how that infant should be managed within the context of clinical circumstances and TSB.3




