Transcription activator-like effector nucleases efficiently disrupt the target gene in Iberian ribbed newts (Pleurodeles waltl), an experimental model animal for regeneration
Abstract
Regeneration of a lost tissue in an animal is an important issue. Although regenerative studies have a history of research spanning more than a century, the gene functions underlying regulation of the regeneration are mostly unclear. Analysis of knockout animals is a very powerful tool with which to elucidate gene function. Recently, transcription activator-like effector nucleases (TALENs) have been developed as an effective technique for genome editing. This technique enables gene targeting in amphibians such as newts that were previously impossible. Here we show that newts microinjected with TALEN mRNAs designed for targeting the tyrosinase gene in single-cell stage embryos revealed an albino phenotype. Sequence analysis revealed that the tyrosinase genes were effectively disrupted in these albino newts. Moreover, precise genome alteration was achieved using TALENs and single strand oligodeoxyribonucleotides. Our results suggest that TALENs are powerful tools for genome editing for regenerative research in newts.
Introduction
An ability to regenerate tissues or organs lost due to disease or injury would be advantageous for most animals. Urodele newts have a remarkable capability to regenerate lost body parts such as limbs, optical tissues (lens, retina, and cornea), brain, spinal cord, intestine, and heart throughout their lives (reviewed in Brockes & Kumar 2002; Agata & Inoue 2012). Therefore, they have been used as a unique experimental model.
Although a lot of research has been performed, the mechanisms of regeneration at the genetic and molecular levels remain mostly unclear. In order to study the mechanisms of regeneration at the molecular and genetic levels, we have adopted the Iberian ribbed newt (Pleurodeles waltl) as a model animal. We established an efficient transgenic technique using P. waltl newts (Hayashi et al. 2013). However, knockout animals that are very useful for understanding gene function in vivo are still not available in newts. This is because gene knockout could be performed only using animals for which embryonic stem cells were established in vertebrates.
Recently, engineered nucleases such as meganuclease, zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and the RNA-guided CRISPR/Cas9 nuculease system have enabled targeted alteration of any genomic sequence in a wide range of organisms (Urnov et al. 2010; Daboussi et al. 2012; Joung & Sander 2013; Wei et al. 2013). These nuclease-mediated genome alterations (called genome editing) make it possible to perform genetic studies that were previously difficult or impossible. TALENs and ZFNs in particular can successfully disrupt targeted genes in the amphibians Xenopus toropicalis and Xenopus laevis (Young et al. 2011; Ishibashi et al. 2012; Lei et al. 2012; Suzuki et al. 2013).
In the present study, we examined whether gene knockout by TALEN is possible in a newt. We generated TALENs targeting newt tyrosinase gene. Tyrosinase is involved in melanin synthesis (Kumasaka et al. 2003), and the disruption of this gene causes an albino phenotype (Ishibashi et al. 2012; Suzuki et al. 2013). We microinjected mRNA of the TALENs into single-cell stage fertilized eggs. The founder (F0) animals, which underwent the microinjection, exhibited an albino phenotype. We confirmed that the tyrosinase gene was disrupted in the albino animals by DNA sequencing. In addition, a loxP sequence was precisely inserted into the TALEN target site using single-stranded oligodeoxyribonucleotides (ssODN). These results indicate that TALENs are useful for functional analysis of regenerative research in newts.
Materials and methods
Animals
Iberian ribbed newts (Pleurodeles waltl) were originally purchased from Tao (Chiba, Japan). The P. waltl newts used in this study were raised in our laboratory from these newts. All animals were kept in a tap-water aquarium at 25–26°C under natural light cycles. The animals were fed more than five times a week; hatched blain shrimp to larvae and combined feed to juvenile/adult newts (Kyorin Corporation, Hyogo, Japan). All procedures were carried out in accordance with the Institutional Animal Care and Use Committee of Tottori University (Tottori, Japan) and national guidelines of the Ministry of Education, Culture, Sports, Science & Technology in Japan.
Generation of TALENs
To design the TALEN target site, the genomic sequence of tyrosinase was amplified by polymerase chain reaction (PCR) using a set of primers and Ex Taq DNA polymerase (Takara BIO, Japan). The primers (5′-CGACTTCGCACACGAGG-3′ and 5′-CTGCCAGGAGGAGAAGAATG-3′) were designed using de novo sequencing database of P. waltl. De novo sequences were obtained from mRNA expressed in the P. waltl larvae and adults hearts using Roche 454. Sequence data were assembled by mimicking intelligent read assembly (MIRA) to develop the database. The PCR products were subcloned into pGEM vector (Promega), and then positive clones were sequenced using BigDye Terminator (Life Technologies). We obtained a 226 bp fragment encoding the 3rd exon of P. waltl tyrosinase.
Two pairs of TALENs against P. waltl tyrosinase were generated using the two-step Golden Gate cloning method (Sakuma et al. 2013a) with various modifications (Sakuma et al. 2013b). TALEN target sites were determined by TAL effector Nucleotide Targeter Ver. 2.0 (Doyle et al. 2012). After construction of the pairs of TALENs, efficiency was examined using a single strand annealing assay (Ochiai et al. 2010; Sakuma et al. 2013a) and a pair of TALENs that exhibited higher efficiency was selected (data not shown).
The TALEN expressing plasmids were linearized by SmaI digestion, and then the mRNAs were synthesized by in vitro transcription using an mMESSAGE mMACHINE T7 Ultra Kit (Life Technologies, Japan). The mRNAs were purified by ethanol precipitation after polyadenylation, and dissolved in nuclease-free water. The mRNAs were stored at −80°C until just before use.
Eighty-nine base single-stranded oligodeoxyribonucleotides (ssOligo-loxP-EcoRI: 5′-GCCTTCCTGCCGTGGCACCGGATCTATAACTTCGTATAGCATACATTATACGAAGTTATGAATTCCTCTGGGAACGCGAGCTCCAGAAG-3′) were ordered from Integrated IDT Technologies and purified by Na+ salt exchange and HPLC.
Microinjection
Fertilized eggs (single cell-stage embryos) were prepared according to Hayashi et al. (2013). The fertilized eggs were treated with 2% cysteine (pH 7.4–7.6) or 2% sodium thioglycolate (pH 7.9) in 0.25 × Holtfreter's solution for 5 min to remove the jelly. De-jellied eggs were rinsed in 0.25 × Holtfreter's solution and transferred into injection medium (4% Ficoll in 0.25 × Holtfreter's solution). The eggshells were removed using fine forceps and stored at 8°C until microinjection.
Various amounts of TALEN mRNA were microinjected into the eggs with 9.4 nl volume/egg of ultra-pure water using a NANOJECT II (Drummond). After microinjection, the fertilized eggs (embryos) were incubated overnight at 25°C.
The next day, the embryos (blastula-stage) were rinsed three times in 0.25 × Holtfreter's solution to remove the Ficoll contained in the injection medium. The embryos were incubated in 0.25 × Holtfreter's solution for 5–7 days (hatching-stage) and then transferred into tap water.
TALEN mutation analysis
Genomic DNAs of individual larvae were prepared by phenol/chloroform extraction. The genomic sequence of the tyrosinase containing TALEN-target site was amplified by PCR using a set of primers (5′-CGAC-TTCGCACACGAGG-3′ and 5′-CTGCCAGGAGGAGAAGAATG-3′) and Ex Taq DNA polymerase (Takara BIO, Japan). To confirm the presence of mutations or insertions, the PCR products were subcloned into pGEM vector (Promega), and then positive clones were sequenced using BigDye Terminator (Life Technologies).
Results
TALENs efficiently produced albino mutants in the newts
We attempted to disrupt the tyrosinase gene in order to examine whether the TALENs induce targeted gene disruption in newts. Tyrosinase is involved in melanin synthesis and is specifically expressed in melanophores and retinal pigmented epithelium (RPE) (Kumasaka et al. 2003). The disruption of this gene causes an albino phenotype (Ishibashi et al. 2012; Suzuki et al. 2013). First, we confirmed that no genetic polymorphism existed in the binding sites of TALENs in the tyrosinase gene of the parental newts (data not shown). F1 and F2 animals derived from these parents were used in this study.
We microinjected mRNA of TALENs into fertilized newt eggs (single-stage embryos). If the TALENs destroy the tyrosinase gene, the albino phenotype can be visually identified. Neither normal melanophores nor RPEs pigmented with melanin were observed in the larva injected with equal amounts of right TALEN (R-TALEN) and left TALEN (L-TALEN) mRNAs (Fig. 1B, F and Fig. 2B). Other types of chromophores (xanthophores or iridophores) normally developed. In contrast, injection with only R-TALEN (200 pg) as negative control showed wildtype phenotype (normal pigmentation, Fig. 1C, G and Fig. 2B) similar to un-injected larvae obtained from the same parents (Fig. 1A,E). When the total amount of R- and L-TALEN mRNA was 200 pg and less, mosaic animals appeared in addition to the albino larvae (Fig. 1D and Fig. 2B). In the mosaic larva, melanophores were greatly reduced, although a small number of melanophores still remained on its body surface (red arrowheads in Fig. 1D).
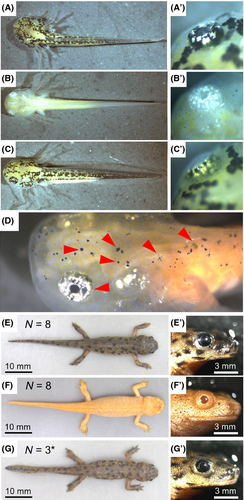
The larvae injected with R- and L-TALENs, or only R-TALEN developed normally, and had metamorphosed within 2–3 months postfertilization similar to the wild type animals (Fig. 1E–G). Normal melanophores and RPE cells were not observed even after metamorphosis in the albino animals.
Optimization of the amount of TALEN mRNA for the microinjection
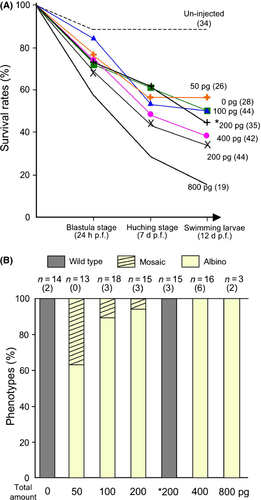
To optimize the amount of TALEN mRNA, the fertilized eggs were microinjected with 50–1600 pg of TALEN mRNA. The survival rates and their phenotypes were then examined (Fig. 2). The total amount of TALENs did not show obvious toxicity in a range between 50 and 400 pg; however, the survival rate was severely decreased by injection of 800 pg (Fig. 2A). As a result of carrying out injection of a total of 1600 pg, all embryos stopped development at the gastrula stage (data not shown). Albino larvae were obtained in all ranges of injection with R- and L-TALENs. All embryos injected with a total of 400 or 800 pg of TALENs (R- and L-, equal amounts) showed albino phenotype.
Confirmation of the disruption of the tyrosinase gene in albino newts
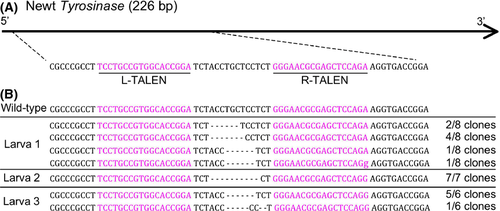
In order to confirm disruption of tyrosinase gene in the albino animals, we sequenced the PCR products amplified from albino larvae (Fig. 3A), and the results indicated mutations in all 21 clones examined (Fig. 3B). Among the sequenced clones, 52% of the clones (11/21) showed frame-shift mutation. The deletion of two amino acids occurred in the remaining clones (10/21).
ssODN-mediated insertion of loxP sequence into TALEN target site
To introduce the loxP site into the tyrosinase locus, we designed a single strand oligo DNA (ssOligo-loxP-EcoRI) that has the loxP sequence and EcoRI site in the center and 26-base homology arms on the 5′ and 3′ ends (Fig. 4A). One hundred picograms of ssOligo-loxP-EcoRI was injected into the fertilized egg with a total of 100 pg of R- and L-TALENs.
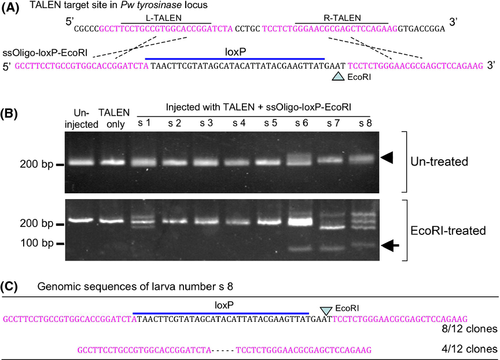
Genomic DNAs were prepared from injected larvae and the TALEN target site was amplified by PCR. The products were then digested with EcoRI. When the ssOligo-loxP-EcoRI was inserted into the TALEN target site, the PCR products became EcoRI sensitive and a fragment of around 100 base pairs was generated (Fig. 4B arrow). After electrophoresis, we detected three positive samples in eight larvae (Fig. 4B, lanes s 6, s 7, and s 8). DNA sequencing analysis of the larva numbered as s 8 in Figure 4B showed that the loxP and EcoRI sites were exactly inserted into the tyrosinase locus (Fig. 4C). The insertion occurred in 66.7% of sequenced clones (n = 8/12). Other clones showed a five base deletion (Fig. 4C). In another positive larva (number s 6), precise insertion was confirmed in 8.2% of the sequenced clones (n = 1/11). In number s 7, the loxP sequence was disrupted although insertion took place in 91.7% of the clones (n = 11/12, sequence data not shown).
Discussion
In this study, we successfully revealed that TALENs worked effectively in newts, and that knockout animals were generated at F0 generation with high efficiency similar to that in Xenopus as reported by Suzuki et al. (2013).
We have developed an experimental system to perform molecular genetics using P. waltl newts (Hayashi et al. 2013). In addition to that system, genome editing by TALENs enables analyses of knockout animals in regenerative studies of newts. Combinations of genome editing and a molecular genetic system would make newts an excellent animal model for understanding the mechanisms of regeneration. TALENs to the tyrosinase mostly destroyed the target sequence in vivo, as there were no melanophores in these animals and all sequenced clones showed the deletions.
Interestingly, a few patterns of mutations were observed in the newts. P. waltl newt embryos require 6 h for the first cleavage at 25°C. This seems to be advantageous for the disruption of a target gene by TALEN. We designed a pair of TALENs to destroy the catalytic core of the enzyme (Fig. 3). It was suggested that an important domain should be chosen as the target site of the TALENs in order to destroy the function of the protein by the deletion of only 1 or 2 amino acids.
Our results showed that efficient TALENs enable functional analysis in F0 individuals in newts. However, it is also important to establish knockout lines. We confirmed that the tyrosinase gene was destroyed in almost all cells in newts injected with more than 200 pg of TALEN mRNA. It is expected that destruction of the tyrosinase gene had also occurred in the cells of germ lines. P. waltl newts have breeding properties comparable to Xenopus tropicalis. They are sexually mature at about 6 months postfertilization. The females lay 150–600 eggs per spawning. Moreover, their ability to regenerate various organs is equivalent to that of the Japanese common newt, Cynops pyrrhogaster (Hayashi et al. 2013). These properties suggest that P. waltl newts are suitable for establishment of the knockout lines generated by genome editing.
Gene disruption throughout the entire body may cause embryonic lethality or a developmental defect. In such a case, a tissue-specific knockout protocol is required for further studies of regeneration. For that purpose, it is possible to directly deliver the mRNA of TALENs into target tissues by electroporation (Sasagawa et al. 2002; Hayashi et al. 2010). In addition, Cre-loxP mediated conditional knockout also would be a powerful strategy. Precise genomic sequence alteration by ssODNs is a promising technique for introducing a specific sequence via a double strand break (Chen et al. 2011). Bedell et al. (2012) have reported that a double strand break mediated insertion of the loxP site into the genome by homologous recombination in Zebrafish. Our results also showed that insertion of the loxP site also occurred in newts using ssODN (Fig. 4). It has already been confirmed that some inducible gene expression protocols including the Cre-loxP system work in urodele amphibians (Whited et al. 2012).
Furthermore, it has been demonstrated that TAL effectors combined with the transcriptional activation domains modulated the transcriptional enhancers of the target genes in vivo and in vitro (Crocker & Stern 2013; Xuefei et al. 2013). Konermann et al. successfully altered the epigenetic states of the target using TALE DNA binding domain and histone modifying components in mouse (Konermann et al. 2013). In addition to genome editing, TAL effector-mediated gene manipulations will elucidate the mechanisms underlying the remarkable ability of newts.
Acknowledgments
This work was supported by JSPS KAKENHI Grant-in-Aid for Young Scientists (B), Grant Number 25840086, and a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT). Dr Kenichi Suzuki (Hiroshima University) provided valuable advice concerning the design of the TALEN for newt tyrosinase. We also would like to thank Kyorin Corporation (Hyogo, Japan) for providing the feed for the newts.




