Magnetic resonance imaging arterial-spin-labelling perfusion alterations in childhood migraine with atypical aura: a case–control study
Abstract
Aim
Atypical migraine with aura can be challenging to diagnose. Arterial-spin-labelling (ASL) is able to non-invasively quantify brain perfusion. Our aim was to report cerebral blood flow (CBF) alterations using ASL, at the acute phase of atypical migraine with aura in children.
Method
Paediatric patients were retrospectively included if (1) referred for acute neurological deficit(s), (2) underwent brain magnetic resonance imaging (MRI) at presentation with ASL sequence, and (3) had subsequent diagnosis of migraine with aura. Neurological symptom-free controls were matched for age. Twenty-eight regions of interest (ROIs) were drawn on CBF maps for each participant/control.
Results
Ten patients were included (median age 13y, range 8–16y). Eight of 10 had multiple aura symptoms during the episode. For every patient, CBF was decreased in a brain region consistent with symptoms when MRI was performed less than 14 hours after onset (n=7 patients) and increased if the MRI was performed 17 hours or more after (n=4 MRIs).
Interpretation
MRI–ASL appears to be a promising tool for the diagnostic workup and differentials exclusion in paediatric migraine with aura. Constant and time-consistent non-territorial CBF modifications were found in our sample providing additional insight to migraine with aura pathophysiology. The authors encourage implementing this sequence at the acute phase of unexplained paediatric neurological deficits, with or without accompanying headache.
What this paper adds
- Children presenting at the acute phase of migraine with aura present measurable alterations of regional cerebral blood flow (CBF) using arterial-spin-labelling (ASL) sequence.
- CBF alterations are related to time post onset, not persistence of symptoms at time of magnetic resonance imaging.
- Patients scanned early show hypoperfusion and those scanned late show hyperperfusion, regardless of symptoms at the time of imaging.
This article is commented on by Abu-Arafeh on pages 897–898 of this issue.
Abbreviations
-
- ASL
-
- Arterial-spin-labelling
-
- CBF
-
- Cerebral blood flow
-
- DWI
-
- Diffusion-weighted imaging
-
- ROI
-
- Region of interest
Atypical presentations of migraine with aura can be difficult to diagnose and can be mistaken for acute stroke.1, 2 Typical aura is a fully reversible visual, sensory, motor, or phasic symptom, developing gradually over 5 minutes, lasting less than an hour per symptom, and accompanied or followed by headache within 60 minutes from symptom resolution.3 With a prevalence ranging from 3% in 3- to 7-year-old children to 8% to 20% in adolescents,4 migraine is a common headache syndrome in the paediatric population. Up to 30% of patients report a preceding aura5 and significant variations of symptoms expression over the span of childhood make migraine with aura a really challenging diagnostic situation, especially for atypical auras. To date, atypical migraine with aura pathophysiology remains elusive. Aura results from neurovascular phenomena comprising cortical spreading depression associated with cerebral regional hypoperfusion accompanied by negative neurological features. Subsequent neuronal hyperpolarization and regional hyperperfusion are concurrent with cephalalgia.6 Several cases and case-series have reported these perfusion modifications in adults,1, 7-10 but literature regarding migraine with aura in children remains scant.
Structural neuroimaging (including T1, T2, and fluid-attenuated inversion recovery) findings in paediatric migraine patients are meagre and largely unspecific. On the other hand, more advanced magnetic resonance imaging (MRI) techniques providing physiological information – such as dynamic susceptibility contrast perfusion-weighted imaging or diffusion-weighted imaging (DWI) – have proven useful in differentiating migraine with aura from differential diagnoses,1, 11-13 and most importantly from acute ischaemic stroke. When investigating an acute neurological deficit, perfusion-weighted imaging and DWI, adjuncts to one another, provide critical elements for the diagnosis workup.
Nevertheless, dynamic susceptibility contrast perfusion-weighted imaging requires a high flow gadolinium contrast agent intravenous injection, which is more invasive and difficult to obtain in children.
MRI arterial-spin-labelling (ASL) sequence uses magnetically labelled blood water to quantitatively map cerebral blood flow (CBF) without contrast injection.14 In a previously published case report, we have shown that ASL can depict brain perfusion changes related to an acute atypical migraine attack.12 ASL may be a powerful tool for positive diagnosis of migraine with atypical aura in clinical practice, may help to differentiate migraine with aura from paediatric acute ischaemic stroke, and might give additional insight into the pathophysiology of perfusion alterations during the aura phase.3
The aim of this study was to report CBF modifications with ASL in the acute setting of paediatric atypical migraine with aura mimicking stroke.
Method
The study conformed to generally accepted scientific principles and to the ethical standards of research and the retrospective review of ASL data was approved by an advisory opinion of the Ethics Committee (CPP Ile de France) at our institution.
Participants and controls
This is a retrospective series of paediatric patients eventually diagnosed with atypical migraine with aura in a tertiary paediatric centre. Inclusion criteria comprised (1) age under 16, (2) referral for acute onset neurological deficit, (3) subsequent diagnosis of migraine with aura, and (4) a diagnostic workup comprising an acute phase brain MRI with ASL and DWI sequences upon the arrival of the patient at the hospital. ASL sequence has been routinely implemented in our MRI protocols since 2012. Thus, the inclusion period ranged from January 2012 to May 2014.
Symptoms and final diagnosis were assessed by a senior paediatric neurologist. All patients were eventually admitted to a paediatric neurology department and symptoms were recorded hourly during hospitalization.
The following demographic and clinical data were retrospectively collected from chart review by a senior paediatric vascular neurologist (MK): age; sex; past medical history, including personal or family history of migraine; ongoing medical treatment; symptoms onset; type; and duration.
Controls matching for age were selected among participants without history of migraine who had a normal brain MRI with ASL sequence during the study period, with a 1:1 ratio. Controls underwent a brain MRI for various non-neurological reasons (non-exhaustively including vestibular symptoms, optic neuritis, amblyopia, arthrogryposis, bowel systemic disease, myopia with suspected ocular complications). Controls were matched 1:1 to participants per age.
MRI and ASL measurement of CBF
All MRI exams were performed according to local standard of care when deemed necessary by the clinical team. ASL perfusion sequence was acquired using a General Electric's 1.5T MRI, and consisted of a concurrent spiral three-dimensional pseudo-continuous ASL (3D volume with a 4mm slice thickness; field of view 40mm; flip angle 155°; repetition time 4431ms; echo delay time 11ms; post-labelling delay 1025ms; number of excitations 3; spiral matrix 512×8mm). Patients also benefited from routine sequences as implemented in our standard protocol: 3D T1, axial T2, axial fluid-attenuated inversion recovery (FLAIR), DWI, 3D-Time of Flight Angiography. Raw ASL data were processed by two trained radiologists (GB, ES), blinded to clinical symptoms, using the 3D-ASL application of the GE Advantage Windows (GE Healthcare, Milwaukee, WI, USA) station Functool post-processing software to obtain two-dimensional CBF maps. Twenty-eight regions of interest (ROIs) corresponding to main anatomical regions were subsequently manually drawn for each participant and control, using a co-registered reference reformatted axial T1-weighted sequence, with pre-specified locations (Fig. S1, online supporting information). The mean values of regional CBF in each ROI were recorded. ASL sequences deemed uninterpretable (artifacts) were excluded from the final analysis.
Interrater agreement between readers was calculated using unbiased intraclass correlation coefficients15 applied to the mean CBF value in each ROI. The absolute CBF values recorded by reader 1 in each ROI for each participant were confronted to the mean CBF values of the matched control corresponding ROI.
Results
During the study period, 14 patients were examined retrospectively for eligibility and 10 patients met inclusion criteria (five males, five females; median age 13y, range 8–16y). Baseline characteristics are displayed in Table 1. Three of 10 participants had a personal history of migraine without aura. Seven of 10 patients had a familial history of migraine. Ten children were included in the comparison group (median age 11y, range 5–16y).
| Participants | |
| Male/female | 5/5 |
| Age in years | 13 (8–16) |
| Controls | |
| Male/female | 5/5 |
| Age | 11 (5–18) |
| Participants: past medical history | |
| Migraine | 3 (30%) |
| Familial migraine | 7/10 (70%) |
| Clinical presentation | |
| Focal neurological deficit | 8 (80%) |
| Confusion | 2 (20%) |
| Duration | |
| Aura | 4 (0.25–20) |
| Episode | 11 (2–36) |
- Values are expressed as n (%), Median (min–max).Total duration of episode/aura symptoms, in hours (min-max).
The median total duration of the aura was 4 hours (0.25–20h). Eight of 10 patients had a total aura duration exceeding 60 minutes.
Eight of 10 participants developed multiple aura symptoms during the episode. Symptoms were moderate to severe aphasia in eight, sensory deficit or tingling in five, motor deficit or numbness in four, and visual disturbances in four, of the 10 cases. Only one patient presented with isolated motor symptoms.
Two patients had headache that lasted over 24 hours. All patients had presented headaches before or during the MRI.
MRI and CBF measures
Median onset-to-MRI delay was 12 hours (interquartile range 11h). Structural MRI sequences performed for stroke suspicion were normal for every patient, noticeably FLAIR and DWI. One patient had a follow-up MRI. Therefore, 11 MRIs were analysed.
All studied children with migraine with aura had alterations of the regional CBF values when compared with the matched controls. All CBF variations were found in anatomical regions consistent with previous or persistent aura symptoms. Summary of CBF alterations per clinical manifestation and delay between symptoms onset and MRI are displayed in Table 2.
| Patient | Symptoms | Delay symptoms-MRI | rCBF | ||||
|---|---|---|---|---|---|---|---|
| Visual | Sensitive | Motor | Aphasia | Confusion | Increase (+)/Decrease (−) | ||
| 1 | − | + | + | + | − | 3h | + |
| 2 | − | − | + | + | − | 4h | + |
| 3 | − | + | + | + | − | 5h | + |
| 4 | − | − | − | + | + | 8h | + |
| 5 | + | − | − | + | + | 9h | + |
| 6 | + | + | − | + | − | 12h | + |
| 7 | + | + | − | + | − | 13h | + |
| 8 | − | − | + | − | − | 17h | − |
| 9 | − | + | − | + | − | 19h | − |
| 10 | + | − | − | − | − | 20h | − |
| 11a | − | − | − | + | + | 31h | − |
- a Patient's second MRI.
When the MRI was performed less than 14 hours after the onset of the symptoms, every study (7/7) displayed a CBF decrease by 25% or more. On the contrary, if the MRI was performed 17 hours or more after the onset, every study (4/4) displayed a CBF increase of 25% or more (for quantitative data, see Fig. S1 and Table SI, online supporting information). Interestingly, a second MRI was performed in one patient 31 hours after symptoms onset. In this patient, ASL first demonstrated a diffuse left-sided CBF decrease followed by an important CBF increase in the same region on follow-up MRI (see Fig. 1).
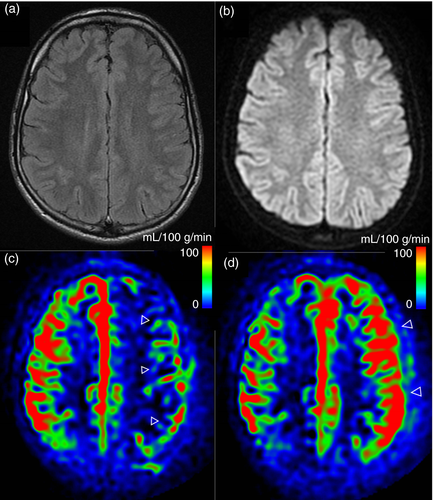
Among the patients scanned before 14 hours (CBF decrease), four of seven had persistent aura symptoms and three of seven were asymptomatic. Among the patients scanned after 17 hours (CBF increase), two of four had decreasing but persistent symptoms, one of four had no symptoms left, and data could not be retrieved for one patient.
Intraclass correlation for CBF values in each predetermined ROI was excellent between the two raters (0.97 CI 95%: 0.94–0.99).
Discussion
The main finding of our study is that all children presenting with migraine with aura demonstrated measurable modifications of regional CBF assessed by ASL sequence whereas structural and DWI MRI sequences were normal. Every patient scanned less than 14 hours after the onset of the aura displayed a decrease of CBF in the brain region consistent with the symptoms, whereas a constant increase was found when the patients were scanned after 17 hours post onset. Although arbitrary and of comparable magnitude, these cut-offs highlight the consistence of the CBF evolution during migraine with aura episodes, being systematically decreased at the early phase and increased at a delayed timing point independently of symptoms evolution.
Interestingly, the time course of CBF alterations did not appear to be linked with the persistence of aura symptoms, neither with the persistence of cephalalgia at the time of scanning in our cohort. These findings are in line with previously published works9, 16, 17 regarding CBF evolution obtained by positron emission tomography MRI or dynamic susceptibility perfusion-weighted imaging during migraine with aura episodes that suggest that a typical episode begins with decreased regional flows during aura, which eventually increase significantly when the headaches appear. Moreover, our results add to those findings, enlightening the critical effect of the delay between onset and MRI, which seems to be more relevant than the symptoms at the time of imaging study. Of note, the duration of hypoperfusion (up to 14h) also appeared to be strikingly long as compared with previous literature,18 also accounting for the specificity of these atypical events. While the pathophysiology of these events is not fully understood, and likely differs by subtype, recent works are paving the way towards a better understanding of the mechanisms involved.6, 10, 11, 19
The duration of symptoms in our cohort was strikingly long for migraine with aura (especially aura, with a median of 4h), reflecting the specificity of these atypical events that misled the initial clinical assessment and eventually led to advanced brain imaging. ASL mapped CBF regional alterations provided critical elements for the positive and differential diagnosis at the acute phase. In these atypical clinical settings, excluding acute ischaemic stroke from the differential list is the first priority. Addition of ASL and DWI sequences provide strong arguments to do so, without the need of contrast injection. Indeed, the absence of DWI lesions, and the presence of CBF alterations lying across different vascular territories, makes the diagnosis of acute ischaemic stroke very much less plausible, given the elapsed time since onset.
Although the perfusion alteration pattern allows discriminating atypical migraine with aura from acute ischaemic stroke (multiple affected regions lying across different vascular territories), one other differential may present similar alterations, namely a prolonged post-ictal state. A careful evaluation of history is mandated to exclude this diagnosis.
To date, MRI–ASL sequence is the only non-invasive perfusion-sensitive routinely available technique. Its reliability has been demonstrated in recent works when compared with the criterion standard for brain perfusion evaluation (015 positron emission tomography).20 Several practical aspects make it of interest in a paediatric environment as mentioned above. Our data show that ASL provides consistent quantitative regional CBF measurements in children with acute phase atypical migraine with aura with excellent interobserver agreement and suggest that this tool is powerful, easily implemented, and efficient in the setting of acute onset neurological deficit in children. Additionally, this work adds insight to our understanding of migraine with aura pathophysiology, revealing the pattern of CBF alterations in this population.
Our study presents several limitations, first of all being the small sample size, explained by the rarity of the condition described. Second, there is no reference atlas for ASL perfusion values in children among ages, explaining our choice to match participants with controls. This choice was further supported by the potential presence of bilateral CBF alterations in migraine with aura prohibiting intra-subject comparisons. We aimed at minimizing biases by matching controls and participants by age during the study period, although it is known that in our age group (not including newborn infants) absolute CBF values do not vary significantly.21
Future studies would benefit from comparing paediatric migraine aura patients with stroke or post-ictal patients, to confirm the ability of ASL to acutely and reliably discriminate these conditions.
Conclusion
Migraine with aura is difficult to diagnose in children, and ASL sequence – in association with a normal diffusion MRI – presents a great help for the positive diagnosis of migraine with aura in children. It helps ruling out acute ischaemic stroke and provides insight in our understanding of its pathophysiology, confirming the timed sequence of hypo followed by hyperperfusion. Therefore, the authors encourage implementing this sequence at the acute phase of unexplained paediatric neurological deficits, with or without accompanying headache.
Further longitudinal studies with larger sample sizes, prospective design, and implementation of quantitative ASL techniques (multiple-delay time sampling) are needed to help refining the dynamics of perfusional changes during migraine with aura in children.
Acknowledgement
The authors have stated that they had no interests which might be perceived as posing a conflict or bias.




