Effects of zoledronic acid on children and young adults with low bone mineral density
L2
K Durkin1, K Shepherd2, E Alexy1, G Noritz2
1Nationwide Children's Hospital, Columbus, OH, USA; 2Nationwide Children's Hospital, The Ohio State University, Columbus, OH, USA
Background and Objective(s): Low bone mass with subsequent fragility fractures is an important problem for patients with neurologic disabilities. Bisphosphonates are the mainstay of therapy in adults with osteoporosis. There is no FDA-approved treatment for pediatric patients with low bone mineral density (B), though pamidronate is commonly used. Zoledronic acid has a higher potency, faster intravenous infusion time, and less frequent need for administration, making it more convenient than pamidronate. The goal of treatment is to reduce fractures as a result of increased B. The primary objective of this study is to measure the change in Lumbosacral Spine (LS) B and Z scores following treatment with zoledronic acid in children with primary or secondary low B.
Study Design: Retrospective Chart Review.
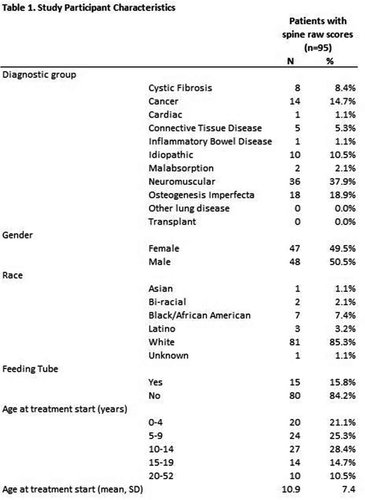
Study Participants & Setting: This study took place at a pediatric institution with a multi-specialty metabolic bone clinic, where bone mass is measured by Dual Energy X-ray Absorptiometry (DXA). Subjects were included if they received intravenous zoledronic acid between January 1, 2009 and October 1, 2016 and had at least one DXA before initiation of therapy and at least one after. Patients were excluded if the DXA was unreadable due to the presence of devices such as spinal rods or catheters.
Materials/Methods: Zoledronic acid has been prescribed clinically at our institution since 2006. The typical dose is 0.025 mg/kg for the first dose, and 0.05 mg/kg in subsequent doses, with a maximum single dose of 4 mg. Baseline demographics included the primary diagnosis, age, gender, race, and medication history. Baseline LS B and Z score values were obtained from DXA scans prior to first infusion and compared to subsequent DXA measurements. Descriptive statistics examined treatment characteristic variables. Linear mixed models accounting for both fixed and random effects were run to look at number of doses of zoledronic acid and cumulative dose of zoledronic acid, separately, as predictors of LS B scores and Z scores. All models included gender, race, diagnosis group, and presence of feeding tube as covariates.
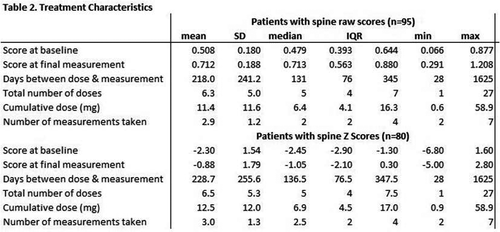
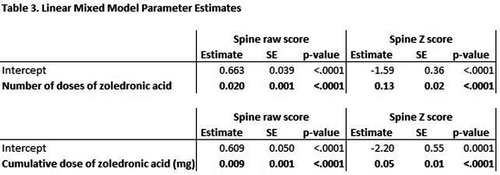
Results: During the study period, 163 patients received zoledronic acid. 95 were included in LS raw score analyses and 80 were included in LS Z score analysis. Table 1 outlines the characteristics of the included participants. An underlying neuromuscular disorder was the most common reason for low bone mass. During the study period, the participants received a median of 5 doses each, and a median total dose of 6.4 mg. Mean scores for LS raw score and LS Z score were higher at final measurement than at baseline (Table 2). The mean increase in B over the study period was 40% (0.508 mg/cm2 to 0.712 g/cm2). The number of doses of zoledronic acid and cumulative dose of zoledronic acid were both significant predictors (p < 0.0001) of increased LS raw scores and spine Z scores, controlling for gender, race, diagnosis group, and feeding tube presence (Table 3). For each dose of zoledronic acid, LS raw scores increased an average of 0.020 g/cm2 and Z scores increased an average of 0.13. For each milligram of zoledronic acid, LS raw scores increased an average of 0.009 g/cm2 and Z scores increased an average of 0.05.
Conclusions/Significance: A significant increase in LS B and Z score were observed in patients with baseline low bone mineral density after treatment with intravenous zoledronic acid at a pediatric institution. The most common reason for low bone mass was an underlying neuromuscular disorder. Further study should have long term follow-up to examine whether these increases in B lead to a reduced risk of fracture.