Establishment of knockout adult sea urchins by using a CRISPR-Cas9 system
Abstract
Sea urchins are used as a model organism for research on developmental biology and gene regulatory networks during early development. Gene knockdown by microinjection of morpholino antisense oligonucleotide (MASO) has been used to analyze gene function in early sea urchin embryos. However, as the effect of MASO is not long lasting, it is impossible to perturb genes expressed during late development by MASO. Recent advances in genome editing technologies have enabled gene modification in various organisms. We previously reported genome editing in the sea urchin Hemicentrotus pulcherrimus using zinc-finger nuclease (ZFN) and transcription activator-like effector nuclease (TALEN); however, the efficiencies of these technologies were not satisfactory. Here, we applied clustered regularly interspaced short palindromic repeat (CRISPR)-CRISPR-associated nuclease 9 (Cas9) technology to knock out the Pks1 gene in H. pulcherrimus. When sgRNAs targeting Pks1, which is required for the biosynthesis of larval pigment, were microinjected into fertilized eggs with SpCas9 mRNA, high-efficiency mutagenesis was achieved within 24 hr post fertilization and SpCas9/sgRNA-injected pluteus larvae had an albino phenotype. One of the sgRNAs yielded 100% mutagenesis efficiency, and no off-target effect was detected. In addition, the albino phenotype was maintained in juvenile sea urchins after metamorphosis, and the knockout sea urchins survived for at least one year and grew to albino adult sea urchins. These findings suggest that knockout adult sea urchins were successfully established and the CRISPR-Cas9 system is a feasible method for analyzing gene functions from late developmental to adult stage.
1 INTRODUCTION
Sea urchins are a model organism for research on developmental biology and has been recently used for the analysis of gene regulatory networks during early development (Davidson et al., 2002; Oliveri & Davidson, 2004; Oliveri, Tu, & Davidson, 2008). Sea urchins are invertebrate deuterostomes, which is a sister group of the chordates. Investigation of the sea urchin genome is important to understand the origin of vertebrate gene functions. The first sea urchin genome sequenced was that of Strongylocentrotus purpuratus (Sea Urchin Genome Sequencing Consortium, 2006). Hemicentrotus pulcherrimus, which is closely related to S. purpuratus, is widely distributed in Japan and also has been used for research on developmental biology. The H. pulcherrimus genome was deciphered in 2018 (Kinjo, Kiyomoto, Yamamoto, Ikeo, & Yaguchi, 2018). The genome size of H. pulcherrimus was estimated to be 800 Mbp, with approximately 25,000 genes.
Gene functional analysis in the sea urchin embryo has relied on gene knockdown by morpholino antisense oligonucleotide (MASO), which blocks translation or RNA splicing of gene transcripts of interest (Angerer & Angerer, 2004). However, the effect of MASO-mediated knockdown is not long lasting. Therefore, it is not possible to perturb gene expression during late developmental stages by MASO.
Recent advances in genome editing technologies have enabled genome modification in various organisms. In recent years, genome editing technologies have been used in sea urchin embryos to knockout genes that are expressed during early development (Hosoi, Sakuma, Sakamoto, & Yamamoto, 2014; Lin & Su, 2016; Mellott, Thisdelle, & Burke, 2017; Ochiai et al., 2010; Oulhen, Swartz, Laird, Mascaro, & Wessel, 2017; Oulhen & Wessel, 2016). Upon targeted genome editing using zinc-finger nuclease (ZFN) in H. pulcherrimus embryos, approximately 10% of ZFN mRNA-injected embryos showed an affected phenotype (Ochiai et al., 2010). Upon targeted genome editing with transcription activator-like effector nuclease (TALEN) in H. pulcherrimus embryos, 12.6% of TALEN pair-injected embryos showed an affected phenotype (Hosoi et al., 2014). These rates of the affected phenotypes are not satisfactory, and a higher mutagenesis efficiency is indispensable for detailed analysis of gene functions during early development of H. pulcherrimus. Moreover, as the adult rudiment is produced from the left side of the coelomic pouch that originates from small micromere and macromere descendants (Cameron, Fraser, Britten, & Davidson, 1991; Cameron, Hough-Evans, Britten, & Davidson, 1987), a higher mutagenesis efficiency is also required for gene functional analysis during late developmental stages. Compared to ZFN and TALEN, which rely on protein/DNA recognition, the CRISPR-Cas9 system uses a single guide RNA (sgRNA), which is more efficient, convenient, and cost-effective (Doudna & Charpentier, 2014). Targeted genome editing using a CRISPR-Cas9 system has been reported in S. purpuratus (Lin & Su, 2016; Mellott et al., 2017; Oulhen & Wessel, 2016; Oulhen et al., 2017), but not in H. pulcherrimus. Furthermore, the effect of genome editing in adult sea urchin after metamorphosis has never been studied.
Polyketide synthases (PKSs) are a large group of enzymes responsible for the biosynthesis of polyketide compounds (Castoe, Stephens, Noonan, & Calestani, 2007; Hopwood, 1997; Hopwood & Sherman, 1990; Staunton & Weissman, 2001). Sea urchins have two PKS genes, Pks1 and Pks2 (Castoe et al., 2007). The onset of Pks1 expression occurs in the blastula stage, and Pks1 expression is restricted to secondary mesenchyme cell (SMC) precursors at the vegetal pole of the embryo and larval pigment cells, which are derived from SMCs (Barsi, Tu, Calestani, & Davidson, 2015; Calestani, Rast, & Davidson, 2003). Pks1 is required for the biosynthesis of the naphthoquinone pigment echinochrome (Griffiths, 1965). Knockdown of Pks1 by MASO resulted in albino embryos (Calestani et al., 2003). Pks2 expression is restricted to skeletogenic cells and their precursors (Beeble & Calestani, 2012), and Pks2 plays a critical role in the formation of calcareous larval skeletons (Hojo et al., 2015).
In this study, we applied the CRISPR-Cas9 system in H. pulcherrimus. We constructed sgRNAs targeting H. pulcherrimus Pks1 (HpPks1). High-efficiency mutagenesis was detected in sgRNA-injected embryos within 24 hr post fertilization (hpf). The mutation rate of one of the sgRNAs reached 100%, and an albino phenotype was observed in all sgRNA-injected embryos. The albino phenotype was maintained in adult sea urchins after metamorphosis, indicating successful establishment of knockout adult sea urchin. These results suggest that the CRISPR-Cas9 system is an effective gene knockout technology in H. pulcherrimus and can be used for the analysis of late developmental processes.
2 MATERIALS AND METHODS
2.1 Sea urchin culture
Adult sea urchins (H. pulcherrimus) were collected from the Seto Inland Sea or Tateyama Bay. Eggs and sperm were obtained by coelomic injection of 0.55 M KCl. Fertilized eggs were cultured in filtered seawater at 16°C. Larvae were fed the microalgae Chaetoceros gracilis and were cultured under rotation. Chaetoceros gracilis was purchased from I.S.C. Co., Ltd. and cultured in filtered seawater supplemented with 1/1,000 volume of marine algae culture medium (KW21; Daiichi Seimo Co., Ltd.) and sodium metasilicate at 16°C under continuous illumination and aeration. After the metamorphosis, juvenile sea urchins were fed dried kelp and natural microalgae attached on oyster shells and cultured at 16°C.
2.2 Preparation and microinjection of sgRNA and Cas9 mRNA
SgRNAs targeting Pks1 were designed following Oulhen and Wessel (2016). Templates for sgRNA synthesis were assembled using a PCR-based strategy (Nakayama et al., 2014; Sakane, Suzuki, & Yamamoto, 2017). Briefly, sgRNA-fwd and reverse-sgRNA were annealed and served as primers for fill-in extension, and the resulting template was amplified with T7-sgRNA-fwd and sgRNA-rev using KOD FX Neo (Toyobo) and purified using a QIAquick PCR Purification Kit (Qiagen). Subsequently, sgRNAs were transcribed using a MEGAshortscript T7 Kit (Thermo Fisher Scientific) and purified using a RNeasy Mini Kit (Qiagen). The nucleotide sequences of all oligonucleotides used in the sgRNA preparations are listed in Table S1.
Humanized codon-optimized SpCas9 cDNA from pX330 (plasmid #42230, Addgene; Cong et al., 2013) was subcloned into a pGreenLantern-derived plasmid, and two SV40 nuclear localization signals were inserted at the 3′ end of the SpCas9 sequence. After linearization of this vector with SpeI, SpCas9 mRNA was synthesized using an mMESSAGEmMACHINE T7 Ultra kit (Thermo Fisher Scientific) and purified using a RNeasy Mini Kit.
Microinjection was carried out as described by Rast (2000) with some modifications. SpCas9 mRNA and sgRNA were dissolved in 30% glycerol at 750 and 150 ng/μl, respectively, and two pl of RNA solution was microinjected into fertilized eggs.
Images of larvae were acquired with an epifluorescence microscope BX50 (Olympus) equipped with a DP72 digital camera and analyzed with DP2-BSW software (Olympus). To obtain a focused image of each larva, Z-stacks of each individual embryo were processed in FIJI software using the Stack Focuser plug-in. After metamorphosis, images of juvenile and adult sea urchins were acquired with a JVC GC-PX1 digital camera (Victor Corporation) mounted on a Leica MZ75 stereo microscope (Leica).
2.3 Isolation of genomic DNA
Genomic DNA was isolated using a simple method described by Lin and Su (2016). At 24 hr post fertilization (hpf), 20 embryos were incubated in 8 μl of lysis buffer (1× NEB#2 buffer; New England Biolabs) at 94°C for 10 min, and then cooled down to 4°C for 10 min. After addition of 1 μl of 18.6 mg/ml proteinase K, PCR Grade (Roche), the sample was incubated at 55°C for 2 hr. After subsequent incubation at 94°C for 10 min to inactivate proteinase K, 8 μl of TE buffer (10 mM Tris-HCl (pH 8.0), 1 mM EDTA) was added for use in PCR.
For extraction of genomic DNA from whole 5-month-old adult sea urchin, an individual sea urchin was added to 30 μl of lysis buffer in a 1.5-ml tube and homogenized. After incubation at 94°C for 10 min and then at 4°C for 10 min, 1 μl of 18.6 mg/ml proteinase K was added and the sample was incubated at 55°C for 2 hr. After inactivation of proteinase K, 30 μl TE buffer was added.
2.4 Heteroduplex mobility assay (HMA) and DNA sequencing analysis
For HMA, target regions were amplified from 1 μl of isolated genomic DNA solution in 35 PCR cycles using KOD FX Neo. The PCR products were separated on a 0.5% agarose gel supplemented with resolution enhancer (patent applied; Dr. Masanobu Obara in Hiroshima University, GeLBio LLC) or 3% agarose gel in 1×TAE buffer (40 mM Tris–acetate, 1 mM EDTA) with mini-sized gel electrophoresis apparatus (Mupid, ADVANCE Co., Ltd.) and stained with ethidium bromide. Nucleotide sequences of the primers used to amplify each target region are listed in Table S2.
For Sanger sequencing, PCR products obtained by 27 PCR cycles were subcloned into the pBluescript SK(−) vector or pTA2 vector (TArget Clone -Plus-; Toyobo). Positive clones were detected by colony PCR and sequenced with the M13 forward or M13 reverse primer using a BigDye Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific).
3 RESULTS
3.1 CRISPR-Cas9-mediated mutagenesis of HpPks1
To evaluate the functionality of the CRISPR-Cas9 system in H. pulcherrimus, we targeted the H. pulcherrimus polyketide synthase Pks1 (HpPks1) because knockout of Pks1 produces an easily observable albino phenotype as recently reported in the closely related sea urchin species, S. purpuratus (Oulhen & Wessel, 2016). To obtain the nucleotide sequence of HpPks1, we searched the H. pulcherrimus Genome and Transcriptome database (HpBase, http://cell-innovation.nig.ac.jp/Hpul/) and we found that the HpPks1 locus is located on scaffold 824.
In this study, we tested three sgRNAs targeting the second coding exon of HpPks1 (Figure 1a, Figure S1) that correspond to those used in S. purpuratus (Oulhen & Wessel, 2016). Although the preceding study using S. purpuratus introduced a combination of three sgRNAs (Oulhen & Wessel, 2016), we used each single sgRNA in this study. The sgRNAs were microinjected with SpCas9 mRNA into fertilized eggs of H. pulcherrimus, and genomic DNA was extracted at 24 hr post fertilization (hpf) from 20 embryos injected with SpCas9/sgRNA or SpCas9 alone. CRISPR-Cas9-mediated mutagenesis was examined by HMA. In the PCR product from embryos injected with SpCas9 alone (control), a clear and distinct band of the expected size was detected for each target site (Figure 1b,c). When sgRNA#1, which corresponds to Sp.PKS1.175(+), was microinjected, no band shift was detected (Figure 1b). However, the PCR products from embryos injected with either sgRNA#2, corresponding to Sp.PKS1.547(−), or sgRNA#3, corresponding to Sp.PKS1.806(−), produced shifted bands, representing heteroduplexes formed by mutated alleles (Figure 1c), indicating that injection of either sgRNA#2 or sgRNA#3 introduced mutations at the target site within HpPks1.
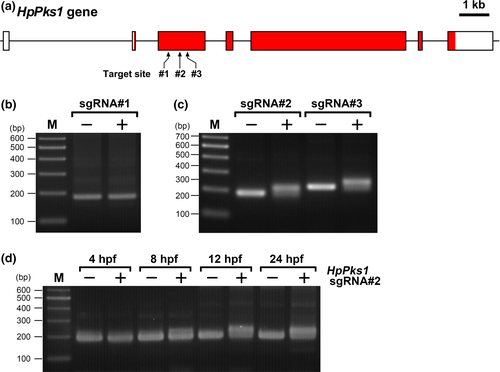
To examine the timing of SpCas9/sgRNA-mediated mutagenesis during sea urchin embryogenesis, HMA was performed at different time points after fertilization using SpCas9/sgRNA#2-injected embryos. The band shift was not detected at 4 hpf, and was first detected at 8 hpf in SpCas9/sgRNA#2-injected embryos. The intensity of the shifted band increased to a maximum at 12 hpf (Figure 1d). Furthermore, in a detailed analysis, the shifted band representing the mutagenesis was first detected at 6 hpf in SpCas9/sgRNA#2-injected embryos (data not shown). These results suggested that the CRISPR-Cas9-induced mutations were introduced during the early stages of development (morula to early blastula stages) in H. pulcherrimus.
To analyze the types and efficiency of mutations induced by CRISPR-Cas9-mediated genome editing, the PCR amplicons were subcloned and sequenced. Among 17 sequenced clones from sgRNA#2-injected embryos, deletions (15 clones) and deletion/insertion (two clones) were observed, and thus, the mutation rate was 100% (Figure 2a). However, as many clones showed deletions of three or multiples of three nucleotides, the frameshift rate was 17.6%. On the other hand, among 15 sequenced clones from sgRNA#3-injected embryos, deletion (seven clones), substitution (one clone), insertion (one clone), and deletion/insertion (three clones) were observed (Figure 2b), indicating a mutation rate of 80%, with 40% being frameshift mutations. Furthermore, deletions seemed to be caused not only by nonhomologous-end joining, but also by microhomology-mediated end joining (Figure 2, underline).

3.2 Off-target analysis in HpPks1 sgRNA-injected embryos
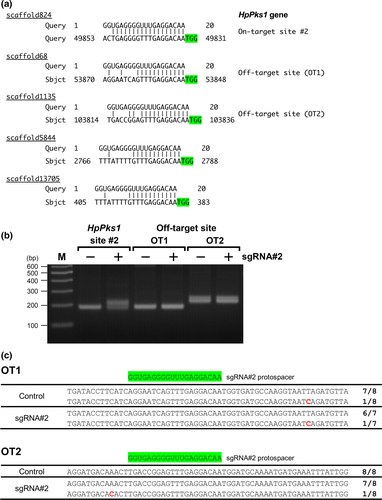
As programmable nucleases used in genome editing can induce undesirable off-target effects, we assessed off-target effects in CRISPR-Cas9-mediated mutagenesis targeting the HpPks1 gene in H. pulcherrimus. To find potential off-target sites for sgRNA#2 and #3, we searched for homologous sequences of the sgRNA target sites in H. pulcherrimus genome in HpBase. We did not identify any homologous sequence of the sgRNA#3 target site. However, potential off-target sites for sgRNA#2 were detected, and four of them (in scaffolds 68, 1135, 5844, and 13705) showed sequence identity in the PAM sequence and 12 nucleotides 3′ of the PAM sequence (Figure 3a). Previous studies have shown that a 12-nucleotide seed region of a sgRNA adjacent to the PAM site is more important than the rest of the target sequence for specific binding (Jiang, Bikard, Cox, Zhang, & Marraffini, 2013; Larson et al., 2013). We carried out HMA and sequencing analyses for two of these potential off-target sites; we did not detect any mutations in these potential off-target sites (Figure 3b,c), suggesting that CRISPR-Cas9-mediated mutagenesis targeting HpPks1 in this study was highly specific to the target site.
3.3 Albino phenotype in HpPks1 sgRNA-injected larvae
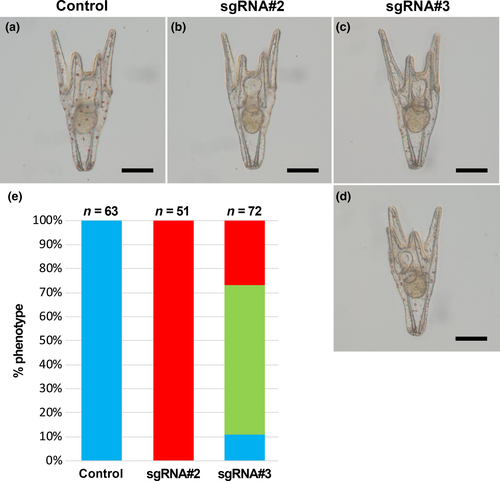
We analyzed the phenotype of the sgRNA-injected embryos. All of the control embryos injected with SpCas9 mRNA alone developed normally (Figure 4a). The sgRNA#1-injected embryos also developed normally and were morphologically indistinguishable from control embryos (data not shown). However, when either sgRNA#2 or sgRNA#3 was injected, pigment deficiency was observed in larvae in the pluteus stage (Figure 4b–d). All of the sgRNA#2-injected pluteus larvae exhibited complete loss of pigment (Figure 4b,e). On the other hand, 28% of sgRNA#3-injected larvae exhibited complete loss of pigment (Figure 4c,e) and 60% exhibited partial loss of pigment (Figure 4d,e). These results indicated that the HpPks1 gene was disrupted in the SpCas9/sgRNA-injected embryos and that gene knockout by the introduction of sgRNA#2 and sgRNA#3 resulted in pigment deficiency.
 Normal pigmentation;
Normal pigmentation;  Partial loss of pigmentation;
Partial loss of pigmentation;  Complete loss of pigmentation
Complete loss of pigmentation3.4 Effect of HpPks1 knockout in late pluteus larvae and adult sea urchins
To gain insights into the effects of CRISPR-Cas9-mediated knockout on late developmental processes of H. pulcherrimus and to establish knockout adult sea urchin, control and SpCas9/sgRNA-injected larvae were further cultured by feeding them the microalgae C. gracilis. The sgRNA#2 or sgRNA#3-injected larvae grew normally and their albino phenotype was maintained during late larval development (Figure 5a–c). Adult rudiment was normally formed on the left side of the larval body (Figure 5d–f) and grew similarly as in the control (Figure 5g–i). In addition, sgRNA-injected larvae showed pigment deficiency not only in the larval body, but also in the adult rudiment. After metamorphosis, sgRNA-injected juvenile sea urchin also exhibited pigment deficiency (Figure 5k,l). In control juvenile sea urchin, pigmentation was normal (Figure 5j).

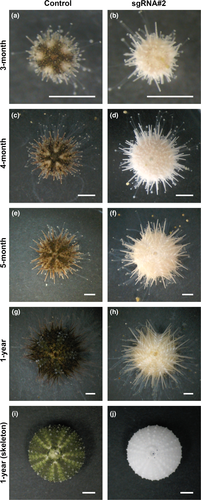
We further monitored the growth of control and sgRNA#2-injected knockout individuals (Figure 6). Whereas 3-month-old adult control sea urchins showed an obvious five-fold symmetric pigmentation pattern, HpPks1-knockout adult sea urchins showed an albino phenotype (Figure 6a,b). Both control and HpPks1-knockout individuals grew normally to adulthood, but the HpPks1-knockout individuals did not exhibit pigmentation on the surface, spines, and tube feet (Figure 6c–f). HpPks1-knockout urchins survived until 1 year of age and grew to more than 1 cm in diameter, and 1-year-old HpPks1-knockout individuals still showed the albino phenotype (Figure 6g,h). Skeletons of HpPks1-knockout adult sea urchins also showed pigment deficiency (Figure 6i,j), suggesting that sgRNA#2-mediated knockout was effective in the entire body.
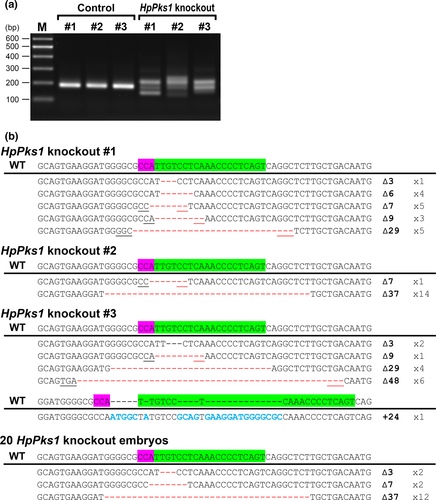
To analyze genotypic variation in individual adult sea urchins, genomic DNA was extracted from three control and three HpPks1-knockout sea urchins of 5 months old. HMA and sequencing analyses revealed that HpPks1-knockout individuals lacked the wild-type allele, whereas each HpPks1-knockout individual had various types of mutations (Figure 7a,b). These results indicated that HpPks1-knockout adult sea urchins were successfully established.
4 DISCUSSION
In this study, we successfully disrupted the HpPks1 gene in the sea urchin H. pulcherrimus using the CRISPR-Cas9 system and established knockout adult sea urchin. The albino phenotype was maintained in HpPks1-knockout adult sea urchin, suggesting that Pks1 is required for pigmentation not only in pluteus larvae, but also in adult sea urchin, and that polyketide compounds are involved in the colorful pigmentation of adult sea urchins.
The preceding study using S. purpuratus introduced a combination of three sgRNAs targeting the Pks1 gene and resulted in efficient mutagenesis and larval albino phenotype, but only a slight portion of embryos showed pigmentation (Oulhen & Wessel, 2016). A similar albino phenotype was reported using CRISPR-Cas9 and CRISPR-Cas9-deaminase by introduction of mixture of 16 sgRNAs targeting the Pks1 gene, but the efficiency was not so high (Shevidi, Uchida, Schudrowitz, Wessel, & Yajima, 2017). Whereas, in our study, we introduced single sgRNA, and the introduction of sgRNA#2 achieved 100% of mutagenesis efficiency and all injected embryos showed the complete loss of pigmentation. We speculate that mixing multiple sgRNA may result in dilution of the effect of highly efficient sgRNA and may increase the rate of undesirable effects, such as off-target effects. Therefore, usage of single highly efficient sgRNA may be desirable for efficient genome editing.
In previous studies, the knockout rates achieved with ZFN- and TALEN-mediated genome editing were 9.5% and 12.6%, respectively (Hosoi et al., 2014; Ochiai et al., 2010). Although the efficiencies cannot be simply compared because different target loci were used, the knockout efficiency of the CRISPR-Cas9 system was much higher than those of ZFN and TALEN in H. pulcherrimus. CRISPR-Cas9-mediated mutagenesis by sgRNA#2 targeting HpPks1 achieved a mutation rate of 100%, with all Cas9/sgRNA-injected embryos exhibiting the albino phenotype, suggesting that the CRISPR-Cas9 system can efficiently induce biallelic genome modifications in H. pulcherrimus embryos. Although the mutation rate of sRNA#2 was 100%, many alleles showed deletions of three or multiples of three nucleotides, and the frameshift rate was 17.6%. Nonetheless, all SpCas9/sgRNA-injected embryos showed the albino phenotype, indicating that the essential residue for HpPKS1 enzymatic function is located in the sRNA#2-targeted site. In other words, it is important to target the gene sequence encoding an essential residue, although the requirement of a PAM sequence may be a restriction for target sequence design in the CRISPR-Cas9 system.
We previously reported that mutations introduced by ZFN were weakly detected at 4 hpf and TALEN-mediated mutagenesis was not observed until 8 hpf (Hosoi et al., 2014; Ochiai et al., 2010). In this study, CRISPR-Cas9-mediated mutagenesis was detected as of 6 hpf. Therefore, the timing of CRISPR-Cas9 mediated genome editing is comparable to those of ZFN and TALEN. Ribonucleoprotein (RNP) consisting of the SpCas9 protein in complex with sgRNA has been used in cultured cells and animals (Kim, Kim, Cho, Kim, & Kim, 2014; Kotani, Taimatsu, Ohga, Ota, & Kawahara, 2015; Lee et al., 2014; Sakane et al., 2018; Shigeta et al., 2016; Sung et al., 2014); it shortens the timing of genome editing and overcomes the mosaic property (Kim et al., 2014; Kotani et al., 2015). We attempted to accelerate mutagenesis in H. pulcherrimus by using SpCas9 RNP. However, upon microinjection of SpCas9 RNP, we did not observe mutations in H. pulcherrimus (Figure S2). Although this may be because of the experimental conditions used, such as the concentration of SpCas9 protein and the ratio between SpCas9 protein and sgRNA, this may suggest that SpCas9 RNP may not be feasible for CRISPR-Cas9-mediated mutagenesis in sea urchin.
Off-target effects are a critical concern for genome editing tools. A mismatch in the seed sequence (12 nucleotides adjacent to the PAM sequence) disrupts target specificity, and SpCas9 tolerates single-base mismatches in the PAM-distal region to a greater extent than in the PAM-proximal region (Cong et al., 2013; Hsu et al., 2013). Furthermore, up to six contiguous mismatches in the 5′-terminal region of the protospacer are tolerated (Jinek et al., 2012), and CRISPR-Cas9 can induce mutations at off-target sites with up to five mismatches (Fu et al., 2013). By screening the H. pulcherrimus genome, we found four potential off-target sites of sgRNA#2 that showed sequence identity in the PAM sequence and 12 nucleotides 3′ of the PAM. Scaffold 68 (OT1) and scaffold 1135 (OT2) have six and five mismatches, respectively. However, HMA and sequencing analysis did not reveal any mutations induced by sgRNA#2 at these potential off-target sites (Figure 3b,c). A previous study showed that the maximum detection limit of HMA for CRISPR-Cas9-mediated mutant alleles was 0.5% in mouse pups and human cultured cells (Zhu et al., 2014). Although nucleotide variations were detected by sequencing, they are probably polymorphisms as they were positioned at a distance from the potential off-target sites and the sea urchin genome is highly polymorphic (Britten, Cetta, & Davidson, 1978; Sea Urchin Genome Sequencing Consortium, 2006; Yamamoto, Kawamoto, Fujii, Sakamoto, & Shibata, 2007). These findings suggest that sgRNA#2 is highly specific to HpPks1 and produces no detectable off-target effect.
During sea urchin larval development, the adult rudiment develops from left side of the coelomic pouch, which originates from small micromere and macromere descendants (Cameron et al., 1987, 1991). The amniotic invagination of the ectoderm merges with the coelomic pouches to form an adult rudiment (Smith, Cruz Smith, Cameron, & Urry, 2008). Consistent with the multiple cell lineages of the adult rudiment, F0 knockout adult sea urchins exhibited multiple genotypes even after metamorphosis (Figure 7). Therefore, to obtain adult sea urchins of a single knockout genotype, an F1 generation should be generated. Although relatively large deletions (29, 37 and 48 bps) were observed in the adult knockout sea urchins (Figure 7) compared with those in the knockout embryos (Figure 2). However, this difference is probably due to the variation between experimental batch because the same batch of Pks1 knockout embryos also showed large deletion (Figure 7).
In conclusion, we showed that the CRISPR-Cas9 system is a highly effective tool for genome editing in H. pulcherrimus. We successfully established not only knockout embryos, but also knockout adult sea urchins with an albino phenotype. Therefore, the CRISPR-Cas9 system can be used for the analysis of late developmental processes, such as the formation of adult rudiment, establishment of the five-fold symmetry of the adult body plan, and the mechanism of metamorphosis. Furthermore, albino adult sea urchins may be useful for the analysis of cell lineages and gene expression by use of fluorescent proteins during the morphogenesis of adult tissues.
ACKNOWLEDGMENTS
We thank Dr. Masato Kiyomoto (Tateyama Marine Laboratory, Ochanomizu University) for supplying live sea urchins and sea water. Special grade agarose was supplied by GeLBio LLC, Japan. Sequencing analysis was carried out in the Gene Science Division, Natural Science Center for Basic Research and Development, Hiroshima University. This work was partially supported by a Grant-in-Aid for Scientific Research (C) (JSPS KAKENHI Grant Number JP17K07241) to NS.
AUTHOR CONTRIBUTIONS
NS conceived and designed the experiments. DL and NS conducted the experiments. AA and TS provided instructions. DL and NS wrote the manuscript with support from all authors. TY supervised the work.




