Par-aPKC-dependent and -independent mechanisms cooperatively control cell polarity, Hippo signaling, and cell positioning in 16-cell stage mouse embryos
Abstract
In preimplantation mouse embryos, the Hippo signaling pathway plays a central role in regulating the fates of the trophectoderm (TE) and the inner cell mass (ICM). In early blastocysts with more than 32 cells, the Par-aPKC system controls polarization of the outer cells along the apicobasal axis, and cell polarity suppresses Hippo signaling. Inactivation of Hippo signaling promotes nuclear accumulation of a coactivator protein, Yap, leading to induction of TE-specific genes. However, whether similar mechanisms operate at earlier stages is not known. Here, we show that slightly different mechanisms operate in 16-cell stage embryos. Similar to 32-cell stage embryos, disruption of the Par-aPKC system activated Hippo signaling and suppressed nuclear Yap and Cdx2 expression in the outer cells. However, unlike 32-cell stage embryos, 16-cell stage embryos with a disrupted Par-aPKC system maintained apical localization of phosphorylated Ezrin/Radixin/Moesin (p-ERM), and the effects on Yap and Cdx2 were weak. Furthermore, normal 16-cell stage embryos often contained apolar cells in the outer position. In these cells, the Hippo pathway was strongly activated and Yap was excluded from the nuclei, thus resembling inner cells. Dissociated blastomeres of 8-cell stage embryos form polar–apolar couplets, which exhibit different levels of nuclear Yap, and the polar cell engulfed the apolar cell. These results suggest that cell polarization at the 16-cell stage is regulated by both Par-aPKC-dependent and -independent mechanisms. Asymmetric cell division is involved in cell polarity control, and cell polarity regulates cell positioning and most likely controls Hippo signaling.
Introduction
Before implantation in the uterus, mouse embryos undergo several rounds of cell division and form a cyst-like structure called the blastocyst (Yamanaka et al. 2006; Sasaki 2010, 2015). The early blastocyst contains two types of cells, the trophectoderm (TE) and the inner cell mass (ICM). The TE is an outer epithelial structure required for implantation that later gives rise to placental tissues, whereas the ICM is a mass of pluripotent cells surrounded by the TE that later forms the embryo proper and some extraembryonic tissues.
Formation of the TE and ICM is the first cell fate specification in mouse development, and it has been the focus of scientific investigation for decades. Over the course of these studies, two models have emerged as central concepts in this field. One model is the “inside-outside (positional) model” proposed by Tarkowski (Tarkowski & Wroblewska 1967), in which the position of the cells within the embryo determines the eventual fate of the cells; the outer layer of cells become the TE, and the inner cells become the ICM. The other model is the “polarity model” proposed by Johnson (Johnson et al. 1981), in which cell polarization at the 8-cell stage and asymmetric inheritance of cell polarity during cell division regulates cell fate. At the 8-cell stage, embryos undergo compaction, and cells establish apicobasal cell polarity (Johnson & McConnell 2004). In the next two rounds of cell division, the divisions are symmetric or asymmetric, contingent on the inheritance of the apical membrane (Graham & Deussen 1978; Johnson & Ziomek 1981a; Pedersen et al. 1986; Fleming 1987). In symmetric division, both daughter cells inherit the apical membrane, which is characterized by the presence of microvilli and maintenance of cell polarity. In asymmetric division, only one daughter cell inherits the apical membrane and maintains cell polarity, whereas the other loses the apical membrane and becomes apolar. Recently, a newer version of the polarity model was proposed, in which the presence of cell polarity or the apical domain regulates cell fate by controlling gene expression independent of cell division (Yamanaka et al. 2006).
Recent studies have revealed that Hippo signaling plays a central role in this cell-fate specification process and have provided a molecular basis for the historical models (Nishioka et al. 2009; Cockburn et al. 2013; Hirate et al. 2013; Leung & Zernicka-Goetz 2013; Hirate & Sasaki 2014; Wicklow et al. 2014). Activation of the Hippo signaling pathway depends on the position of the cells; Hippo signaling is active in the inner cells and inactive in the outer cells. In the outer cells, inactive Hippo signaling promotes nuclear accumulation of the coactivator proteins Yap1 and Taz (hereafter collectively designated as Yap), which activates the transcription factor Tead4 by forming a Tead4-Yap complex. Active Tead4 induces the TE-specific transcription factor Cdx2, and expression of Cdx2 promotes TE development (Niwa et al. 2005; Strumpf et al. 2005; Dietrich & Hiiragi 2007; Ralston & Rossant 2008). In contrast, in the inner cells, activation of the Hippo pathway inhibits nuclear accumulation of Yap, and Tead4 remains inactive. As Tead4 does not induce Cdx2, these cells adopt the alternative ICM fate. This cell position-dependent Hippo signaling provides evidence for the inside-outside model.
Studies at the 32-cell stage have revealed that position-dependent Hippo signaling is established through a combination of cell–cell adhesion and cell polarity. The inner cells are apolar; therefore, cell–cell adhesion activates the Hippo pathway. At adherens junctions (AJs), a scaffold protein angiomotin (Amot), which associates with the intracellular domain of E-cadherin through catenins and the tumor suppressor Nf2/Merlin, strongly interacts with the protein kinase Lats1/2 and activates the Hippo pathway (Cockburn et al. 2013; Hirate et al. 2013; Hirate & Sasaki 2014). The outer cells have apicobasal polarity, and cell polarity regulated by the Par-aPKC system (Suzuki & Ohno 2006) sequesters Amot from basolateral AJs to the apical domains (Hirate et al. 2013; Hirate & Sasaki 2014). Due to the absence of Amot in the E-cadherin complex, cell–cell adhesion does not activate the Hippo pathway. Regulation of Hippo signaling by cell polarity indicates that the newer version of the polarity model can explain the molecular events that unfold at the 32-cell stage.
Although the mechanisms that control position-dependent Hippo signaling at the 32-cell stage have been extensively studied, it is unclear if these mechanisms also operate at earlier stages. It is important to elucidate the mechanisms acting at the 16-cell stage, because inner cells are first formed during the 8- to 16-cell transition (Morris et al. 2010). Recently, a correlation was reported between cell polarity and Hippo signaling in 16-cell stage embryos (Anani et al. 2014). However, the roles of cell polarity in Hippo signaling have not been directly examined.
In this study, we investigated the role of cell polarity at the 16-cell stage. Disruption of the Par-aPKC system had limited effects on cell polarity and regulation of the Hippo signal/Yap. We also found that 16-cell stage embryos often contain apolar and Hippo-active cells in the outer position. These results highlight the differences in the regulation of cell polarity and Hippo signaling between 16- and 32-cell stage embryos. The relationship between these results and historical models of embryo development is also discussed.
Materials and methods
Mouse lines
Wild-type embryos were obtained by crossing F1 (C57BL/6xDBA/2; BDF1) female mice with C57BL/6 or BDF1 male mice. Mice were housed in environmentally controlled rooms in the Laboratory Animal Housing Facility of the RIKEN Center for Developmental Biology (CDB) and the Center for Animal Resources and Development (CARD) of Kumamoto University. All experiments were carried out according to the regulations for animal and recombinant DNA experiments of RIKEN CDB and Kumamoto University and the laws and protocols outlined by the Japanese government. All studies were approved by the institutional committees for animal and recombinant DNA experiments at the RIKEN CDB and Kumamoto University.
Embryo culture and embryo manipulation
One- or two-cell stage embryos were recovered from oviducts following standard protocols (Hogan et al. 1994) and were cultured as previously described (Nishioka et al. 2008). Dissociation of 8-cell stage embryos was performed as previously described (Suwinska et al. 2008).
RNA injection
Poly(A)-tailed RNA was injected into both blastomeres of 2-cell stage embryos as previously described (Nishioka et al. 2009). For dominant-negative experiments, RNA encoding dnPKCλ was injected at a concentration of 400 ng/μL.
Gene knockdown by injection of shRNA-encoding plasmids
Gene knockdown was achieved by pronuclear injection of verified shRNA plasmids (Sigma-Aldrich, MO, USA), as described previously (Alarcon 2010; Hirate et al. 2013). Plasmids were purified by electrophoresis on a low melting point agarose gel, followed by agarose digestion with a Thermostable β-agarase (Wako pure chemical industries, Osaka, Japan) (Hogan et al. 1994). The purified plasmid DNA solution (10 ng/μL) was injected into male pronuclei according to standard protocols (Hogan et al. 1994).
Immunofluorescent staining
Immunofluorescent staining of preimplantation embryos was performed as previously described (Nishioka et al. 2009), with slight modifications. Primary antibodies against Yap (Abnova, H00010413-M01), p-Yap (Cell Signaling, 4911), p-ERM (Cell Signaling, 3141), PKCλ/ζ (Santa Cruz, sc-216), E-cadherin (Takara, M108), Pard6b (Santa Cruz, sc-67393), Scribble (Santa Cruz, sc-28737), Cdx2 (BioGenex, MU392-UC), and Amot (Hirate et al. 2013) were used. For secondary antibodies, goat anti-mouse, anti-rabbit, or anti-rat IgG antibodies labeled with either Alexa Fluor 488, 555, or 647 (Invitrogen) were used. For nuclear staining, Hoechst33258 (Dojindo, Kumamoto, Japan) was used. Confocal images were obtained by using either a Zeiss LSM 510 or Nikon A1 microscope.
Quantitative data analysis and statistics
Quantification of the signal intensities in confocal images was performed using ImageJ software. Statistical analyses were performed using Prism 6 software (Graphpad, CA, USA).
Results
Polarization correlates with the nuclear localization of Yap
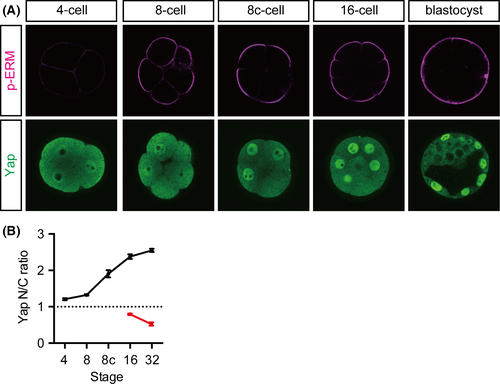
To determine the role of cell polarity in the subcellular distribution of Yap at the 16-cell stage, we first examined the correlation between cell polarity and the levels of nuclear Yap. Mouse embryos start to develop cell polarity at the 8-cell stage after compaction (Johnson et al. 1986; Fleming & Johnson 1988). Polarization was visualized as the distribution of phosphorylated-Ezrin/Radixin/Moesin (p-ERM) proteins, which are localized to the apical microvilli (Fig. 1A) (Yonemura et al. 1999). The p-ERM proteins were first detected throughout the plasma membrane in uncompacted 8-cell stage embryos. After compaction, the strong signal was mostly restricted to the apical membrane. Nuclear Yap also became evident in the outer cells after compaction (Fig. 1A). To quantify the levels of Yap in the nuclei, we determined the nuclear to cytoplasmic (N/C) ratio of the Yap signals. Basal levels of nuclear Yap were observed from the 1-cell stage. In the outer cells, the nuclear Yap signal began to increase after compaction and kept increasing over time, whereas in the inner cells, it decreased over time (Fig. 1B) as previously reported (Nishioka et al. 2009). The coincidence of the emergence of cell polarity and the increase in the nuclear Yap signal at the 16-cell stage likely reflects their close relationship.
Nuclear localization of Yap is partially perturbed by disruption of the Par-aPKC system in 16-cell stage embryos
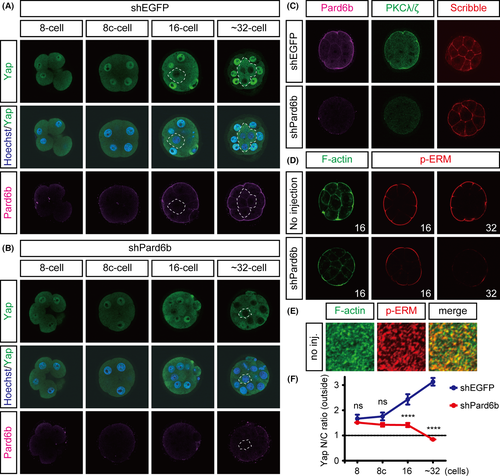
To directly examine the involvement of cell polarity in the regulation of Yap at the 16-cell stage, we knocked down Pard6b, a component of the Par-aPKC system. This was done by injecting a Pard6b shRNA expression plasmid. Following knockdown, we observed the development of the embryos. Consistent with previous observations, in control embryos, Pard6b gradually accumulated at the apical membrane during compaction and remained there in the outer cells up to the 32-cell stage (Fig. 2A) (Vinot et al. 2005). In Pard6b-knockdown (KD) embryos, apical Pard6b was clearly reduced by the 16-cell stage (Fig. 2B). Loss of an apical regulator PKCζ/λ (n = 7/7) and apical expansion of a basolateral regulator Scribble (n = 3/4) in Pard6b-KD embryos confirmed disruption of the Par-aPKC system at this stage (Fig. 2C). Reproducing previous results, the outer cells of Pard6b-KD embryos showed clear exclusion of Yap from the nuclei at 32-cell stage (Fig. 2B,F). In contrast, at the 16-cell stage, Pard6b-KD embryos did not clearly exclude Yap from the nuclei of the outer cells, even though the nuclear Yap signals were significantly weaker than those in control embryos (Fig. 2B,F). Therefore, the effects of disruption of the Par-aPKC system on the regulation of Yap are weaker at the 16-cell stage than at the 32-cell stage.
Cdx2 expression is moderately affected by disruption of the Par-aPKC system at the 16-cell stage
At the 16-cell stage, the effect of Pard6b KD on the subcellular localization of Yap was weak. Because Yap protein forms a complex with Tead4 in the nucleus and activates Cdx2 enhancer, we hypothesized that the effect of Pard6b KD on the expression of Cdx2 would also be weak. Although Pard6b-KD embryos showed reduced levels of Cdx2 at the 16-cell stage (Fig. 3A,C,D), the reduction was less prominent than previously observed at the 32-cell stage (Alarcon 2010; Hirate et al. 2013). These results supported our hypothesis.
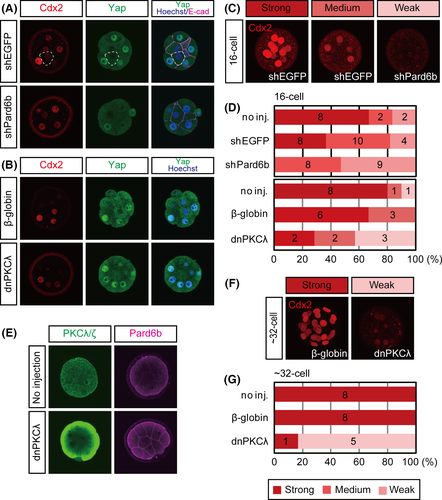
To further confirm the weak effects of Par-aPKC system disruption at the 16-cell stage, we also used a second approach. We injected RNAs encoding the dominant-negative form of PKCλ (dnPKCλ) into both blastomeres of two-cell stage embryos. Similar to that observed in previous studies, overexpression of dnPKCλ resulted in clear exclusion of Yap from the nuclei and a strong reduction of Cdx2 at the 32-cell stage (Fig. 3F,G) (Hirate et al. 2013). Immunostaining between the 8- and 16-cell stages revealed strong, widespread expression of dnPKCλ protein (Fig. 3E; n = 6/6). Pard6b protein was distributed throughout the plasma membrane, indicating disruption of the Par-aPKC system (Fig. 3E; n = 10/10). In 16-cell stage embryos, the effects of dnPKCλ were similar to those of Pard6b KD; both the nuclear localization of Yap and the expression of Cdx2 were only moderately reduced (Figs 2F and 3B,D). These results suggest that the contribution of the Par-aPKC system to the regulation of Yap and Cdx2 expression differs between the 16- and 32-cell stages. Whereas the Par-aPKC system determines the subcellular localization of Yap and strongly regulates the expression of Cdx2 at the 32-cell stage, it has a limited role at the 16-cell stage.
Disruption of the Par-aPKC system at the 16-cell stage activates Hippo signaling in the outer cells
At the 16-cell stage, disruption of the Par-aPKC system resulted in limited exclusion of Yap from the nuclei and moderate Cdx2 expression in the outer cells. At the 32-cell stage, disruption of the Par-aPKC system activates Hippo signaling leading to clear exclusion of Yap from the nuclei in the outer cells (Hirate et al. 2013). Therefore, we hypothesized that activation of Hippo signaling was incomplete in polarity-disrupted embryos at the 16-cell stage. Upon activation of the Hippo pathway, protein kinase Lats1/2 phosphorylates five serine residues in Yap, including serine 112 (S112, which is equivalent to serine 127 in human Yap). To monitor Hippo signaling, we examined the distribution of S112-phosphorylated Yap (p-Yap). In control embryos, a strong p-Yap signal was only observed in the inner cells (Fig. 4A; n = 20/20). In the dnPKCλ-injected embryos, a strong p-Yap signal was also observed in the outer cells, indicating activation of the Hippo pathway in these cells (Fig. 4A,B; n = 16/19). Double knockdown (DKD) of the basolateral regulators Par1a and Par1b by shRNA also resulted in increased p-Yap levels in the outer cells (Fig. 4A,D; n = 20/20). Therefore, similar to that observed at the 32-cell stage, disruption of the Par-aPKC system ectopically activates Hippo signaling in the outer cells at the 16-cell stage.
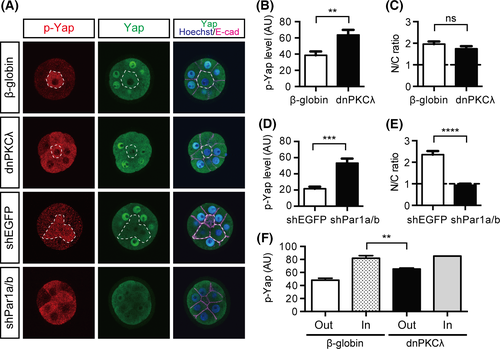
Despite the clear activation of Hippo signaling in the outer cells, neither dnPKCλ-injected (n = 24/24) nor Par1a/b-DKD (n = 20/29) embryos showed clear exclusion of Yap from the nuclei in the outer cells (Fig. 4A,C,E). We hypothesized that the increase in p-Yap levels in the outer cells is less than that in the inner cells and is not sufficient to exclude Yap from the nuclei. Therefore, we compared the Yap phosphorylation levels in control and dnPKCλ-injected embryos semi-quantitatively by acquiring and analyzing p-Yap signals under the same experimental conditions. Indeed, at the 16-cell stage, the outer cells of dnPKCλ-injected embryos (n = 5; 79 cells) showed lower p-Yap levels than the inner cells of control embryos (n = 5; 8 cells; Fig. 4F). These results are consistent with the hypothesis that disruption of the Par-aPKC system does not fully activate Hippo signaling in the outer cells to the extent required to exclude Yap from the nuclei at the 16-cell stage.
Disruption of the Par-aPKC system does not completely disrupt the organization of the apical cortex at the 16-cell stage
Because disruption of the Par-aPKC system showed stage-dependent differential effects on the regulation of Hippo signaling, Yap, and Cdx2, we investigated whether other stage-dependent differences are also present in Pard6b-KD embryos. We found that the effects of Pard6b KD on the apical p-ERM signals differed depending on the developmental stage. As previously reported, at the 32-cell stage, the apical p-ERM signal was strongly reduced (Fig. 2D) (Hirate et al. 2013), whereas at the 16-cell stage, the p-ERM signal was not significantly altered (Fig. 2D). Phosphorylated or activated ERM proteins crosslink actin filaments and membrane proteins to promote the formation of microvilli in the apical domain of epithelial cells (Yonemura et al. 1999). Indeed, in the apical domain of 16-cell stage embryos, p-ERM signals were distributed as distinct dots or pillars perpendicular to the cortex, and these signals partially overlapped with the F-actin signals (Fig. 2E). In Pard6b-KD embryos, at the 16-cell stage, as expected from the presence of strong apical p-ERM signals, the F-actin signals in the apical cortex were also largely unaffected, while the F-actin signals in the basolateral cortex and the apical edge of cell–cell boundaries were clearly reduced (Fig. 2D). These results suggest that, at the 16-cell stage, establishment and/or maintenance of the cortical apical pole, which consists of p-ERM proteins, F-actin, and microvilli, does not fully depend on the Par-aPKC system. This implies that cell polarity at the 16-cell stage is controlled by at least two mechanisms, the Par-aPKC system and a yet unknown mechanism that controls the cortical apical pole independent of the Par-aPKC system.
The presence of outer apolar cells with intense Hippo signaling in 16-cell stage embryos
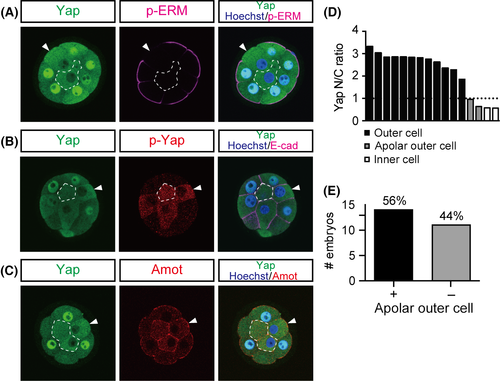
At the 32-cell stage, subcellular localization of Yap always correlates with cell position in embryos, and manipulation of cell position alters Yap localization (Nishioka et al. 2009; Hirate et al. 2012). A correlation between Yap and cell position was also observed at the 16-cell stage (Nishioka et al. 2009; Hirate et al. 2012). However, detailed examination of embryos at this stage revealed that 56% of embryos (n = 14/25) contain at least one outer cell that shows nuclear exclusion of Yap (Figs 5A,D,E and S1). These cells show strong p-Yap signals, which are indicative of active Hippo signaling (Fig. 5B). Furthermore, these cells are apolar, as revealed by the absence of apical p-ERM signals (Fig. 5A). Therefore, these Hippo-active outer cells resemble inner cells. At the 32-cell stage, the distribution of Amot is regulated by cell polarity. In the outer (polar) cells, Amot is restricted to the apical domain, whereas in the inner (apolar) cells, Amot is distributed throughout the plasma membrane (Hirate et al. 2013). In 16-cell stage embryos, Hippo-active outer cells also showed Amot throughout the plasma membrane (Fig. 5C), further confirming that these cells are apolar. Morphologically, the polar outer cells were flattened and their nuclei were located in the periphery of the embryo, whereas the apolar outer cells were columnar and their nuclei were more centrally located (Fig. 5A–C). Taken together, these results suggest that at the 16-cell stage, some embryos have outer cells with characteristics of inner cells, such as the absence of cell polarity and active Hippo signaling.
Asymmetric cell division regulates cell polarity, Yap, and cell positioning during the division of dissociated blastomeres of 8-cell stage embryos
Studies at the 32-cell stage revealed that cell position controls cell polarity and Hippo signaling. The presence of apolar and Hippo-active cells in the outer position of 16-cell stage embryos differentiates them from 32-cell stage embryos. In 16-cell stage embryos, cell position does not necessarily control cell polarity and Hippo signaling. Therefore, we hypothesized that the cell division processes during the 8- to 16-cell transition are also involved in the regulation of cell polarization and Hippo signaling, independent of cell position.
To test this hypothesis, we studied the process of the 8- to 16-cell transition by culturing individual blastomeres of dissociated 8-cell stage embryos (1/8 cells) until they divide and give rise to 2/16 couplets. As reported previously, cell division resulted in either couplets of two polar cells, in which p-ERM signals were present in the outer cortex of both cells (symmetric division, 29/43; 67%), or couplets of polar and apolar cells, in which only one cell showed a p-ERM signal in the outer membrane (asymmetric division, 14/43; 33%; Fig. 6A) (Johnson & McConnell 2004; Dietrich & Hiiragi 2007). Cell positions are equivalent in freshly formed couplets, and the generation of polar-apolar couplets suggests that cell division controls cell polarization in dissociated blastomeres.
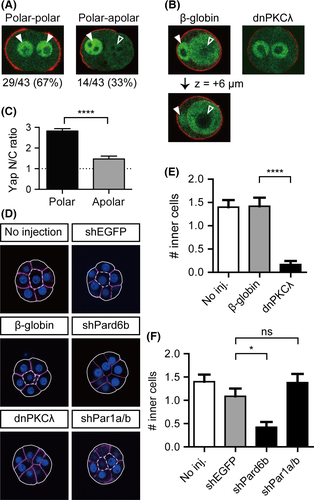
In polar-polar couplets, Yap was distinctly nuclear in both cells (Fig. 6A). In polar-apolar couplets, only the polar cells marked by apical p-ERM signals showed strong nuclear Yap signals, and the apolar cells showed weaker nuclear Yap signals (Fig. 6A). Therefore, Hippo signaling correlates with cell polarity. While apolar cells in 16-cell stage embryos showed clear exclusion of Yap from the nuclei, the apolar cells of the freshly formed polar-apolar 2/16 couplets did not exclude Yap from the nuclei (Fig. 6A,C). This difference is likely caused by the limited cell–cell adhesion in couplets and consequent insufficient activation of Hippo signaling. Indeed, after longer incubation, the apolar cells of 2/16 couplets were engulfed by the polar cells and Yap was clearly excluded from the nuclei of the internalized apolar cells (n = 4/8 polar-apolar couplets; Fig. 6B).
All the couplets generated from dnPKCλ-injected embryos underwent symmetric cell division (n = 17/17), suggesting that the Par-aPKC system is required for asymmetric cell division. In these couplets, both cells exhibited weak p-ERM signals. This is different from that observed in embryos with disrupted Par-aPKC systems, in which strong apical p-ERM signals were maintained (Fig. 2D). Because cell–cell adhesion restricts the positioning of microvilli (Johnson & Ziomek 1981b), this difference may reflect the requirement of cell–cell adhesion for the establishment and/or maintenance of apical p-ERM in the absence of the Par-aPKC system.
The couplets generated from dnPKCλ-injected embryos did not undergo engulfment (n = 0/17). Therefore, polar cells with active Par-aPKC systems are required to internalize the apolar cells. In further support of this observation, disruption of the Par-aPKC system in embryos after injection of dnPKCλ or shPard6b reduced the number of inner cells at the 16-cell stage (Fig. 6D–F) (Dard et al. 2009). The Par-aPKC system establishes apicobasal cell polarity through mutual inhibition of the apical regulator complex (Par3-Par6-aPKC) and the basolateral regulator Par-1 (Suzuki & Ohno 2006). Because disruption of the apical regulator complex by targeting aPKC and Pard6b compromised cell positioning, we next asked whether disruption of the basolateral regulator has similar effects. Remarkably, when the basolateral regulators Par-1a/b were knocked down, a decrease in the number of inner cells was not observed. This result suggests that the apical Par-aPKC complex is required for engulfment and internalization of the apolar cells. In summary, at the 16-cell stage, cell division, which is regulated by the Par-aPKC system, is an important determinant of cell polarity. Cell polarity correlates with the subcellular localization of Yap, and the apical Par-aPKC complex controls the positioning of cells within embryos.
Discussion
Cell polarity but not cell position regulates Hippo signaling in 16-cell stage embryos
Previous studies on Hippo signaling have shown that at the 32-cell stage, cell position determines whether cells acquire polarity, and the resulting cell polarity, in combination with cell–cell adhesion, establishes position-dependent Hippo signaling (Nishioka et al. 2009) (see 1). Therefore, the evidence from studies of 32-cell stage embryos (Hirate et al. 2013; Hirate & Sasaki 2014) supports the inside-outside model (Tarkowski & Wroblewska 1967) and the newer version of the polarity model (Yamanaka et al. 2006). In this study, we examined whether similar mechanisms also operate at 16-cell stage, and we found several differences.
The first important difference is that 16-cell stage embryos often contain apolar, Hippo-active cells, i.e., cells with characteristics of inner cells, in the outer position. Therefore, at least for these cells, cell position determines neither cell polarity nor Hippo signaling. In experiments examining 2/16 couplets, some 1/8 blastomeres formed polar-apolar couplets, indicating that the process of cell division (asymmetric cell division) plays a role in the regulation of cell polarity. In both normal 16-cell stage embryos and 2/16 couplets, polar and apolar cells always exhibited inactive and active Hippo signaling, respectively. These observations are consistent with the hypothesis that cell polarity regulates Hippo signaling. Indeed, partial disruption of cell polarity through inhibition of the Par-aPKC system altered Hippo signaling in 16-cell stage embryos. Therefore, cell division is an important regulator of cell polarity, and cell polarity likely controls Hippo signaling.
The nuclei of the apolar, Hippo-active outer cells in 16-cell stage embryos are located in the deep interior of these cells. Therefore, they are probably identical to the transient outer cells that have been observed in time-lapse imaging of nuclei (McDole et al. 2011). Recently, gradual internalization of apolar outer cells at the 16-cell stage was observed by live cell tracking (Anani et al. 2014). Furthermore, in polar-apolar 2/16 couplets, the apolar cell was engulfed by the polar cell, and embryos with disrupted Par-aPKC systems have fewer inner cells at the 16-cell stage. Therefore, cell polarity regulated by the Par-aPKC system also controls cell position in embryos. These results demonstrate that at the 16-cell stage or during the 8- to 16-cell transition, cell division is involved in the regulation of cell polarity, cell polarity likely controls Hippo signaling, and the Par-aPKC system controls cell position. This implies that the original polarity model, in which asymmetric cell division generates differences in cell polarity, as well as the newer version of the polarity model, in which cell polarity controls gene expression, are supported by our findings at the 16-cell stage. However, unlike at the 32-cell stage, the inside-outside model cannot explain our observations at the 16-cell stage.
The effects of disruption of the Par-aPKC system in 16- and 32-cell stage embryos are different
The second important difference between the 16- and 32-cell stages is the role of the Par-aPKC system in the regulation of cell polarity and Hippo signaling. Disruption of the Par-aPKC system in 32-cell stage embryos completely disrupted cell polarity, activated Hippo signaling, and excluded Yap from the nuclei of the outer cells (Hirate et al. 2013). In contrast, at the 16-cell stage, embryos with a disrupted Par-aPKC system clearly maintained apical p-ERM and F-actin, the key regulators of apical microvilli, and Yap was not excluded from the nuclei of the outer cells. These results show two important differences between the 16- and 32-cell stages (summarized in Fig. 7).
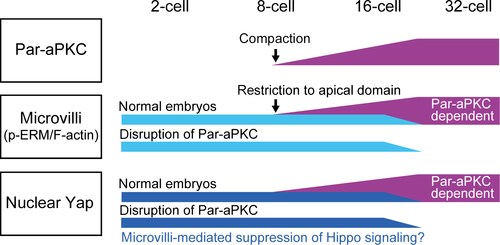
First, at the 16-cell stage, p-ERM and F-actin, key components of microvilli, are not tightly regulated by the Par-aPKC system. Consistent with this notion, microvilli are present at the 2-cell stage, prior to initiation of the Par-aPKC system at the 8-cell stage (Calarco & Epstein 1973; Ducibella et al. 1977; Reeve & Ziomek 1981). At the early 8-cell stage, cell–cell contacts restrict the position of microvilli. However, after 4–5 h, the position of the microvilli becomes fixed (Johnson & Ziomek 1981b), and they remain localized during mitotic division. It is likely that once fixed, microvilli, which consist of F-actin, p-ERM, and membrane proteins, constitute a stable cortical apical architecture that is independent of the Par-aPKC system. Therefore, we propose that cell polarity in 16-cell stage embryos is regulated by two independent mechanisms, the cortical apical domain, represented by microvilli/p-ERM/F-actin, and the Par-aPKC system. After the 32-cell stage, p-ERM becomes dependent on the Par-aPKC system.
Second, at the 16-cell stage, the effects of disruption of the Par-aPKC system on the regulation of Yap were not evident. Importantly, embryos with disrupted Par-aPKC systems exhibited clear activation of Hippo signaling in the outer cells, suggesting that the mechanisms by which Par-aPKC suppresses Hippo signaling in the outer cells commonly operate at both the 16- and 32-cell stages. In support of this hypothesis, Amot, the junctional Hippo component that connects the Par-aPKC system and Hippo signaling at the 32-cell stage, shows a similar cell position-dependent distribution at both the 16- and 32-cell stages (Hirate et al. 2013). Then, the question that arises is why activation of the Hippo pathway does not promote exclusion of Yap from the nuclei. Since both the cortical apical pole, which is characterized by dense stable microvilli (Johnson & Mcconnell 2004), and nuclear Yap (Nishioka et al. 2009) are important in the regulation of cell fate, it is likely that the remaining apical microvilli contribute to the regulation of Yap. F-actin is known to promote the nuclear localization of Yap by inhibiting Lats kinase (Sansores-Garcia et al. 2011; Wada et al. 2011; Yu et al. 2012; Zhao et al. 2012). Because F-actin bundles are the major components of microvilli, these F-actins may contribute to the suppression of Hippo signaling and promote nuclear localization of Yap. Indeed, activation of Hippo signaling in the outer cells of Par-aPKC-disrupted 16-cell stage embryos was weaker than that in the inner cells of normal 16-cell stage embryos. One candidate molecule that connects microvillar F-actin to Hippo signaling is Amot. While Amot activates Hippo signaling at adherens junctions, Amot activity is suppressed by its interaction with F-actin (Hirate et al. 2013; Hirate & Sasaki 2014; Mana-Capelli et al. 2014). In 32-cell stage embryos, Amot localizes to microvilli and is required for suppression of Hippo signaling in the outer cells (Hirate & Sasaki 2014). Therefore, it is likely that F-actin in microvilli restricts the distribution of Amot in the absence of the Par-aPKC system, which reduces Hippo signaling.
Considering the small differences between the Hippo signaling level in the outer cells of the Par-aPKC-disrupted embryos and that in the inner cells of normal cells, it is also possible that the remaining apical microvilli, p-ERM, and/or F-actin promote nuclear localization of Yap independent of Hippo signaling through yet unknown mechanisms. In either case, the involvement of apical microvilli, p-ERM, and/or F-actin should be directly examined in the future by experimentally manipulating these molecules.
Multiple mechanisms cooperatively establish the TE and ICM lineages
Previous studies have established that the Hippo signaling pathway plays a central role in the regulation of TE and ICM fates (Nishioka et al. 2009; Cockburn et al. 2013; Hirate et al. 2013; Leung & Zernicka-Goetz 2013; Hirate & Sasaki 2014; Wicklow et al. 2014). While our previous studies focused on the mechanisms that regulate the signaling pathway at the 32-cell stage, our current study revealed the mechanisms that partially regulate Hippo signaling and Yap at the 16-cell stage. Combining these findings with other known mechanisms, we propose a model for the role and regulatory function of the Hippo signaling pathway in the development of TE and ICM.
At the 8-cell stage, embryos undergo compaction, and each blastomere acquires apicobasal cell polarity. Cell polarity is regulated by two independent mechanisms, the Par-aPKC system and the cortical apical domain, which is represented by microvilli (p-ERM/F-actin). During the 8- to 16-cell transition, some blastomeres undergo asymmetric cell division, which is regulated by the stable cortical apical domain and the Par-aPKC system. The cells that inherit the apical domain become polar cells, in which Hippo signaling is suppressed and nuclear localization of Yap is promoted. The cells that do not inherit the apical domain become apolar cells, in which Hippo signaling is activated and Yap is excluded from the nuclei. Nuclear Yap increases the activity of Tead4, which initiates TE fate. Recently, Notch signaling has been reported to be involved in promoting TE fate. Tead4-Yap and the Notch transcription factor RBPJ cooperatively activate the Cdx2 enhancer (Rayon et al. 2014). Activation of Notch signaling is initially stochastic at the morula stage, which likely contributes to the stochastic expression of Cdx2 at this stage (Dietrich & Hiiragi 2007). Asymmetric cell division also includes asymmetric inheritance of Cdx2 mRNA to the polar cells (Skamagki et al. 2013). The presence of early bias among blastomeres at the 4-cell stage also contributes to the generation of different lineages (Piotrowska-Nitsche et al. 2005). However, the relationship between early bias and cell division during the 8- to 16-cell transition has yet to be elucidated. Notch signaling becomes gradually restricted to the outer cells by the blastocyst stage (Rayon et al. 2014). After the 32-cell stage, continuous, position-dependent Hippo signaling likely functions as a safeguard mechanism for the establishment of two distinct cell fates. Hippo signaling maintains and enhances the position-dependent differences between TE and ICM cells until these fates are fixed at the late blastocyst stage.
Acknowledgements
We thank M. Shibata, T. Ideue, M. Harano, A. Katsuyama, and N. Fujita for their excellent technical assistance; K. Yamagata, V. Alarcon, and A. Shitamukai for plasmids; and A. Shimono for the Amot antibody. We are also grateful to the Laboratory for Animal Resources Development and Genetic Engineering, RIKEN CDB for collecting mouse embryos and housing mice, as well as the CARD, Kumamoto University for housing mice. This work was supported by grants from RIKEN (to H.S.), MEXT KAKENHI (Grant Number 21116003 to H.S.), and JSPS KAKENHI (Grant Number 25440112 to Y. H.).