Alternative splicing for germ cell-specific Mga transcript can be eliminated without compromising mouse viability or fertility
Communicating Editor: Harukazu Nakamura
Funding information: Ministry of Education, Culture, Sports, Science and Technology, Grant/Award Numbers: 19H03426, 20G10148, 20K16147, 21K06843
Abstract
The stimulated by retinoic acid gene 8 (STRA8)/MEIOSIN complex and polycomb repressive complex (PRC) 1.6, a PRC1 subtype, are believed to be positive and negative regulators of meiotic onset, respectively. During meiotic initiation, the transcription repressive activity of PRC1.6 must be attenuated so that meiosis-related genes can be effectively activated by the STRA8/MEIOSIN complex. However, the molecular mechanisms that control the impairment of PRC1.6 function remain unclear. We recently demonstrated that the Mga gene, which encodes a scaffolding component of PRC1.6, produces variant mRNA by alternative splicing specifically during meiosis. Furthermore, the anomalous MGA protein encoded by the variant mRNA bears an intrinsic ability to function as a dominant negative regulator against the construction of PRC1.6 and is therefore assumed to be, at least in part, involved in impairment of the complex. Therefore, to unequivocally evaluate the physiological significance of Mga variant mRNA production in gametogenesis, we examined the consequences of a genetic manipulation that renders mice unable to produce Mga variant mRNA. Our data revealed that mutant mice were equivalent to wild-type mice in terms of viability and fertility. Our detailed examination of spermatogenesis also revealed that this genetic alteration is not associated with any apparent abnormalities in testis size, spermatogenic cycle, timing of meiotic onset, or marker gene expression of spermatogonia and spermatocytes. Taken together, these data indicate that the production of germ cell-specific Mga variant mRNA is dispensable not only for viability but also for gametogenesis.
1 INTRODUCTION
Germ cells are the only cell type that can transmit genetic information to the subsequent generation. While spermatogonial stem cells maintain their cell number through their self-renewal ability, some undergo differentiation into mature sperm formation via numerous processes, including meiosis (de Rooij, 2017; Kanatsu-Shinohara & Shinohara, 2013; Makela & Hobbs, 2019; Tan & Wilkinson, 2020).
Stimulated by retinoic acid gene 8 (STRA8) is known to be a crucial positive regulator of meiosis (Anderson et al., 2008; Baltus et al., 2006; Dokshin et al., 2013; Mark et al., 2008). Although the molecular basis of the action of STRA8 has long been enigmatic, a recent molecular cloning of MEIOSIN, which interacts with STRA8, has profoundly changed the situation (Ishiguro et al., 2020; Oatley & Griswold, 2020). Indeed, identification of MEIOSIN as a binding partner for STRA8 has led to the discovery that STRA8 is directly involved in the transcriptional activation of numerous meiosis-related genes, including Stra8 and Meiosin, with the aid of the DNA binding ability of MEIOSIN. Contrary to the STRA8/MEIOSIN complex, one of the polycomb repressive complex1 (PRC1) subtypes, i.e., PRC1.6, has been shown to function as a strong blockade against meiotic onset thus preventing germ cells and pluripotent early embryonic cells from precocious and ectopic onset of meiosis, respectively (Endoh et al., 2017; Maeda et al., 2013; Suzuki et al., 2016). PRC1.6 exerts its effect by repressing transcription of a number of meiosis-related genes. To be subjected to STRA8/MEIOSIN complex-mediated transcriptional activation effectively for meiotic onset, meiosis-related genes must be liberated from the transcriptional repression enforced by PRC1.6. However, the molecular mechanisms that govern inactivation or attenuation of the transcriptionally repressive activity of PRC1.6 are completely unknown.
MGA is the largest component of PRC1.6, containing more than 3000 amino acids, and functions as a scaffolding component in PRC1.6 (Gao et al., 2012). A previous knockout study demonstrated that Mag gene-null embryos show an early embryonic lethal phenotype (Washkowitz et al., 2015), suggesting a crucial role for Mga in sustaining viability. MGA bears two independent DNA-binding domains, which are termed T-box and basis-helix–loop–helix (bHLH) domains. The former has intrinsic DNA-binding activity by itself, but the DNA-binding activity of the latter is dependent on its interaction with MAX (Hurlin et al., 1999; Stielow et al., 2018; Uranshi et al., Uranishi et al., 2021). We recently demonstrated that germ cells produce a variant of the Mga splice variant (Mga SV) from the Mga gene by alternative splicing during meiosis (Kitamura et al., 2021). The MGA protein encoded by Mga SV mRNA lacks a carboxy-terminal portion, including the bHLH domain, owing to the presence of a premature stop codon in a variant-specific exon (Exon 19a). Due to the lack of the bHLH domain, the anomalous MGA has the potential to exert a dominant negative function against the construction of PRC1.6 by sequestering components of the complex, such as PCGF6 and RING1B. Based on this, it is logical to assume that anomalous MGA impedes PRC1.6 function towards the onset of meiosis. Therefore, we deleted the Mga SV-specific exon sequence from the genome such that mice do not produce Mga SV mRNA and instead exclusively produce canonical Mga mRNA. Notably, depletion of Mga SV mRNA through deletion of a variant-specific exon sequence from the genome did not lead to obvious abnormalities in viability and fertility. Taken together, this implies that germ cells bear at least an additional molecular means other than the production of an anomalous MGA with no bHLH domain for the impediment of function of PRC1.6 to attain secure meiosis.
2 MATERIALS AND METHODS
2.1 Mice
Wild-type C57BL/6J mice (6–12 weeks-old) and those in which the genomic sequence corresponding to Exon19a and its flanking regions of Mga gene (Mga ΔExon19a mice) were deleted were inbred at the Saitama Medical University animal facility. Animal experiments were carried out in strict accordance with international and institutional guidelines. The study protocol was approved by the Institutional Review Board of the Ethics of Animal Experiments of Saitama Medical University (permission numbers 2579, 3032, and 3143).
2.2 Generation of Mga ΔExon19a mice
The sequences (5′-CTA TTC CAC TGA TAA GAC GC-3) and (5′-TCA GTA GTA TTG CAG ACA GA-3′) were selected as guide RNA (gRNA) targets. gRNAs were synthesized and purified according to the manufacturer′s instructions (Thermo Fisher Scientific). Unfertilized oocytes were collected from super-ovulated female C57BL/6J mice (>10 weeks-old) with gonadotropin and chorionic gonadotropin and were subjected to in vitro fertilization with sperm from C57BL/6J mice. Five hours after treatment, the resultant zygotes were subjected to gRNAs (5 ng/μl) and GeneArt Platinum Cas9 Nuclease (100 ng/μl) (Thermo Fisher Scientific) by electroporation using a NEPA 21 electroplater (NEPAGNENE) as described previously (Sato et al., 2018). Two-cell stage embryos developed by subsequent in vitro culture were transferred into the oviducts of pseudopregnant ICR female mice and allowed to develop for delivery. One of the originally obtained heterozygous Mga ΔExon19a mutant mice, but not the homozygous mutant mice, was used as a founder to maintain the colony by crossing it with wild-type C57BL/6J mice. Heterozygous mutant mice were backcrossed three times before being used to generate homozygous mutant mice via heterozygous intercrosses. To distinguish between wild-type and deletion, PCR was performed using oligonucleotides in which 963 bp and 711 bp PCR products were obtained in the presence and absence of Mga Exon19a genomic sequence, respectively.
Forward: 5′-TGG GTA ACA GAC CCA TTT CC-3′.
Reverse: 5′-CAG CCC CAA ACC AAT TTT AG-3′.
2.3 Fertility analyses
For fertility examination of Mga ΔExon19a mutant mice, 2- or 6-month-old male mutant and 2-month-old female mutant mice were mated with 2-month-old wild-type (C57BL/6J) female and male mice, respectively. Intercrosses between 2-month-old male and female mice were conducted in parallel as controls. Once pregnancy was indicated by vaginal plug formation, the female mice were individually transferred to new cages.
2.4 Sperm counting
For sperm counting, the cauda epididymis was isolated by dissection on a petri dish and then minced with a small amount of PBS using tweezers. Mature sperm-containing PBS solution was diluted with additional PBS, and the total number of sperm was counted using a hemocytometer.
2.5 RT-PCR
Total RNA from wild-type and Mga ΔExon19a mutant mice was used to generate cDNA by reverse transcription. The cDNA was then used for PCR to examine the presence or absence of Mga exon19a as described previously (Kitamura et al., 2021).
2.6 Antibodies
The guinea pig anti-mouse MEIOSIN antibody was kindly provided by Dr. Kei-ichiro Ishiguro (Kumamoto, Japan), and information on the manufacturer and catalog number of the purchased antibodies is as follows: goat anti-PLZF antibody (AF2944, R&D Systems), mouse anti-SYCP3 antibody (sc74569, Santa Cruz Technology), and rabbit anti-γH2AX antibody (2577, Cell Signaling Technology).
2.7 Histological HE- and immuno-stainings
Testes and epididymides from wild-type and Mga ΔExon19a mutant mice were fixed with Bouin's solution, dehydrated, and treated with ethanol with rotation at 4°C overnight. The samples were embedded in paraffin after treatment with xylene and sliced to a thickness of 4 μm. For HE staining, the slides were soaked in hematoxylin solution for 10 min after deparaffinization and then washed under running water for 30 min. Subsequently, the slides were stained with eosin, followed by 70% ethanol and xylene treatment. For immunostaining, the slides were subjected to antigen retrieval treatment using 10 mM sodium citrate buffer with 0.05% Tween20 after deparaffinization and permeabilization. Subsequently, the slides were blocked with 5% BSA and incubated with specific primary antibodies at 4°C overnight. After washing, the slides were incubated with appropriate fluorescence-conjugated secondary antibodies and mounted.
2.8 Lenti virus production for short hairpin RNA-medicated knockdown of Mga gene expression in GC2-spd cells
The sequences of oligonucleotides subcloned into the pLKO.1-puro lentiviral vector for short hairpin RNAs (shRNAs) against Mga expression and a scrambled control have been described previously (Suzuki et al., 2016). To produce the viruses for each specific shRNA, vectors for the knockdown of Mga expression and that for the control were individually introduced into HEK293 cells together with psPAX2(Addgene) and pLP-VSVG (Invitrogen) plasmids using Lipofectamine 2000 (Invitrogen). GC2-spd cells were infected with the generated lentivirus in the presence of 8 ug/ml polybrene and then selected with 1 ug/ml puromycin for 6 days.
2.9 Quantitative PCR analysis
Total RNA was isolated using an RNeasy Mini kit from Qiagen based on the manufacturer's instructions. Next, cDNA was generated using ReverTra Ace qPCR RT Master Mix with gDNA remover which was then used for quantitative PCR on the StepOnePlus Real-Time PCR System (Applied Biosystems). A TaqMan-based reaction (Invitrogen) was used to quantify the expression levels of Max (Mm00476449_m1), Stra8 (Mm00486473_m1), and Meiosin (Mm00473176_m1). The expression levels of Mga, Stag3 and Zcwpw1 genes were quantified using the SYBR Green-based method. The oligonucleotides used for Mga knockdown were the same as those previously described (Mga sh2) (Suzuki et al., 2016), whereas the oligonucleotides used for knockdown of Stag3 and Zcwpw1 genes were as follows.
Stag3: 5′-GCA AGC AGC TGA CCC GAC T-3′ (forward)
5′-GGT CCA ACC CAT GCT CAC TGG-3′ (reverse).
Zcwpw1: 5′-CTG GGA GGG AAG GAA GAG CA-3′ (forward).
5′-CTC CTC AGA TAA GTT GGT GGC TGA-3′ (reverse).
3 RESULTS AND DISCUSSION
3.1 Deletion of a specific exon that is exclusively used for Mga variant mRNA
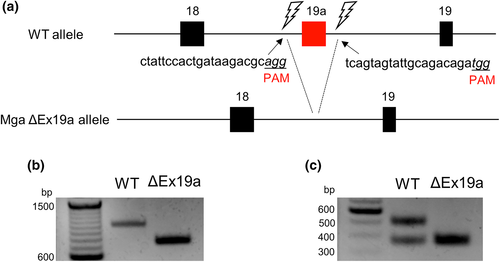
We have previously demonstrated that Mga SV mRNA is generated rather specifically in meiotic and post-meiotic germ cells by alternative splicing, in which a specific portion of the 18th intron of the Mga locus (designated as Mga Exon 19a) is used as the variant-specific exon (Kitamura et al., 2021). To decipher the physiological importance of this alternative splicing in gametogenesis, mice lacking the Mga Exon19a sequence in the genome were generated using the CRISPR Cas9 system (Figure 1a). Two different guide RNAs were microinjected into fertilized eggs along with Cas9 endonuclease so that double-strand breaks were incorporated into both the upstream and downstream sides of Mga Exon 19a. Next, the genetically manipulated oocytes were transferred to pseudopregnant mice to generate genome-edited mice. These procedures yielded a total of 35 pups. PCR and subsequent DNA sequence analyses revealed that, although abnormal genetic alterations that were assessed by unexplainable length of PCR products occurred in six pups, exactly designed heterozygous and homozygous deletion of the Mga Exon 19a genomic sequence (Mga ΔEx19a) occurred in four and 19 pups, respectively, while no alteration occurred in six pups (Figure 1b, Figure S1). Both heterozygous and homozygous mutant mice were viable, indicating that the exon 19a sequence essential for the production of Mga SV mRNA is dispensable for mouse viability. RT-PCR with RNA from the testes of wild-type and homozygous mutant mice showed that Mga SV mRNA carrying the Mga Exon 19a sequence is present only in wild-type mice, whereas canonical Mga mRNA missing the Mga Exon 19a sequence was present in both wild-type and Mga ΔEx19a mutant mice (Figure 1c), validating that the mutant mice are defective in alternative splicing for producing Mga SV mRNA.
3.2 Loss of Mga SV mRNA does not result in any apparent abnormalities in spermatogenesis
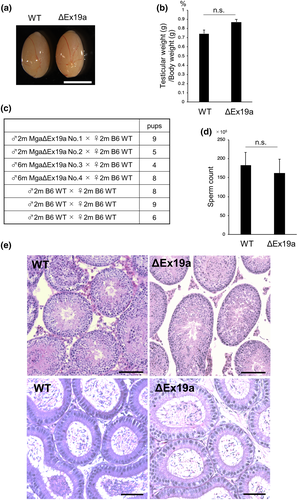
Since deletion of the exon 19a sequence did not affect apparent viability, we explored the possible role of Mga SV mRNA in spermatogenesis. To this end, one of the heterozygous mutant mice that was originally obtained was backcrossed with wild-type C57BL/6J mice three times. Homozygous mutant mice used for the rest of the studies were generated by intercrossing descendants of the original heterozygous mutant mouse. Comparison of testis size between wild-type and homozygous Mga ΔEx19a mutant mice did not show an apparent difference between them (Figure 2a,b). Next, we examined the fertility of male homozygous mutant mice by mating them with wild-type female mice. This intercross yielded reasonable numbers of viable newborn mice (Figure 2c). Homozygous Mga ΔEx19a mutant mice were indistinguishable from wild-type mice in terms of the amount of mature sperm in the epididymis (Figure 2d). Furthermore, histological analyses of HE-stained testis sections revealed no apparent differences between wild-type and homozygous Mga ΔEx19a mutant mice (Figure 2e). Taken together, these results indicate that homozygous Mga ΔEx19a mutant male mice are equivalent to wild-type mice in terms of fertility. We also examined the fertility of female mutant mice by crossing homozygous mutant female mice with wild-type male mice. Again, this intercross did not show any apparent abnormalities in viability or number of pups, indicating that the production of Mga SV mRNA is also dispensable for oogenesis (Figure S2).
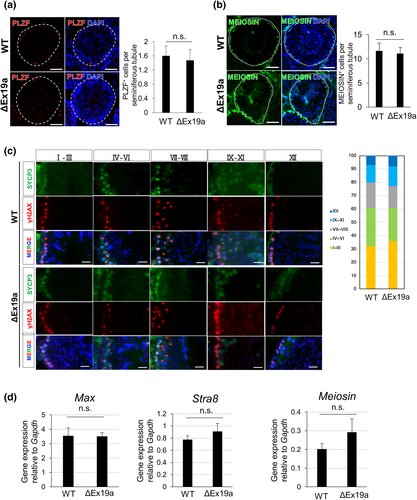
3.3 Deprivation of Mga SV mRNA does not affect the spermatogenic cycle
We further examined whether Mga SV mRNA is dispensable for normal spermatogenesis. To this end, we examined whether homozygous Mga ΔEx19a male mutant mice showed any abnormalities in the spermatogenic cycle. We first compared the number of undifferentiated spermatogonia that were positive for PLZF (Rossi, 2013) between wild-type and homozygous Mga ΔEx19a mutant mice within seminiferous tubules of the testes. These analyses revealed no statistically significant differences between wild-type and homozygous Mga ΔEx19a mutant mice (Figure 3a). MEIOSIN was recently identified as a protein that plays a crucial role in meiotic onset by interacting with STRA8 (Ishiguro et al., 2020). Therefore, we compared the number of MEIOSIN-positive cells within a section of the seminiferous tubule between the groups by immunohistochemistry. Similarly, these analyses revealed no appreciable difference in the number of MEIOSIN-positive cells between the wild-type and homozygous mutant mice (Figure 3b). Since the mouse seminiferous cycle comprises 12 stages (I-XII) (de Rooij, 1998), we next examined whether there were any differences in the relative distribution of each stage in the homozygous mutant mice compared to that of wild-type mice. To this end, we conducted co-immunohistochemistry on testis sections from wild-type and homozygous mutant mice with antibodies against SYCP3 and γH2AX. Although the actual stage of individual seminiferous tubule sections cannot be determined unequivocally, this procedure allows for the classification of at least five groups: I–III, IV-VI, VI–VIII, IX-XI, and XII. Therefore, we compared the proportions of these five different stage groups between the wild-type and homozygous mutant mice. Again, there was no apparent difference in the proportion of these groups between the wild-type and homozygous mutant mice (Figure 3d, Figure S3). Next, we examined whether there was any indication of a delay in meiotic onset among homozygous mutant mice. Since meiotic onset in male germ cells occurs around postnatal day 10 (P10) in testes, we compared the expression levels of two key players of meiotic onset, Stra8 and Meiosin, and Max which decreases its expression levels immediately prior to meiotic entry between wild-type and mutant mice. Our quantitative PCR analyses revealed that the expression levels of these three genes in mutant mice were comparable to those in wild-type mice, suggesting that the timing of meiotic onset in mutant mice was not delayed (Figure 3d). Taken together, these results indicate that Mga SV mRNA generated by alternative splicing with the exon 19a sequence is dispensable for spermatogenesis. Next, to exclude the possibility that Mga itself is not related to meiotic gene expression in germ cells, we conducted a lentivirus-mediated knockdown of Mga gene expression in GC2-spd cells, which are immortalized spermatogenic cell lines. These analyses revealed that the decrease in Mga expression levels by knockdown was accompanied by significant upregulation of meiosis-related genes (Figure S4), confirming that Mga is involved in the repression of meiosis-related genes in germ cells and ESCs.
In summary, our data revealed that the production of this Mga variant was dispensable for both viability and fertility. These data indicate that alternative splicing that generates Mga SV mRNA has no physiological significance on meiotic onset. However, it is also possible that alternative mechanisms other than the production of anomalous MGA protein which functions as a dominant negative regulator against the construction of PRC1.6, may be operating in parallel and therefore inactivation of only one of the mechanisms is not accompanied by obvious abnormalities. Indeed, we have previously demonstrated that MAX, which constitutes the core of PRC1.6 with MGA, shows a significant reduction in expression levels at the time of meiotic onset physiologically (Suzuki et al., 2016). Therefore, we plan to generate mice that produce constant amounts of MAX protein during the meiotic process by genetic manipulation and then examine the consequences of the combination of this mutation with the mutation generated in this study (loss of alternative splicing for Mga SV transcript generation). In parallel, we intend to explore the possibility of additional mechanisms that are involved in the inactivation and/or attenuation of the transcriptional repressing activity of PRC1.6 on meiosis-related genes.
ACKNOWLEDGMENTS
The authors thank to Drs. Takeshi Kajihara and Yumi Mizuno at Saitama Medical University, Japan for helpful discussion. Anti-MEIOSIN antibody was kindly provided by Dr. Kei-Ichiro Ishiguro at Kumamoto University, Japan. We also thank Kazumi Ubukata for his technical assistance. This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. K.U., A.S, and A.O. are recipients of grants from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers 20K16147, 21K06843, and 19H03426, respectively). This work was also supported in part by the Research Fellowship for Young Scientists (DC2) from JSPS to Y.K (Grant Number 20G10148).
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article or its supplementary information files.