Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer
Abstract
The Japan Gastroenterological Endoscopy Society has developed endoscopic submucosal dissection/endoscopic mucosal resection guidelines. These guidelines present recommendations in response to 18 clinical questions concerning the preoperative diagnosis, indications, resection methods, curability assessment, and surveillance of patients undergoing endoscopic resection for esophageal cancers based on a systematic review of the scientific literature.
Introduction
Endoscopic mucosal resection (EMR)1-3 and endoscopic submucosal dissection (ESD)4 are endoscopic resection (ER) methods developed in Japan for the treatment of patients with esophageal cancer. These methods have gained widespread popularity in Asia and various Western countries. However, although ER represents an excellent treatment option, misjudgment of the indications or curability assessment can result in unfavorable patient outcomes, indicating the need for due attention. Various recent reports have provided accumulating scientific evidence regarding ER. We therefore developed the current guidelines based on up-to-date evidence-based recommendations for preoperative diagnosis, indications, resection methods, curability assessment, and post-resection surveillance in patients treated with ER for esophageal cancer. The guidelines also aim to suggest future research questions.
Clinical practice guidelines are defined as “a document that presents optimal recommendations to support decisions on patient care in highly important situations based on systematic review of scientific literature, strength of evidence, and benefit-to-harm balance.”5 The current guidelines were created in accordance with the methodology described in Minds Manual for Guideline Development 2017.5 The guidelines were developed based on scientific evidence, but also taking account of the benefit-to-harm balance, patient preferences, and medical economics. We also aimed to incorporate useful information for daily practice into these guidelines. The level of recommendation was determined by the guidelines working and internal review committees using an anonymous voting system, with committee members with a conflict of interest abstaining from voting. The poll results have been listed in the guidelines to allow readers to understand the level of agreement among committee members in greater detail. The strength of evidence supporting each recommendation was graded based on the integrated evidence for several different outcomes resulting from a certain medical intervention.
The details of the specific procedures and equipment used for ESD/EMR are given in the Gastroenterological Endoscopy Handbook,6 and the current guidelines specifically present the recommendations for preoperative diagnosis, indications, resection methods, curability assessment, and post-resection surveillance in patients undergoing ER for esophageal cancer. In addition, the Esophageal Cancer Practice Guidelines 20177 were previously published by the Japan Esophageal Society and the items examined in these previous guidelines have not been re-examined in the present guidelines. However, a summary of some of the recommendations in the 2017 guidelines is provided in the current guidelines. Moreover, there are two aspects of diagnosis: i.e., a “clinical diagnosis” based on imaging modalities before treatment, and a “pathological diagnosis” based on pathologic examination after treatment. These clinical and pathological diagnoses were not clearly distinguished in the descriptions of endoscopic treatment in the previous guidelines. However, these two diagnoses often differ from each other and should thus be treated differently. We therefore clearly distinguished between these two diagnoses and presented recommendations for indications based on the clinical diagnosis, and for curability assessment based on the pathological diagnosis.
The present guidelines serve as a guide for standard treatments under the scope of the Japanese health insurance system and are not intended to coerce practitioners into performing certain medical procedures. Clinical decisions in daily clinical practice should be made on an individual basis in accordance with the patient's condition and situation at the institution. This guideline on gastrointestinal endoscopy was published in the Japanese language in 2020.8
Preparation Procedures for these Guidelines
Committees
Eleven gastrointestinal endoscopists were commissioned as working committee members for the guidelines. A systematic review was conducted by 10 members of the systematic review committee together with these 11 working committee members. Assessment was made by an internal review committee comprising two gastrointestinal endoscopists, one gastrointestinal surgeon, and one physician in charge of guideline-development methodology, while the external review committee comprised two gastrointestinal endoscopists, one gastrointestinal pathologist, and one epidemiologist (Table 1).
| Japan Gastroenterological Endoscopy Society Guidelines Committee | |
| President | Haruhiro Inoue (Digestive Diseases Center, Showa University Koto Toyosu Hospital) |
| Senior advisor | Hisao Tajiri (Department of Innovative Interventional Endoscopy Research, The Jikei University School of Medicine) |
| Responsible director | Kazuma Fujimoto (International University of Health and Welfare) |
| Chairperson | Kazuma Fujimoto (International University of Health and Welfare) |
| Committee of the endoscopic submucosal dissection/endoscopic mucosal resection Guideline for Esophageal Cancer | |
| Working committee chairperson | Ryu Ishihara (Department of Gastrointestinal Oncology, Osaka International Cancer Institute) |
| Guideline working committee members | Miwako Arima (Division of Gastroenterology, Saitama Cancer Center) |
| Toshiro Iizuka (Department of Gastroenterology, Toranomon Hospital) | |
| Tsuneo Oyama (Department of Endoscopy, Saku Central Hospital Advanced Care Center) | |
| Chikatoshi Katada (Department of Gastroenterology, Kitasato University School of Medicine) | |
| Motohiko Kato (Division of Gastroenterology, Keio University School of Medicine) | |
| Kenichi Goda (Department of Gastroenterology, Dokkyo Medical University) | |
| Osamu Goto (Department of Gastroenterology, Nippon Medical School, Graduate School of Medicine) | |
| Kyosuke Tanaka (Department of Endoscopy, Mie University Hospital) | |
| Tomonori Yano (Department of Gastroenterology and Endoscopy, National Cancer Center Hospital East) | |
| Shigetaka Yoshinaga (Endoscopy Division, National Cancer Center Hospital) | |
| Internal review committee chairperson | Manabu Muto (Department of Therapeutic Oncology, Graduate School of Medicine, Kyoto University) |
| Internal review committee members | Hirofumi Kawakubo (Department of General and Gastroenterological Surgery, Keio University Hospital: The Japan Esophageal Society) |
| Mitsuhiro Fujishiro (Department of Gastroenterology and Hepatology, Nagoya University Graduate School of Medicine) | |
| Masahiro Yoshida (Department of Hepato-Biliary-Pancreatic & Gastrointestinal Surgery, International University of Health and Welfare) | |
| External review committee members | Haruhiro Inoue (Digestive Diseases Center, Showa University Koto Toyosu Hospital) |
| Tomio Arai (Department of Pathology, Tokyo Metropolitan Geriatric Hospital and Institute of Gerontology) | |
| Tomoyuki Koike (Division of Gastroenterology, Tohoku University Hospital) | |
| Hideo Tanaka (Fujiidera Public Health Center, Osaka Prefecture) | |
| Systematic review committee members | Teppei Akimoto (Cancer Center, Keio University School of Medicine/Department of Gastroenterology, Graduate School of Medicine, Nippon Medical School) |
| Youhei Ikenoyama (Department of Gastroenterology, Cancer Institute Hospital, Japanese Foundation for Cancer Research) | |
| Kenji Ishido (Department of Gastroenterology, Kitasato University School of Medicine) | |
| Taro Iwatsubo (Department of Gastroenterology, Moriguchi Keijinkai Hospital/2nd Department of Internal Medicine, Osaka Medical College) | |
| Yugo Iwaya (Department of Gastroenterology, Shinshu University Hospital) | |
| Tomohiro Kadota (Department of Gastroenterology and Endoscopy, National Cancer Center Hospital East) | |
| Yuto Shimamura (Digestive Disease Center, Showa University Koto Toyosu Hospital) | |
| Yugo Suzuki (Department of Gastroenterology, Toranomon Hospital) | |
| Atsushi Nakayama (Cancer Center, Keio University School of Medicine) | |
| Yasuhiko Mizuguchi (Endoscopy Division, National Cancer Center Hospital) | |
| Related societies | The Japan Esophageal Society, The Japanese Society of Gastroenterology, The Japanese Gastroenterological Association |
Target users
These guidelines are primarily intended for use by health professionals involved in gastrointestinal endoscopy. The subjects of the guidelines are adult patients with esophageal cancer.
Clinical practice guidelines development method
Clinical questions (CQs)
Working committee members selected questions related to ESD/EMR for esophageal cancer with answers or recommendations that would improve the quality of clinical practice as CQs. The CQs were formulated in the patients, interventions, comparisons, and outcomes (PICO) format. For each CQ, outcomes considered important for patients receiving the intervention were determined by the working committee members. The importance of each outcome was classified as “very important”, “important”, or “not important”. Very important and important outcomes were subjected to systematic review.
Literature search and systematic review
Search terms were extracted via the PICO framework and a search strategy was determined in cooperation with the librarians of the Japan Medical Library Association. The literature was searched from January 2005 to April 2019. Articles that could not be collected through the systematic search were found by manual searching. Regarding the inclusion criteria for systematic review, randomized controlled trials (RCTs) were given priority, but non-randomized and observational studies were also included. Article inclusion was determined by two individuals. The validity (or quality) of the evidence was evaluated in accordance with the method proposed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group9 and ultimately graded on a four-point scale as high, moderate, low, or very low. The strength of the evidence for each recommendation was determined based on the validity of the evidence (Table 2).
| A (Strong) | Strong confidence that estimated effect adequately support the recommendation |
| B (Moderate) | Moderate confidence that estimated effect adequately support the recommendation |
| C (Weak) | Weak confidence that estimated effect adequately support the recommendation |
| D (Very weak) | Very weak confidence that estimated effect adequately support the recommendation |
Determination of recommendations and their strength of evidence
- Performing or not performing is “strongly recommended”.
- Performing or not performing is “weakly recommended”.
The present guidelines were then completed after revision in response to external review committee comments and public comments.
Funding
Funds related to the development of these guidelines were provided by the Japan Gastroenterological Endoscopy Society.
References
Part 1: Esophageal Squamous Cell Carcinoma
Chapter 1: Preoperative diagnosis of esophageal squamous cell carcinoma and indications for ER
Introduction
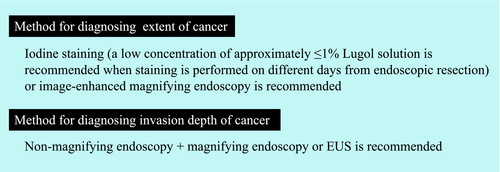
The treatment strategy for superficial squamous cell carcinoma of the esophagus is determined based on preoperative diagnosis of cancer invasion depth, lateral extent of the cancer, and metastasis. The Esophageal Cancer Practice Guidelines 20171 suggest “performing endoscopic ultrasound (EUS) or magnifying endoscopy in addition to non-magnifying endoscopy” to diagnose the cancer invasion depth. This recommendation is based on a systematic review2 of the diagnosis of cancer invasion depth in esophageal cancer, which revealed that EUS and magnifying endoscopy had a higher diagnostic accuracy than non-magnifying endoscopy. The guidelines also recommend ER for clinically diagnosed T1a-epithelial/lamina propria (EP/LPM) cancers. Furthermore, the guidelines report that the extent of ER is closely related to the risk of stenosis, and it is therefore “strongly recommended to evaluate the circumferential extent of the lesion preoperatively.” It is also noted that stenosis might develop following ER if the circumferential extent of the lesion is extensive. Image-enhanced magnifying endoscopy or iodine staining is recommended to diagnose the lateral extent of the lesion, whereby the lesion border can be clearly delineated by the latter. However, use of iodine solution at a high concentration may cause the superficial epithelium to peel off, making a subsequent diagnosis difficult; hence, iodine solution is recommended to be used at a low concentration of ≤1%.
The chapter on endoscopic treatment in the Esophageal Cancer Practice Guidelines 2017 considers ER as a relative indication for T1a-MM/T1b-SM1 (MM/SM1) cancer. However, it is unclear if the diagnosis of MM/SM1 is a clinical or pathological diagnosis. There can be considerable discrepancy between clinical and pathological diagnoses, and these should thus be treated separately. As mentioned above, the policy of the present guidelines was to describe the indication for ER based on the clinical diagnosis and the curability assessment based on the pathological diagnosis. However, the validity of ER in subjects limited to those with clinical (c) MM/SM1 cancers has not been investigated adequately to date. Furthermore, the validity of ER for esophageal cancer occupying the entire circumference with an extremely high risk of stenosis is also unclear. In this chapter, we therefore performed a systematic review of the CQs pertaining to these two issues and the newly created recommendations. The recommendation summaries are presented in Figures 1-3.

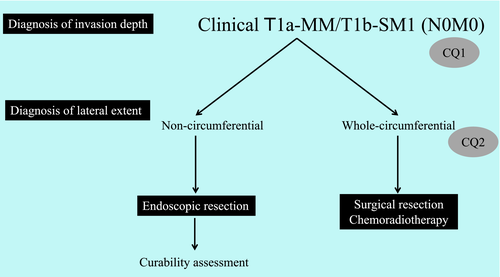
CQ1: Is endoscopic resection recommended as first-line treatment for preoperatively diagnosed cT1a-MM/T1b-SM1 (N0M0) non-circumferential esophageal squamous cell carcinomas?
Recommendation statement: Endoscopic resection is weakly recommended as first-line treatment for preoperatively diagnosed cT1a-MM/T1b-SM1 non-circumferential esophageal squamous cell carcinomas.
Modified Delphi scores: median = 9, lowest = 8, highest = 9 Strength of evidence: C
Commentary: The indication for ER is determined based on the preoperative diagnosis of cancer invasion depth. cMM cancers have been considered as a relative indication for ER in previous guidelines; however, if post-resection histological findings reveal pathological (p) EP/LPM invasion and negative vascular invasion, the resection is judged as curative. Surgical resection or chemoradiotherapy is recommended for cT1b cancers according to the Esophageal Cancer Practice Guidelines 2017; however, some cSM1 cancers may result in pT1a-M (pM) following surgical resection, and ER may have been sufficient for such lesions. Clarification of the validity of performing ER for cMM and cSM1 cancers would therefore help to determine the appropriate treatment strategies for these cancers, and we therefore examined the effectiveness of ER for such cancers. Furthermore, it can be difficult to distinguish between cMM and cSM1 cancers during preoperative diagnosis using endoscopy, and these two entities were therefore treated as being in the same category (i.e., cMM/SM1 cancer) in this manuscript.
Our literature search found no reports comparing the outcomes of ER with those of other treatments for cMM/SM1 superficial squamous cell carcinoma of the esophagus. A search for studies examining the surgical resection and ER of cMM/SM1 cancers and the associated pathological results produced 262, 15, and 78 articles from the PubMed, Cochrane, and Japan Medical Abstracts Society (JAMAS), respectively. These 355 articles underwent primary screening and 57 articles were selected for secondary screening. After applying stringent selection criteria, we performed a systematic review of seven articles3-9 and also investigated articles on chemoradiotherapy as an additional treatment10 and articles comparing ER with surgical resection.11, 12
Diagnostic accuracy of cancer invasion depth
We first examined the diagnostic accuracy of image-enhanced magnifying endoscopy and EUS for preoperative diagnosis. Analysis of studies examining the pathological diagnosis following the resection of cancers diagnosed as cMM/SM1 based on type B2 vessels according to the magnified endoscopic classification of the Japan Esophageal Society identified 212 lesions from six articles,3-8 among which the pathological diagnoses were pEP/LPM in 27.4% (58/212), pMM/SM1 in 55.7% (118/212), and pSM2 in 17.0% (36/212) lesions (Table 3). The post-resection pathological diagnoses of cMM/SM1 cancers diagnosed based on type V3 blood vessels of intrapapillary capillary loops9 were pEP/LPM in 29.8%, pMM/SM1 in 42.3%, and pSM2 in 27.9% lesions. The post-resection pathological diagnoses of cMM/SM1 cancers diagnosed based on EUS findings3 were pEP/LPM in 55.2%, pMM/SM1 in 29.3%, and pSM2 in 15.5% lesions (Table 4).
| Magnifying endoscopic diagnosis | pEP/LPM | pMM/SM1 | pSM2 |
|---|---|---|---|
| cEP/LPM (B1 vessels) | 92.4% (661/715 lesions) | 6.0% (43/715 lesions) | 1.5% (11/715 lesions) |
| cMM/SM1 (B2 vessels) | 27.4% (58/212 lesions) | 55.7% (118/212 lesions) | 17.0% (36/212 lesions) |
| cSM2 (B3 vessels) | 0% (0/43 lesions) | 9.3% (4/43 lesions) | 90.7% (39/43 lesions) |
| EUS diagnosis | pEP/LPM | pMM/SM1 | pSM2 |
|---|---|---|---|
| cEP/LPM | 84.0% (89/106 lesions) | 14.2% (15/106 lesions) | 1.9% (2/106 lesions) |
| cMM/SM1 | 55.2% (32/58 lesions) | 29.3% (17/58 lesions) | 15.5% (9/58 lesions) |
| cSM2 | 30.0% (3/10 lesions) | 30.0% (3/10 lesions) | 40.0% (4/10 lesions) |
The above results indicated that, even among cancers diagnosed as cMM/SM1 before treatment, 27.4–55.2% of cases were pEP/LPM cancers, for which ER is highly likely to be curative, whereas 15.5–27.9% cases included pSM2 cancers. The accuracy of preoperative diagnosis for cMM/SM1 cancers is thus poor, and the least-invasive treatment option (usually ER) should therefore be selected as the first-line treatment in these cases.
Safety of ER and additional treatment for non-curative resection cases
The JCOG0508 trial was a confirmatory study for the efficacy of ER followed by chemoradiotherapy in patients with cSM1/SM2 cancer.10 In that study, patients with “pMM, negative vascular invasion, and negative resection margin” based on the pathological results following ER underwent follow-up observation, while patients with “pMM, positive vascular invasion, and negative resection margin” or “pSM and negative resection margin” underwent prophylactic chemoradiotherapy (41.4 Gy), and patients with a “positive resection margin” underwent definitive chemoradiotherapy. As a result, the 3-year overall survival rate for all patients was 92.6% [90% confidence interval (CI): 88.5–95.2%] and the 3-year progression-free survival rate was 89.7% (90% CI: 84.2–93.4%). Favorable results were obtained in the prophylactic chemoradiotherapy group, with a 3-year overall survival rate of 90.7% (90% CI: 84.0–94.7%). ER-related grade ≥3 adverse events (CTC-AE 3.0) included esophageal stenosis in only 0.6% of patients. These results showed that ER can be performed safely for cSM1/SM2 cancer, which is more advanced than cMM/SM1 cancer. Furthermore, even when such ER does not lead to curative resection, a good prognosis can be expected if suitable additional treatment is administered based on the pathological findings.
Comparison of ER and surgical resection
Two articles compared the outcomes of ESD and surgical resection for pT1 squamous cell carcinoma of the esophagus, both of which were single-center, retrospective studies. A report from Shanghai11 found fewer treatment-related deaths in patients in the ESD compared with the surgery group, although the difference was not significant (0.3% vs. 1.5%; P < 0.186). Furthermore, there were significantly fewer severe complications in the ESD group than in the surgical resection group (15.2% vs. 27.7%; P < 0.001), particularly esophageal fistulas (0.3% vs. 16.4%; P < 0.001) and respiratory complications (0.3% vs. 3.6%; P < 0.004). Post-treatment stenosis was more common in the ESD group but the difference was not significant (13.4% vs. 9.9%; P < 0.203). However, the treatment duration and length of hospital stay were significantly shorter (49 min vs. 240 min; P < 0.001 and 3 days vs. 11 days; P < 0.001, respectively) and the cost of hospitalization was significantly lower (median 2813 USD vs. 10,001 USD; P < 0.001) in the ESD compared with the surgical resection group. There was no significant difference between the two groups in terms of all deaths, disease-specific death rates, or metastasis rates over a mean observation period of 21 months. The results were comparable after adjusting for confounding factors including age, sex, invasion depth, other organ cancers, and the presence or absence of radiotherapy.
Similarly, a report from Korea12 found no difference between the ESD and surgical resection groups after mean observation periods of 43 and 63 months, respectively, in terms of overall survival, disease-specific survival, or recurrence-free survival. ER is therefore considered safer and less invasive than surgical resection in patients with pT1 cancers, as well as being superior in terms of medical economics. Furthermore, patients are likely to prefer ER over surgical resection and this choice therefore coincides with the patients’ wishes.
Summary
CQ2: Is endoscopic resection recommended for superficial squamous cell carcinomas involving the entire circumference of the esophagus?
Recommendation statement: Endoscopic resection is weakly recommended for cT1a-EP/LPM superficial squamous cell carcinomas with a major axis length ≤50 mm and involving the entire circumference of the esophagus, upon implementing preventive measures for stenosis.
Modified Delphi scores: median = 7, lowest = 3, highest = 9 Strength of evidence: C
Commentary: ER for esophageal cancer is a minimally invasive treatment with high curative potential. However, whole-circumferential ER can result in intractable stenosis, considerably reducing the patient's quality of life (QOL). Recent reports indicated that stenosis following whole-circumferential resection may be prevented in some cases. Clarification of the recommendations for and against ER for circumferential esophageal cancer could help to determine the appropriate treatment strategies.
Obtaining a favorable prognosis without reducing QOL are the most important factors when considering ER for superficial squamous cell carcinoma involving the whole circumference of the esophagus. However, our literature search failed to identify any reports directly comparing the outcomes of ER and other treatments. We therefore investigated the risk of stenosis following whole-circumferential ER, the outcomes of ER for widespread superficial esophageal squamous cell carcinoma, and the outcomes of surgical resection and chemoradiotherapy for cT1N0M0 esophageal squamous cell carcinoma.
Our literature search for studies reporting therapeutic outcomes of whole-circumferential ESD identified 151, 18, and 26 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 195 articles underwent primary screening and 27 articles were selected for secondary screening. After applying stringent selection criteria, one RCT13 and 12 retrospective observational studies14-25 on preventive endoscopic balloon dilatation, steroid injection therapy, oral steroid therapy, and polyglycolic acid sheets were reviewed.
ESD outcomes for circumferential esophageal cancer
Multiple studies have reported on the survival rates following ESD for esophageal squamous cell carcinoma; however, our literature search showed that none of these studies described the specific survival rates of patients with circumferential esophageal squamous cell carcinoma. One study of 22 patients who underwent subtotal or greater circumferential resection (including seven patients who underwent whole-circumferential resection) reported recurrence-free survival of all patients over a median follow-up period of 15.5 months.26 In another study, 51 patients underwent subtotal or greater circumferential resection (including 11 patients who underwent whole-circumferential resection) and were followed up for a median of 29 months.16 In this study, stenosis was successfully eliminated via balloon dilatation (a median of four sessions) in all seven patients who developed stenosis, and no serious adverse events were observed.16 The complete resection rate (negative resection margin) was 100% using ESD in all studies reporting this rate.14, 19 Although curative resection rates have not been reported after ESD for circumferential esophageal cancer, curative resection was achieved in 70% of patients with cEP/LPM widespread superficial esophageal cancer, which is an extensive lesion resembling circumferential esophageal cancer.27
Stenosis rates following ESD for circumferential esophageal cancer (without stenosis prevention)
In the absence of stenosis-prophylaxis measures following ER,13, 15, 17, 18, 22, 23 stenosis developed in all 31 patients, and a mean of 26 balloon dilatation sessions were required to eliminate the stenosis. These results do not support a recommendation to perform ER without prophylaxis for stenosis.
Stenosis rates following ESD for circumferential esophageal cancer (with stenosis prevention using steroids)
When stenosis-prophylaxis strategies were used following ER, stenosis rates were 76% in 45 patients who received steroid injection therapy,13, 15, 16, 20, 21 55% in 44 patients who received oral steroid therapy,14, 16, 18-20, 22, 23 and 71% in 14 patients who received both steroid injection therapy and oral steroid therapy.23 However, these studies included widespread esophageal cancers with a mean major axis length of 6 cm.
Miwata et al.20 examined patients who received stenosis prophylaxis using steroid injection therapy or oral steroid therapy following whole-circumferential resection, and found that the stenosis risk was increased when the resection diameter was >50 mm. Accordingly, 85% of patients (11/13 patients) required at least six sessions when the resection major axis length was >50 mm, compared with only 17% of patients (1/6 patients) with a resection major axis length ≤50 mm. Although the specific numbers of dilatation sessions were not reported for the second group (resection ≤50 mm), these were likely to be relatively few. Furthermore, Yamaguchi et al.16 reported that, even among patients who underwent whole-circumferential resection, the administration of oral steroids with prednisolone at a starting dose of 30 mg and tapered for 12–18 weeks limited the stenosis rate to 27.3% (3/11 patients), and a mean of only 1.6 sessions of balloon dilatation were required in patients who developed stenosis. Shibagaki et al.26 treated seven patients with whole-circumferential resections and prevented stenosis in all patients by filling the esophagus with steroids immediately after and 1 week after ESD, as well as when mild stenosis developed.
Dilatation can therefore be achieved in relatively few sessions even if stenosis develops, as long as it is limited to short segments ≤50 mm in length. Furthermore, an effective method has been developed to prevent stenosis following whole-circumferential stenosis.
Adverse events related to ESD and steroid therapy
The use of steroids to prevent stenosis has reportedly been associated with a risk of perforation, caused by fragility of the esophageal wall due to steroid therapy.28, 29 Moreover, oral steroid therapy has been associated with diabetes and, extremely rarely, with serious infection.30 ESD for widespread compared with small lesions is considered to be a risk factor for procedure-related complications. Although no significant differences were reported in a study of this comparison,27 it is preferable for ESD to be performed by an expert endoscopist, considering the potentially high incidence of complications.
Radiofrequency ablation
Radiofrequency ablation (RFA) has recently been used for the treatment of Barrett's esophagus or superficial esophageal squamous cell carcinoma overseas, but has not been approved for these indications in Japan. In a Chinese study,31 90 patients with moderate-grade intraepithelial neoplasia (MGIN) – cT1a cancer received one to four sessions of RFA over a 1-year period. Subsequent endoscopic evaluations revealed that the lesions disappeared in 78 of these patients, whereas 12 patients had residual lesions. Among the 12 patients with residual lesions, five were cured by additional RFA or EMR, while the lesions in six patients progressed; four of these six patients underwent surgical resection, one received chemoradiotherapy, and one received ESD. The other patient withdrew from the study. Among the 78 patients in whom the lesions disappeared, SM cancer recurred in three patients and moderate- to high-grade intraepithelial neoplasia recurred in eight patients. Based on this report, we consider that the effects of RFA for MGIN – cT1a esophageal cancer are insufficient.
Chemoradiotherapy
In a phase II trial (JCOG9708)32 of chemoradiotherapy including 72 patients with cT1N0M0 esophageal squamous cell carcinoma, over 90% of patients achieved a complete response, with a 4-year overall survival rate of 80.5%. However, local recurrences (including metachronous cancers of the esophagus) were observed in 31% of patients, with a 4-year disease-free survival rate of only 52.8%.
More recently, a retrospective study of definitive chemoradiotherapy in 36 patients with cT1bN0M0 esophageal squamous cell carcinoma found that local and metastatic recurrences were common, with a 5-year overall survival rate of 86% and a 5-year disease-free survival rate of 59%.33
Data from the JCOG9708 trial32 showed that grade ≥2 adverse events included dyspnea in 11.1%, esophagitis in 2.7%, ischemic heart disease in 2.7%, myocarditis in 2.7%, and arrhythmia in 1.4% of patients. A recent report found33 grade ≥2 adverse events including esophageal stenosis in 11% and pleural effusion in 14% of patients, with grade 4 pericardial effusion in 3% and grade 5 pneumonia in 3% of patients.
Surgical resection
Our literature search failed to identify any recent Japanese reports describing the outcomes of surgical resection for cT1aN0M0 esophageal squamous cell carcinoma alone. The outcomes of surgical resection in patients with cT1bN0M0 esophageal cancer (JCOG0502 trial)34 showed a good 5-year survival rate of 86.5%. However, grade 3 and 4 adverse events including anastomotic leak occurred in 6.3%, pneumonia in 7.7%, recurrent nerve palsy in 2.9%, and fistula in 1.9% of patients.
Summary
Endoscopic submucosal dissection and chemoradiotherapy both enable organ preservation and are relatively less-invasive treatments compared with surgical resection. However, the high incidence of postoperative stenosis following ESD presents a problem. Nevertheless, stenosis following prophylactic measures and in patients with resections with a major axis length of ≤50 mm can be resolved by five or fewer sessions of dilatation in most cases. Furthermore, more effective stenosis-prophylaxis measures have recently been developed and serious procedural accidents have become rare. Meanwhile, chemotherapy may be associated with serious adverse events such as dyspnea and pericardial effusion, and death from pneumonitis was recently reported in 3% of patients receiving chemoradiotherapy.33
In terms of outcomes, complete resection can be achieved by ESD in most patients with esophageal cancer, and ESD can be curative in approximately 70% of cases of cEP/LPM cancers ≤50 mm in size, based on preoperative diagnosis. Conversely, chemoradiotherapy can achieve a complete response in 90% of cases, but it carries a relatively high risk of local recurrence. Although salvage therapies such as photodynamic therapy and ESD are often possible for localized recurrences, intensive surveillance is required to detect local recurrences.
The benefit and harm profiles of ESD and chemoradiotherapy therefore differ, making a simple comparison impossible. However, the benefit-to-harm balance of ESD limited to subjects with cEP/LPM cancers ≤50 mm in size was equivalent or superior to that of chemoradiotherapy, reflecting the minimal invasiveness of ESD. Thus, if either ESD or chemoradiotherapy is indicated, we recommend ESD as the first-line treatment and chemoradiotherapy as a possible after-treatment option.
Considering the overall benefits and harms of ESD and surgical resection, surgical resection is superior in terms of curability. However, relatively serious procedural adverse events, such as anastomotic leak, occur in approximately 19% of patients and reduced postoperative QOL is a cause for concern following surgical resection. Conversely, curative resection can be expected in approximately 70% of cEP/LPM cancers ≤50 mm in size based on preoperative diagnosis, and even if curative resection is not achieved, a good prognosis can be expected if suitable additional treatment is administered. Hence, balancing the benefits of organ preservation and the harm of postoperative complications, we believe that ESD can be recommended for selected lesions.
The efficacy of ESD for circumferential esophageal cancer needs to be clarified in a prospective, multicenter study including analyses of post-treatment prognosis, QOL, and stenosis rates. Based on the above information, ESD is weakly recommended due to insufficient supporting evidence; however, it is recommended for cEP/LPMs ≤50 mm in size and for circumferential esophageal cancer.
References
Chapter 2: ER for esophageal squamous cell carcinoma
Introduction
Endoscopic resection has gained widespread popularity for the treatment of superficial esophageal squamous cell carcinoma in Asia and various Western countries. This procedure is conducted to completely remove tumors and to obtain a specimen for histological diagnosis. En bloc resection is required for curability because piecemeal resection increases the risk of local recurrence. Furthermore, en bloc-resected specimens can provide an accurate histological diagnosis. ESD is an ER method that enables en bloc resection of lesions that underwent piecemeal resection in EMR procedures. New technologies that facilitate ESD, such as traction devices, have been introduced to support the further widespread use of ESD. We therefore addressed a CQ regarding the use of traction devices and established a relevant recommendation.
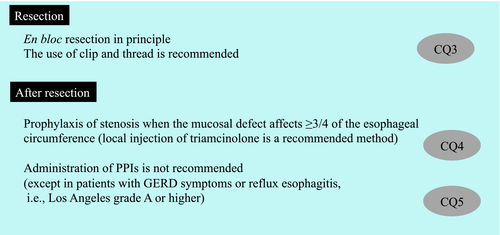
CQ3: Is the use of a traction device recommended when performing ESD for superficial esophageal squamous cell carcinoma?
Recommendation statement: Use of a traction device using a clip and thread is weakly recommended when performing ESD for superficial esophageal squamous cell carcinoma.
Modified Delphi scores: median = 8.5, lowest = 7, highest = 9 Strength of evidence: B
Commentary: It is challenging to perform ESD for superficial esophageal squamous cell carcinoma safely in the narrow esophageal lumen, and serious adverse procedure-related events, such as perforation, could occur during ESD. Traction devices are expected to improve procedural safety, and the efficacy and safety of these devices therefore need to be investigated in terms of shortening procedure times and decreasing adverse events.
Our literature search for studies reporting the usefulness of traction devices in ESD for esophageal cancer identified 337, 138, and 62 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 537 articles underwent primary screening and 17 were included in secondary screening. After applying stringent selection criteria, one RCT and two case–control studies that examined the usefulness of clip-and-thread traction devices were included in a qualitative systematic review.
Evaluation of therapeutic outcomes
All three included studies involved treatments conducted by two or more experts (Table 5). In the RCT,2 the use of traction devices was associated with a significantly shorter procedure time (traction 19.8 min vs. no traction 31.8 min; P = 0.044). One of the case–control studies also found a significant difference in procedure times.3 In the other study,4 analysis of a subset of lesions less than half of the circumference showed a significant difference in procedure times (traction 22.0 min vs. no traction 26.5 min; P = 0.018), while no significant difference was reported when widespread lesions such as whole-circumferential lesions were included.
Evaluation of adverse events
We evaluated muscular layer damage and perforation as adverse events (Table 6). In the RCT,2 the rates of muscular layer damage were similar in both treatment groups (traction 40% vs. no traction 55%; P = 0.34) and no cases of perforation were reported. There were no instances of perforation in the case–control studies,3, 4 and the use of a clip-and-thread device reduced the rate of muscular layer damage from 15% to 0% in one study and from 30% to 10% in the other study (P = 0.007).
Summary
CQ4: Is local injection of triamcinolone recommended compared with no prophylaxis in patients with mucosal defects affecting ≥3/4 of the esophageal circumference after endoscopic resection for superficial esophageal squamous cell carcinoma?
Recommendation statement: Local injection of triamcinolone is weakly recommended when mucosal defects affecting ≥3/4 of the esophageal circumference occur after endoscopic resection for superficial esophageal squamous cell carcinoma.
Modified Delphi scores: median = 9, lowest = 5, highest = 9 Strength of evidence: B
Commentary: A search of the literature relating to this CQ identified 190, 81, and 73 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 344 articles underwent primary screening and 39 articles were selected for secondary screening. After applying stringent selection criteria, one RCT and three case–control studies were included in a systematic review. The severity of stenosis following local injection of triamcinolone differs depending on whether the mucosal resection is non-circumferential or whole-circumferential, and these two instances were therefore considered separately.
Preventive effects of triamcinolone acetonide injection against stenosis after non-circumferential mucosal defects
The stenosis rate following local injection of triamcinolone for non-circumferential lesions was 10–45%,5-8 which tended to be lower than the stenosis rate of 61–82% without local triamcinolone injection (Table 7). Furthermore, the mean number of balloon dilatation sessions required after stenosis tended to be fewer following local injection (injection 0–1.7 sessions vs. non-injection 2–6 sessions).5-8
| Degree of mucosal defect | Injection | Injection dose (mg) | Injection time (day) | Stenosis rate (%) | Number of dilatation sessions | Reference | ||
|---|---|---|---|---|---|---|---|---|
| >2/3 | TA | 40 | 0 | 45 (5/11) | P = 0.18 | 6.1† | P < 0.05 | 5 |
| Control | 0 | ― | 82 (9/11) | ― | 12.5† | ― | ||
| >3/4 | TA | 50 | 3, 7, 10 | 36 (17/47) | P = 0.06 | ― | ― | 6 |
| Control | 0 | ― | 61 (17/28) | ― | ― | ― | ||
| >3/4 | TA | 100 | 0 | 10 (3/30) | P < 0.01 | 0 | P < 0.01 | 7 |
| Control | 0 | ― | 66 (19/29) | ― | 2 | ― | ||
| >3/4 | TA | 18–62 | 3, 7, 10 | 19 (4/21) | P < 0.01 | 1.7 | P < 0.01 | 8 |
| Control | 0 | ― | 75 (15/20) | ― | 6.6 | ― | ||
- †Including the entire circumference.
- TA, triamcinolone acetonide.
Preventive effects of triamcinolone acetonide injection against stenosis after whole-circumferential defects
Triamcinolone did not prevent stenosis after whole-circumferential lesions, based on two studies that reported stenosis rates of 100% after local injection of triamcinolone (Table 8).5, 6 However, these studies only used relatively low doses considering the extent of the mucosal defect. The mean number of balloon dilatation sessions required following stenosis tended to be fewer following local injection (injection 6–10.4 sessions vs. non-injection 12.5–22.2 sessions),5, 6 but there was no significant difference in the durations required for repeated dilatation in either study.
| Injection | Injection dose (mg) | Injection time (day) | Stenosis rate (%) | Number of dilatation sessions | Reference | ||
|---|---|---|---|---|---|---|---|
| TA | 40 | 0 | 100 (5/5) | P = 0.99 | 10.4 | P < 0.05 | 5 |
| Control | 0 | ― | 100 (5/5) | 22.2 | |||
| TA | 50 | 3, 7, 10 | 100 (6/6) | P = 1.0 | 6.0† | P < 0.05 | 6 |
| Control | 0 | ― | 100 (5/5) | 12.5† | |||
- †Lesions affecting 7/8 of the circumference (entire circumference).
- TA, triamcinolone acetonide.
Adverse events
Regarding safety, the identified studies did not make it clear if any of the adverse events were directly related to the local triamcinolone injections. We therefore considered adverse events at the time of dilatation as well as at the time of ER. The reported incidences of perforation or bleeding were 0–6.25% (1/16 patients)5-8 in the case of non-circumferential resection and 0–33.3% (2/6 patients) in the case of whole-circumferential resection.5, 6
Summary
Perforation after local triamcinolone injection can likely be avoided by not injecting into the muscular layer, and perforation during balloon dilatation can likely be avoided by using balloons with smaller diameters. Local triamcinolone injection thus significantly reduced the number of dilatation sessions, although stenosis rates were unaffected following whole-circumferential resections but were significantly reduced following non-circumferential resections. These findings suggest that local triamcinolone injection might help to prevent stenosis, and is thus weakly recommended.
CQ5: Is PPI therapy recommended after endoscopic resection for superficial esophageal squamous cell carcinoma?
Recommendation statement: It is weakly recommended that PPIs should not be administered to prevent bleeding and promote ulcer healing following endoscopic resection for superficial esophageal squamous cell carcinoma, except in patients with gastroesophageal reflux disease symptoms or reflux esophagitis of grade A or higher according to the Los Angeles Classification at the time of endoscopic resection.
Modified Delphi scores: median = 9, lowest = 7, highest = 9 Strength of evidence: C
Commentary: The incidence of superficial esophageal squamous cell carcinoma has increased in recent years and it is generally treated with ER. The postoperative management of such cases is often based on the management procedure for ER of early-stage gastric cancer. However, unlike the stomach, the esophagus is less-exposed to gastric acid and the use of PPI therapy therefore needs to be considered in terms of its advantages and cost effectiveness.
We systematically searched for studies that assessed the effectiveness of PPIs following ER for esophageal cancer and extracted 187, 120, and 187 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 494 articles underwent primary screening and three articles were selected for secondary screening. After applying stringent selection criteria, one RCT was included. This study9 included patients with a frequency scale for symptoms of GERD of >7 points and excluded patients with a history of surgery, radiotherapy, or anticancer drugs for esophageal squamous cell carcinoma, those with reflux esophagitis of grade A or higher according to the Los Angeles Classification, and those requiring steroids or non-steroidal anti-inflammatory drugs.
Proton pump inhibitor treatment had no apparent effects on ulcer-healing rates (84% without PPI vs. 85% with PPI) or GERD symptom-appearance rates (25% without PPI vs. 30% with PPI). There were also no differences between the groups in terms of adverse events, including treatment-related bleeding, pain, perforation, and stenosis. Observed adverse events of grade 3 or higher included esophageal pain in 4% of patients without PPI treatment and 2% of patients with PPI treatment, and pharyngeal pain in 3% of patients with PPI treatment. In this trial, a PPI was administered for 5 weeks starting immediately after ESD, which increased the financial burden on these patients compared with those without PPI treatment. However, in this study, PPI treatment was administered when GERD symptoms appeared following ER, and symptoms improved in 90% of patients.
It is therefore weakly recommended that PPIs should not be administered except in patients with GERD symptoms or reflux esophagitis of grade A or higher according to the Los Angeles Classification, because the financial costs outweigh the effectiveness.
References
Chapter 3: Assessment of curability following ER for superficial esophageal squamous cell carcinoma and recommendations for additional treatments
Introduction
Curability following ER is determined based on the histological findings of the resected specimens. Lymph node metastasis occurs in some patients with esophageal squamous cell carcinoma, including those with pT1a cancer. However, the incidence of lymph node metastasis is extremely low in patients with pEP/LPM cancers without vascular invasion and with negative resection margins, and curative resection is indicated and additional treatments are considered unnecessary.1 Conversely, there is an increased risk of metastasis in patients with pMM cancers, and because the incidence of metastasis depends on the presence or absence of vascular invasion, the Esophageal Cancer Practice Guidelines 20171 state that “for pMM cancer with vascular invasion, it is strongly recommended to administer additional treatment.” In this section, we posed CQs concerning how to determine curability in patients with pMM cancers who are negative for vascular invasion, as well as patients with pSM cancers. We generated corresponding recommendations, as summarized in Figure 5.
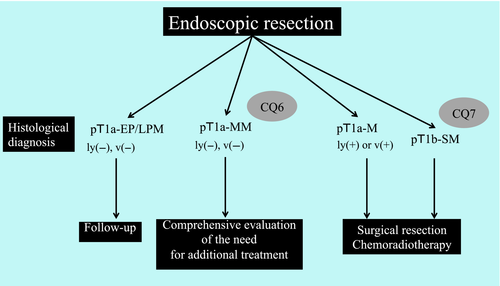
CQ6: Is additional treatment with surgical resection or chemoradiotherapy recommended in patients with pT1a-MM esophageal squamous cell carcinoma who are negative for vascular invasion according to histological findings following endoscopic resection?
Recommendation statement: We could not determine a recommendation for or against the administration of additional treatments in patients with pT1a-MM esophageal squamous cell carcinoma and negative vascular invasion following endoscopic resection.
Strength of evidence: D
Commentary: We searched the literature for studies that addressed the CQ regarding the additional use of surgical resection or chemoradiotherapy in patients with pMM esophageal squamous cell carcinomas who are negative for vascular invasion following ER. We identified 188, 24, and 19 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 231 articles plus 14 manually searched articles underwent primary screening, after which 44 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of 27 articles was performed.
The incidence of metastasis was examined in terms of concurrent lymph node metastasis rates in surgically resected specimens and metastatic recurrence rates after ER. Adverse events related to surgical resection and chemoradiotherapy were primarily examined in terms of treatment-related deaths. Improvements in survival were initially assessed as mortality after treatment; however, many deaths were unrelated to esophageal cancer and we accordingly did not include survival analyses after treatment in the review of this CQ. We were also unable to assess reduced QOL, prolonged hospital stay, and cost of treatment due to a lack of relevant literature.
Incidence of metastasis of pMM cancers in surgically resected patients
Analyses of resected specimens of pMM esophageal squamous cell carcinomas from patients who received surgical resection as first-line treatment, including patients with vascular invasion, revealed concurrent lymph node metastasis in 0–26.7% of cases. In our summary of the main reports, concurrent lymph node metastasis occurred in 29 of 199 patients (14.6%, 95% CI: 10.0–20.3%).2-9 A report of 50 patients with pMM cancer found that the incidence of lymph node metastasis was increased in patients with vascular invasion (invasion negative 4/38 (10.5%) vs. invasion positive patients: 5/12 (41.7%)).2
However, pMM cancers treated with surgical resection may have higher rates of lymph node metastasis than those treated with ER for the following reasons. First, in pathological diagnosis, the slice width of surgical specimens is thicker than that of ER specimens, and it is possible that the lesions evaluated as pMM using surgical specimens included some pSM lesions. Second, although not stated explicitly, it is possible that some patients who receive surgical resection as first-line treatment are diagnosed as lymph node metastasis-positive (cN-positive) prior to treatment. The incidence of concurrent lymph node metastasis for pMM will thus be higher in patients undergoing surgical resection because of the inclusion of cN-positive cases, while only cN-negative cases are included in the ER group.
Incidence of metastasis of pMM cancers in ER cases
No RCTs or case–control studies have elucidated the effectiveness of additional treatments for pMM vascular invasion-negative esophageal squamous cell carcinoma based on histological findings after ER. However, several case-series studies reported on follow-up observation, additional surgical resection, and chemoradiotherapy groups including patients who were positive for vascular invasion and pSM cases following ER. We made enquiries to the authors and tabulated the subsequent incidences of metastasis in patients with pMM cancer without vascular invasion and pVM0 esophageal squamous cell carcinoma.
The metastasis rates for each group were 12/216 (5.6%, 95% CI: 2.9–9.5%) in the follow-up observation group (six reports; Table 9),3-8 0/6 in the additional surgical resection group (three reports; 0%, 95% CI: 0–46.0%),9-11 and 1/17 in the additional chemoradiotherapy group (six reports; 5.9%, 95% CI: 0.2–28.7%)7, 11-15 (Table 10).
| Invasion depth | Vascular invasion | Incidence of metastasis in the follow-up observation group | Reference |
|---|---|---|---|
| pMM | Negative | 0% (0/42 patients) | 3 |
| pMM | Negative | 16.7% (1/6 patients) | 4 |
| pMM | Negative | 9.5% (2/21 patients) | 5 |
| pMM | Negative | 1.8% (1/55 patients) | 6 |
| pMM | Negative | 8.3% (2/24 patients) | 7 |
| pMM | Negative | 8.8% (6/68 patients) | 8 |
| Invasion depth | Vascular invasion | Metastasis rate | ||
|---|---|---|---|---|
| Follow-up observation group | Additional surgical resection group | Additional chemoradiotherapy group | ||
| pMM | Negative | 5.6% (12/216 patients) | 0% (0/6 patients) | 5.9% (1/17 patients) |
| pMM | Positive | 21.4% (3/14 patients) | 5.0% (1/20 patients) | 15.6% (7/45 patients) |
Furthermore, after contacting the authors, we also tabulated the data for the follow-up observation, additional surgical resection, and additional chemoradiotherapy groups for patients with pMM cancer with vascular invasion and pVM0 esophageal squamous cell carcinoma following ER. Metastasis occurred in 3/14 patients (21.4%, 95% CI: 4.7–50.8%) in the follow-up observation group (four reports),5-8 1/20 patients in the additional surgical resection group (four reports; 5.0%, 95% CI: 0.1–24.9%),8-11 and 7/45 patients in the additional chemoradiotherapy group (six reports; 15.6%, 95% CI: 6.5–29.5%)6-8, 11-14 (Table 10).
These data may be underestimated by including patients with insufficient follow-up, given that the metastasis rates would possibly increase with further follow-up. Moreover, it is unclear if immunostaining was used to assess vascular invasion in the reported histological analyses, although one report indicated that the rate of vascular invasion-positive cases increased after performing immunostaining in addition to hematoxylin and eosin staining.13
Adverse events due to additional treatments
Few reports summarized the adverse events following additional surgical resection after ER. We therefore also reviewed primary surgical resection (including some reports of esophageal adenocarcinoma) for cT1 cancers. Regarding adverse events following additional chemoradiotherapy, we examined additional chemoradiotherapy following ER (including pMM cancer patients with vascular invasion and pSM cancer patients) irrespective of the pathology results after ER.
The rates of treatment-related death following surgical resection were 0–2.0% in four case-series studies2, 16-18 and one non-RCT.19 The rate of treatment-related death in our summary of all reports was 1.3% (95% CI: 0.7–2.2%). Delayed adverse events following additional chemoradiotherapy were tabulated from eight case-series studies4, 11-13, 15, 20-22 and one single-arm prospective trial,23 with radiation pneumonitis of ≥grade 3 in 1.0% (3/302) of patients, grade 3 thromboembolism in 0.3% (1/302) of patients, and myocardial infarction of ≥grade 3 in 1.3% (4/302) of patients. A total of four treatment-related deaths (4/302: 1.3%, 95% CI: 0.4–3.4%) were reported, comprising radiation pneumonitis in one, sudden death in one, and myocardial infarction in two patients.
Summary
Because most of the present reports were retrospective case-series studies and did not provide high-level evidence, we assigned a strength of evidence D to these data. The metastasis rate was 5.6% in the follow-up observation group of patients with pMM cancer without vascular invasion and pVM0 esophageal squamous cell carcinoma following ER. However, considering the reduced QOL and the possibility of treatment-related deaths due to additional surgical resection, as well as delayed adverse events and treatment-related deaths following additional chemoradiotherapy, the guidelines committee proposed the following statement: “as additional treatment for pMM esophageal squamous cell carcinoma without vascular invasion following ER, it is weakly recommended to not perform surgical resection and chemoradiotherapy.” However, no consensus was achieved among the guidelines committee members (modified Delphi scores: median = 5, lowest = 2, and highest = 8) despite in-depth discussion, and no conclusion could be reached regarding the recommendation on whether or not to perform additional treatments. The recommendation statement thus indicates that “the recommendation could not be concluded regarding whether or not to perform additional treatment with surgical resection or chemoradiotherapy for pMM esophageal squamous cell carcinoma without vascular invasion following ER.”
In routine clinical practice, patients and their family members are presented with the known metastasis rates and the decision regarding additional surgical resection or chemoradiotherapy is then made considering the patient's performance status, age, main organ function, and comorbidities, as well as the wishes of the patient and their family members. In the case of follow-up observations without additional treatments, patients should be informed that metastasis can occur at certain rates and that it is crucial to perform careful follow-up including screening for metastasis.
CQ7: Is additional treatment with surgical resection or chemoradiotherapy recommended in patients with pT1b-SM esophageal squamous cell carcinoma based on histological findings following endoscopic resection?
Recommendation statement: Additional treatment with surgical resection or chemoradiotherapy is strongly recommended in patients with pT1b-SM esophageal squamous cell carcinoma following endoscopic resection.
Modified Delphi scores: median = 9, lowest = 7, highest = 9 Strength of evidence: D
Commentary: To address this CQ, we performed a literature search to assess if surgical resection or chemoradiotherapy was recommended as additional treatment for patients with pSM esophageal squamous cell carcinomas based on histological findings following ER. We extracted 188, 24, and 19 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 231 articles and 13 manually searched articles underwent primary screening and 42 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of 28 articles was performed.
The incidence of metastasis was examined in terms of concurrent lymph node metastasis rates in surgically resected specimens and metastatic recurrence rates after ER. Adverse events related to surgical resection and chemoradiotherapy were primarily examined in terms of treatment-related deaths. Improvements in survival were initially assessed as mortality after the treatment; however, many deaths were unrelated to esophageal cancer and we therefore did not include survival analyses after the treatment in the review of this CQ. Moreover, we were unable to assess reduced QOL, prolonged hospital stay, and cost of treatment due to a lack of relevant literature.
Incidence of metastasis of pSM cancers in surgically resected patients
Analyses of resected specimens from patients with pSM1/SM2 esophageal squamous cell carcinoma who received surgical resection as first-line treatment, including patients with vascular invasion, showed that the incidences of concurrent lymph node metastasis were 8.3–53.1% for pSM1 cancer and 18.5–30.0% for pSM2 cancer. After tabulating data from the main reports, metastasis was found to occur in 43/170 patients (25.3%, 95% CI: 19.0–32.5%)2, 3, 16, 24-28 with pSM1 cancers and 49/196 patients with pSM2 cancers.3, 24-28 One of these reports analyzed 32 patients with pSM1 cancer and reported that the incidence of lymph node metastasis was increased in patients with vascular invasion [invasion negative 6/20 (28.6%) vs. invasion positive 11/11 (100%)].2
However, the lymph node metastasis rate among patients with pSM cancer treated with surgical resection may be higher than in patients treated with ER for the following three reasons. First in pathological diagnosis, the slice width of surgical specimens is thicker than that of ER specimens, and it is possible that lesions with deeper invasion might be included in cases of surgical resection. Second the pathological definition of pSM1/pSM2 differs between surgical and ER specimens. In surgically resected specimens, the invasion depth of pSM1 is defined as “lesions limited to the upper third of the submucosal layer subdivided into three equal parts”, whereas pSM2 is defined as “lesions limited to the middle third of the submucosal layer subdivided into three equal parts”. In contrast, the distance at which the submucosal layer is subdivided into three equal parts is unclear in endoscopically resected specimens, and pSM1 is therefore defined as “cancer invading into submucosa ≤200 μm from the muscularis mucosae” and pSM2 as “cancer invading into submucosa >200 μm from the muscularis mucosae”. It is therefore surmised that lesions with an invasion depth >200 μm may be included for patients diagnosed with pSM1 cancer in surgical specimens. Finally, although it was not described in the extracted articles, it is possible that some patients who undergo surgical resection as a primary treatment are diagnosed as positive for lymph node metastasis (cN-positive) prior to treatment. The incidence of concurrent lymph node metastasis for pSM will therefore be higher among patients undergoing surgical resection because of the inclusion of cN-positive cases in this group, while only cN-negative cases are included in the ER group.
Incidence of metastasis of pSM cancers in endoscopically resected patients
No RCT or case–control studies demonstrated the effectiveness of additional treatments for pSM esophageal squamous cell carcinoma based on histological findings following ER. However, we found several observational case-series studies of pSM cancer with follow-up observation, additional surgical resection, and chemoradiotherapy groups following ER. After enquiries to the authors for data, we tabulated metastasis rates for pSM and pVM0 esophageal squamous cell carcinomas. Because the risk of metastasis depends on the presence or absence of vascular invasion and pSM1 or pSM2 cancers, we tabulated metastasis rates separately based on invasion depth and the presence or absence of vascular invasion (Table 11).
| Invasion depth | Vascular invasion | Metastasis rate | ||
|---|---|---|---|---|
| Follow-up observation group | Additional surgical resection group | Additional chemoradiotherapy group | ||
| pSM1 | Negative | 13.2% (5/38 patients) | 0% (0/5 patients) | 2.9% (1/35 patients) |
| pSM2 | Negative | 18.8% (3/16 patients) | 8.3% (1/12 patients) | 9.3% (8/86 patients) |
| pSM1 | Positive | 60.0% (3/5 patients) | 0% (0/14 patients) | 17.9% (5/28 patients) |
| pSM2 | Positive | 0% (0/4 patients) | 0% (0/21 patients) | 28.1% (23/82 patients) |
Metastasis among patients with pSM1/SM2 cancer without vascular invasion occurred in 8/54 patients in the follow-up observation group (six reports; 14.8%, 95% CI: 6.6–27.1%),4-8 1/17 patients in the additional surgical resection group (six reports: 5.9%, 95% CI: 0.2–28.7%),5, 6, 9-11, 29 and 9/121 patients in the additional chemoradiotherapy group (11 reports 7.4%, 95% CI: 3.5–13.7%).4, 6-8, 11-15, 21, 30
Metastasis among patients with pSM1/SM2 cancer with vascular invasion occurred in 3/9 patients in the follow-up observation group (four reports; 33.3%, 95% CI: 7.5–70.1%),5-8 0/35 patients in the additional surgical resection group (six reports; 0%, 95% CI: 0.0–10.0%),5, 6, 9-11, 29 and 28/110 patients in the additional chemoradiotherapy group (11 reports; 25.5%, 95% CI: 17.6–34.7%).6-8, 11-15, 21, 22, 30
These data may be underestimated by including patients with insufficient follow-up, given that the metastasis rates would possibly increase with further follow-up. Moreover, it is unclear if immunostaining was used for the assessment of vascular invasion in the histological analyses.
Adverse events following additional treatments
No report summarized adverse events following additional surgical resection after ER. We therefore reviewed primary surgical resection (including some reports of esophageal adenocarcinoma) for cT1 cancer. Regarding adverse events of additional chemoradiotherapy, we examined additional chemoradiotherapy following ER (including pMM cancer patients with vascular invasion and pSM cancer patients) irrespective of the pathology results after ER.
The rates of treatment-related death following surgical resection were 0–2.0% in four case-series studies2, 16-18 and 1.3% in a non-RCT19 (1.3%, 95% CI: 0.7–2.2%). Delayed adverse events of additional chemoradiotherapy were tabulated from eight case-series studies4, 11-13, 15, 20-22 and one single-arm prospective trial,23 with radiation pneumonitis grade ≥3 in 1.0% (3/302) of patients, grade 3 thromboembolism in 0.3% (1/302) of patients, and myocardial infarction grade ≥3 in 1.3% (4/302) of patients. A total of four treatment-related deaths (4/302: 1.3%, 95% CI: 0.4–3.4%) were reported, comprising pneumonitis in one, sudden death in one, and myocardial infarction in two patients.
Summary
Most of the present reports were retrospective case-series studies and did not provide high-level evidence, and we therefore assigned a strength of evidence D to these data. A metastasis rate of 13.2–18.8% was observed in the follow-up observation group of pSM cancer patients without vascular invasion and pVM0 esophageal squamous cell carcinoma following ER, compared with rates of only 5.9% in the additional surgery group and 7.4% in the additional chemoradiotherapy group. Despite the reduced QOL and possibility of treatment-related death due to additional surgical resection, as well as delayed adverse events and treatment-related deaths following additional chemoradiotherapy, the effectiveness of additional treatment was considered to surpass the adverse events and it was therefore stated that “as additional treatment for pSM esophageal squamous cell carcinoma without vascular invasion following ER, surgical resection and chemoradiotherapy are strongly recommended.”
The effectiveness of additional treatment was also considered to surpass the adverse events for patients with pSM cancer with vascular invasion and pVM0 esophageal squamous cell carcinoma following ER. It was therefore stated that “as additional treatment for pSM esophageal squamous cell carcinoma with vascular invasion following ER, surgical resection or chemoradiotherapy are strongly recommended.” Metastasis or recurrence rates differed between the additional chemoradiotherapy (25.5%) and additional surgical resection (0.0%) groups based on our detailed survey of previous publications. However, most of the studies were small and were non-randomized comparisons, and the results may therefore have been affected by differences in background factors. Moreover, data regarding chemoradiotherapy were not adjusted for irradiation field and radiation dose. The preferred type of additional treatment, i.e., surgical resection or chemoradiotherapy, was therefore not specified in the recommendations. Further studies are needed to identify the most suitable additional treatment method for these patients.
References
Chapter 4: Surveillance following ER for esophageal squamous cell carcinoma
Introduction
Local, lymph node, and distant recurrence, metachronous esophageal cancers, and metachronous cancers in other organs can develop after ER for esophageal squamous cell carcinoma. To improve prognosis, it is therefore important for patients to abstain from drinking and smoking, and to receive proper surveillance. In this chapter, we proposed CQs related to surveillance for recurrence, metachronous esophageal cancers, and metachronous cancers in other organs, and generated corresponding recommendations. The recommendation summary is presented in Figure 6.
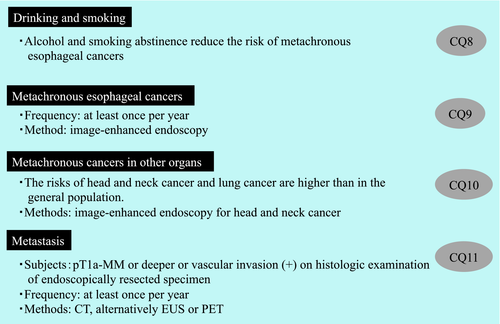
CQ8: Is it recommended to abstain from drinking alcohol and smoking after endoscopic resection of superficial esophageal squamous cell carcinoma?
Recommendation statement: It is strongly recommended that the patient abstains from drinking alcohol and smoking after endoscopic resection of superficial esophageal squamous cell carcinoma.
Modified Delphi scores: median = 9, lowest = 7, highest = 9 Strength of evidence: B
Commentary: The occurrence of metachronous esophageal cancers and metachronous cancers in other organs can dramatically affect outcomes after ER of superficial esophageal squamous cell carcinoma. In this chapter, we investigated the importance of abstaining from drinking and smoking after ER. Our search extracted 44, eight, and 12 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 64 articles underwent primary screening and 20 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of six articles, comprising five cohort studies and one retrospective study, was performed.
Effects of alcohol consumption
Katada et al.1 conducted a prospective cohort study of 331 patients who received ER for superficial esophageal squamous cell carcinoma and reported that alcohol abstinence significantly reduced the cumulative incidence of metachronous esophageal cancers [hazard ratio (HR): 0.47, 95% CI: 0.25–0.91; P = 0.025]; the risk was reduced further by abstinence in patients with multiple iodine unstained lesions (Lugol voiding lesions: LVL; HR: 0.23, 95% CI: 0.09–0.6; P = 0.003; Table 12).
| Evaluation item | Subjects | Status | No. of subjects | Risk | Reference |
|---|---|---|---|---|---|
| Metachronous esophageal cancers | Patients who have undergone endoscopic resection of esophageal cancer | Current drinker | 154 | 1 (reference) | 1 |
| Recent quitter | 69 | 0.47 (0.25–0.91) | |||
| Metachronous esophageal cancers | Male patients who have undergone endoscopic resection of esophageal cancer (Post hoc analysis above) | Current drinker | 149 | 1 (reference) | 2 |
| Recent quitter | 63 | 0.45 (0.22–0.89) |
Yokoyama et al.2 conducted a post hoc analysis of 278 men from the above-mentioned prospective cohort study. The cumulative incidence of metachronous esophageal cancers in high-risk patients, identified by a total score ≥12 in a medical questionnaire evaluating drinking habits, smoking habits, alcohol flushing, and dietary habits, was significantly reduced (HR: 0.37, 95% CI: 0.14–0.97; P = 0.042).
No studies examined the relationship between alcohol abstinence and survival rate in patients who underwent ER of superficial esophageal squamous cell carcinoma.
Alcohol abstinence is therefore strongly recommended based on the reduced risk of metachronous esophageal cancers after ER of superficial esophageal squamous cell carcinoma.
Effect of smoking
Katada et al.3 conducted a prospective cohort study of 331 patients who underwent ER for superficial esophageal squamous cell carcinoma and reported that smoking abstinence significantly reduced the cumulative incidence of metachronous esophageal cancers (HR: 0.47, 95% CI: 0.26–0.91; P = 0.024; Table 13).
| Evaluation item | Subjects | Status | No. of subjects | Risk | Reference |
|---|---|---|---|---|---|
| Metachronous head and neck cancer | Cancer patients after first-line treatment | Current smoker | – | 1 (reference) | 4 |
| Recent quitter | – | 0.7 (0.33–1.48) | |||
| Metachronous esophageal cancers | Patients after endoscopic resection of esophageal cancer | Current smoker | 60 | 1 (reference) | 3 |
| Recent quitter | 69 | 0.49 (0.26–0.91) | |||
| Metachronous esophageal cancers | Male patients after endoscopic resection of esophageal cancer (Post hoc analysis above) | Current smoker | 54 | 1 (reference) | 2 |
| Recent quitter | 65 | 0.71 (0.35–1.45) | |||
| Metachronous esophageal cancers | Cancer patients after first-line treatment | Current smoker | – | 1 (reference) | 4 |
| Recent quitter | – | 0.49 (0.28–0.86) | |||
| Metachronous cancers overall | Cancer patients after first-line treatment | Current smoker | 9833 | 1 (reference) | 4 |
| Recent quitter | 2645 | 0.82 (0.69–0.96) | |||
| Smoking-related metachronous cancer† | Cancer patients after first-line treatment | Current smoker | 9833 | 1 (reference) | 4 |
| Recent quitter | 2645 | 0.74 (0.61–0.90) | |||
| Mortality rate | General population | Never smoker | – | 1 (reference) | 5 |
| Current smoker | – | 1.84 (1.74–1.96) |
- †Oral cancer/pharyngeal cancer, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, laryngeal cancer, lung cancer, renal cancer, ureter cancer, and bladder cancer.
In an epidemiological study of smoking in 29,795 patients initially diagnosed for first cancer, smoking abstinence significantly reduced the risk of the development of all secondary cancers (HR: 0.82, 95% CI: 0.69–0.96) and of metachronous esophageal cancers (HR: 0.49, 95% CI: 0.28–0.86)4 However, there was no significant difference in the risk of developing metachronous head and neck cancers (HR: 0.70, 95% CI: 0.33–1.48).
A cohort study was performed to examine the relationship between smoking and mortality rates in 27,311 Japanese men and 40,662 Japanese women. The mortality rate was significantly higher among men and women who were smokers than among nonsmokers (odds ratio: 1.84, 95% CI: 1.74–1.96).5 Furthermore, a cohort study examining the relationship between smoking status and mortality rates in 34,439 English male doctors found that the mortality rate was significantly higher among smokers than nonsmokers.6
CQ9: Is endoscopic examination at least once a year recommended for the surveillance of metachronous esophageal cancers after endoscopic resection of superficial esophageal squamous cell carcinoma?
Recommendation statement: Endoscopic examination at least once a year is strongly recommended as surveillance after endoscopic resection of superficial esophageal squamous cell carcinoma.
Modified Delphi scores: median = 8, lowest = 7, highest = 9 Strength of evidence: C
Commentary: The occurrence of metachronous esophageal cancers adversely affects outcomes after ER of superficial esophageal squamous cell carcinoma. However, there is currently no consensus regarding how surveillance should be performed for metachronous esophageal cancers after ER. Our literature search relating to this CQ identified 66, 17, and 66 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 149 articles and one manually searched article underwent primary screening and 14 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of seven articles, comprising one cohort study and six retrospective studies was performed.
No reports examined the impact of different surveillance methods in terms of surveillance interval on the early detection rate and mortality rate in patients with metachronous esophageal cancers. Furthermore, it was difficult to examine the impacts of different surveillance methods on the costs incurred and the stress and adverse events experienced by patients.
Studies examining the detection of metachronous esophageal cancers1, 7-12 (Table 14) found an extremely high annual incidence of cancer development, i.e., 2.2–9.0% (based on the median, rather than the mean, follow-up period). The interval of endoscopic examinations was every 6 months in three articles, every 12 months in two articles, every 6–12 months in one article, and not reported in one article.1, 7-12 Furthermore, Katada et al.1 conducted a prospective cohort study of patients who underwent curative ER of superficial esophageal squamous cell carcinoma and showed that multiple iodine unstained lesions of the esophagus were associated with a high cumulative incidence of metachronous esophageal cancers.
| Subjects | Study design | No. of patients | Median follow-up period (months) | Annual incidence of esophageal cancer (reference value)† | Interval of endoscopic examinations | Reference |
|---|---|---|---|---|---|---|
| Post-EMR and -ESD patients | Cohort study | 331 | 49.4 | 5.4% | Every 3 months up to 6 months after EMR or ESD and every 6 months thereafter | 1 |
| Mainly post-EMR male patients | Retrospective study | 110 | 41 | 2.2% | Every 6 months | 2 |
| Post-EMR patients | Retrospective study | 96 | 62.7 | 2.4% | At least once a year | 3 |
| Post-ESD patients | Retrospective study | 208 | 28 | 5.2% | At 2 months post-ESD and then every 6–12 months after the heeling of ESD ulcer | 6 |
| Post-ESD patients | Retrospective study | 117 | 38.8 | 9.0% | Once a year | 7 |
- †Number of patients with metachronous esophageal cancers/median follow-up period × number of patients.
- ESD, endoscopic submucosal dissection.
Most reports indicated that endoscopic examinations were performed every 6–12 months as surveillance for metachronous esophageal cancers after ER of superficial esophageal squamous cell carcinoma. In these reports, although the stage of metachronous esophageal cancer was not clearly reported, most cases could be treated with minimally invasive endoscopic treatment.7, 11, 12
CQ10: Is surveillance of metachronous cancers in other organs using imaging tests recommended following endoscopic resection of superficial esophageal squamous cell carcinoma?
Recommendation statement: Surveillance of metachronous cancers in other organs using upper gastrointestinal endoscopy is strongly recommended following endoscopic resection of superficial esophageal squamous cell carcinoma.
Modified Delphi scores: median = 9, lowest = 7, highest = 9Strength of evidence: C
Commentary: The occurrence of metachronous cancers in other organs following ER of superficial esophageal squamous cell carcinoma remains a cause for concern. However, which other organs are at high risk of developing metachronous cancers and whether surveillance of other organs improves outcomes is currently unclear. Improved understanding of these issues will facilitate clinical decisions.
A literature search for studies pertaining to this CQ identified 171, 32, and 15 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 218 articles underwent primary screening, 17 articles were selected for secondary screening, and nine articles were finally extracted. A further two manually searched papers were identified as important and were included with the nine articles in a qualitative systematic review of a total of 11 articles.
Ishihara et al.13 conducted a cohort study using a cancer registry and reported a high standardized mortality ratio due to malignant tumors (3.14, 95% CI: 1.79–5.09) in patients who had undergone ER for superficial esophageal squamous cell carcinoma. In studies examining the standardized incidence ratios (SIRs)14-17 of metachronous cancers in other organs, the SIRs of head and neck cancers (6.7–20.1)14-17 and lung cancers14-16 were significantly higher than in the general population, and remained significantly higher for both sites even after 5 years.15-17
Furthermore, elevated SIRs of gastric cancer (1.5–3.3)15, 16 and renal cancer (1.9–2.2)14-16 have been reported. However, the SIRs of these cancers decreased over time and ceased to be significantly elevated. These studies14-16 suggest the presence of bias in SIRs, with a temporary elevation upon detection via various tests performed during the initial staging of esophageal cancers. No reports indicated a significantly elevated SIR for colorectal cancer.14, 16 We examined two additional articles on metachronous cancers in other organs in Japanese patients with esophageal squamous cell carcinoma. Matsubara et al.18 compared patients who received surgery for esophageal squamous cell carcinoma with healthy controls and reported 34.9-, 3.2-, and 2.0-fold increased risks of head and neck cancer, lung cancer, and gastric cancer, respectively. In a study on metachronous cancers in other organs in patients with esophageal squamous cell carcinoma, Tabuchi et al.19 demonstrated that the SIRs were significantly increased for head and neck cancer (21.6) and lung cancer (1.71). Based on these results, we considered that the risks of head and neck cancer and lung cancer were almost certainly increased and that the risks of gastric cancer and renal cancer might be increased in patients with esophageal cancer.
Regarding surveillance methods, many studies reported on the use of endoscopy for head and neck cancer, and the cumulative incidence of head and neck cancer was 3.2–12.1%.1, 13, 20-22 Katada et al. examined the incidence of metachronous head and neck cancer according to the number of iodine unstained lesions of the esophagus in a prospective cohort of 331 patients, in whom curative resection was achieved by ER for superficial squamous cell carcinoma. In their study, the incidence of head and neck cancer increased with increasing numbers of iodine unstained lesions, with 2-year cumulative detection rates of 0.0%, 1.7%, and 8.6% in patients without iodine unstained lesions, moderately affected patients (1–9 lesions per endoscopic visual field), and severely affected patients (≥ 10 lesions), respectively.1
Regarding the endoscopic modality, endoscopic surveillance with narrow-band imaging (NBI) and white-light imaging improved the detection of head and neck cancers compared with white-light imaging alone, according to Morimoto et al. (9.8% vs. 3.9%; P = 0.008)20 and Nonaka et al. (10.9% vs. 1.2%; P < 0.0001).21 Furthermore, Morimoto et al. also reported that all metachronous head and neck cancers detected during surveillance with NBI were treated by local resection. The mortality rate was significantly lower in patients with metachronous head and neck cancers detected during surveillance with NBI compared with patients detected by white-light imaging (0% vs. 60%; P < 0.001).20 Moreover, Onochi et al.22 revealed a high 5-year cumulative detection rate of gastric cancer of 4.1% in patients with esophageal squamous cell carcinoma treated with ER. These results suggest that endoscopic surveillance is useful for detecting both gastric cancer and head and neck cancer.
Collectively, the reviewed studies indicated that patients with esophageal squamous cell carcinoma were at risk of developing metachronous cancers in various other organs. Periodic upper gastrointestinal endoscopic examinations are recommended because they may improve patient prognosis and enable organ-preserving treatment for head and neck cancers. Periodic upper gastrointestinal endoscopic examinations also facilitate the detection of metachronous esophageal cancers. In addition to head and neck cancers, long-term surveillance is particularly needed for detecting lung cancer. However, public measures have already been adopted to reduce mortality rates due to lung cancer, including in high-risk groups, and no particular recommendation is therefore offered in the guidelines in this regard.
This review failed to evaluate the disadvantages of surveillance, such as patient stress, cost, and adverse events. Surveillance methods should therefore be selected taking into consideration patient-background factors such as age, presence or absence of comorbidities, and financial status.
CQ11: Are imaging tests using computed tomography (CT) at least once a year recommended for surveillance of metastasis following endoscopic resection for superficial esophageal squamous cell carcinoma in patients with pathological (p)MM or deeper invasion?
Recommendation statement: Imaging diagnosis using CT at least once a year is weakly recommended for surveillance of metastasis following endoscopic resection for superficial esophageal squamous cell carcinoma in patients with pMM or deeper invasion.
Modified Delphi scores: median = 7, lowest = 5, highest = 9 Strength of evidence: C
Commentary: Our literature search pertaining to CQ11 identified 344, 17, and 46 articles from the PubMed, Cochrane, and JAMAS databases, respectively. These 407 articles and one manually searched article underwent primary screening and 52 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of six articles was performed. These six observational studies reported the period of imaging diagnosis and presented long-term outcomes in terms of metastasis rates for ≥100 patients with pMM or deeper invasion.10, 23-27
The metastasis rates in follow-up studies of patients with superficial esophageal squamous cell carcinoma were 1.9% (2/104 patients) in pMM patients (including those with vascular invasion)10 and 5.1–11.8% in patients with pT1 cancers beyond pMM invasions.23, 24, 27 Furthermore, the 5-year cumulative metastasis rates in 402 patients who underwent ER for esophageal cancer25 were 0.4% for pEP/LPM cancer, 8.7% for pMM cancer, 7.7% for pSM1 cancer, and 36.2% for pSM2 cancer, indicating increasing incidence with deeper invasion depths (P < 0.0001). Multivariate analyses also identified invasion depth as an independent risk factor for metastasis, with HRs of 13.1 in pMM cancer (95% CI: 1.3–133.7), 40.2 in pSM1 cancer (95% CI: 2.9–552.7), and 196.3 in pSM2 cancer (95% CI: 10.9–3523.6) compared with pEP/LPM. The only prospective study in this systematic review was the JCOG0508 trial,26 which analyzed outcomes after ER for clinically suspected submucosal cancer. This study included a total of 176 patients divided into three subgroups: 74 patients in the low-risk group with pT1a cancer and without vascular invasion (Group A) without additional treatment, 87 patients in the high-risk group with pT1a cancer and with vascular invasion or pT1b cancer (SM1/2; Group B), and 15 patients with positive vertical margins (Group C). Group B received prophylactic chemoradiotherapy and Group C received definitive chemoradiotherapy. CT was performed every 4 months over 3 years after ER and every 6 months thereafter. The 3-year overall survival rate in group B, which was considered to be at high risk for metastasis despite achieving complete ER, as assumed in this CQ, was 90.7% (95% CI: 84.0–94.7%). Recurrence and metastasis were also observed in 15 patients overall (8.7%: Groups A/B/C: 1/10/4 patients, respectively), including five who already had distal metastases at the time of detection. Of the seven patients who underwent salvage surgery due to metastases in the lymph nodes alone, only two remained alive at the final follow-up observation.
Among patients who have undergone ER for superficial esophageal squamous cell carcinoma, those with pMM or deeper invasion are thus at higher risk of metastasis than those with pEP or pLPM cancer. Moreover, some patients develop metastatic recurrences even after additional treatment with chemoradiotherapy. Although no studies showed that early detection of metastasis improved patient survival, some retrospective studies with relatively small numbers of cases found that salvage chemoradiotherapy and lymph node dissection improved survival in patients with localized recurrence after esophagectomy,28-36 suggesting that early detection of metastatic recurrence and consequent early therapeutic interventions could improve prognosis.
Computed tomography surveillance at least once a year was performed in many studies and patients were also expected to choose to receive surveillance in many instances.37 Diagnostic imaging using CT at least once a year should therefore be recommended. In addition to CT, EUS was also used for surveillance following ER in one article, with six of seven patients who exhibited lymph node recurrence following ER diagnosed by EUS, compared with only three of the seven patients by CT.27 A meta-analysis comparing the diagnostic performances of EUS, CT, and fluorodeoxyglucose positron emission tomography (PET) for regional lymph nodes in patients with esophageal cancer38 reported sensitivities of 80%, 50%, and 57% and specificities of 70%, 83%, and 85%, respectively. PET has comparable sensitivity and specificity to CT, whereas EUS has inferior specificity but superior sensitivity. It is therefore possible that the effectiveness of surveillance could be improved by using EUS or PET rather than CT. Future studies are required to compare the effectiveness of these surveillance modalities.
Our recommendation statement is thus as follows: “following ER for superficial esophageal squamous cell carcinoma in patients with a pMM or deeper invasion, it is weakly recommended to perform imaging diagnosis using CT at least once a year for surveillance of metastasis.”
References
Part 2: Esophageal Adenocarcinoma
The definition of Barrett's esophagus differs considerably between Japan and Western countries. In Japan, Barrett's esophagus refers to a columnar epithelium extending from the stomach continuous with the esophagus, with the esophagogastric junction defined as the distal end of the esophageal palisade vessels, and irrespective of the length and histological presence of intestinal metaplasia. In contrast, most Western guidelines define the esophagogastric junction as the proximal end of the gastric folds, with segments <1 cm not considered as Barrett's esophagus and with the presence of intestinal metaplasia as a histological requirement except in the United Kingdom (UK). Furthermore, in Japan, long-segment Barrett's esophagus (LSBE) refers to segments of Barrett's mucosa circumferentially extending for ≥3 cm, whereas LSBE is defined in Western countries as segments of Barrett's mucosa with a maximum length of ≥3 cm. Because most studies cited in the current report were from Western countries, the Western definition of LSBE (Barrett's mucosa with a maximum length of ≥3 cm) was mainly used in these guidelines. Furthermore, the pathological evaluation of Barrett's esophagus-related neoplasms differs between Japan and Western countries, with Western countries including histological diagnoses not included in Japan, such as low-grade dysplasia (LGD) and high-grade dysplasia (HGD). In the current guidelines, we created a table comparing the modified Vienna classification with the Japanese pathologists’ diagnosis (Table 15) and described each classification based on this table.
| Modified Vienna classification, Category | Japanese pathologists’ diagnosis | |
|---|---|---|
| 3 | LGD | Adenoma or well differentiated adenocarcinoma with low-grade atypia (noninvasive)† |
| 4.1 | HGD | Adenocarcinoma with high-grade atypia (noninvasive) |
| 4.2 | Noninvasive carcinoma (Ca in situ) | |
| 4.3 | Suspicion for invasive carcinoma | Intramucosal adenocarcinoma (suspicion of stromal invasion) |
| 4.4 | Intramucosal carcinoma | Intramucosal adenocarcinoma (with stromal invasion) |
| 5 | Submucosal carcinoma | Invasive adenocarcinoma (with submucosal invasion) |
- †It can be difficult to differentiate category 3 from inflammatory change.
- HGD, High-grade dysplasia; LGD, Low-grade dysplasia.
Chapter 1: Preoperative diagnosis of esophageal adenocarcinoma and indications for ER
Introduction
Regarding endoscopic treatment for esophageal adenocarcinoma, the Esophageal Cancer Practice Guidelines 20171 recommend performing endoscopic treatment for intramucosal adenocarcinoma; however, there is currently no description of how to conduct preoperative examinations.
A study on the therapeutic outcomes of ESD for superficial esophageal adenocarcinoma and HGD in 524 lesions reported an R0 resection rate of 74.5% and a positive horizontal margin in 54 lesions (40.3%) among 134 R1 resection cases, suggesting the difficulty in diagnosing the lateral extent of cancer using endoscopy, especially those developing in LSBE.2, 3 Furthermore, when superficial esophageal adenocarcinomas are adjacent to squamous epithelium, approximately half of the lesion spreads under the squamous epithelium, making the diagnosis of lateral extent even more difficult.4 We therefore addressed CQs regarding the preoperative diagnosis of superficial esophageal adenocarcinoma and established relevant recommendations.
In a systematic review relating to CQ12 and CQ13, we searched for articles that considered the preoperative diagnosis of the lateral extent of the cancer. Reports from Japan were extremely limited because of the low incidence of esophageal adenocarcinoma in this country. Furthermore, Barrett's esophagus surrounding a cancer is usually treated with RFA following EMR in Western countries; diagnosing lateral extent of the cancer is therefore of little importance and has not been reported. However, several studies from Western countries did report on the detection of superficial esophageal adenocarcinoma. Diagnosing the lateral extent of esophageal adenocarcinoma is conducted in the same manner as cancer detection, with continuous differential cancer/noncancer diagnosis to determine the cancer border. To address CQ12 and CQ13, we therefore conducted a systematic review of reports, primarily from Western countries, pertaining to the detection of esophageal adenocarcinoma, and produced commentaries and recommendation statements in response to the CQs. A summary of the recommendations is presented in Figure 7.
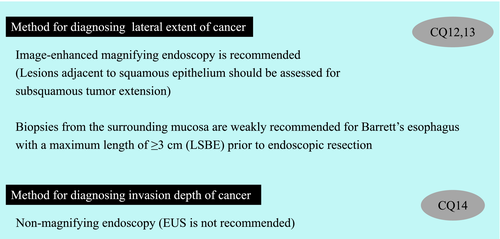
CQ12: Is image-enhanced magnifying endoscopic examination recommended for diagnosing lateral extent of superficial esophageal adenocarcinoma prior to endoscopic resection?
Statement: Image-enhanced magnifying endoscopic examination is weakly recommended for diagnosing lateral extent of superficial esophageal adenocarcinoma prior to endoscopic resection.
Modified Delphi scores: median = 8, lowest = 3, highest = 9 Strength of evidence: D
Commentary: Although ESD is widely performed for superficial esophageal adenocarcinoma, diagnosing lateral extent of the cancer using endoscopy is not easy. Some cases of LSBE have extensive LGD surrounding the cancer and HGD, making the diagnosis of lateral extent of the tumor extremely difficult. Furthermore, when superficial esophageal adenocarcinomas are adjacent to the squamous epithelium, approximately half of the lesion invades under the squamous epithelium (i.e. subsquamous tumor extension),4 making the accurate endoscopic diagnosis of subsquamous tumor extension. The application of acetic acid and magnifying observation combined with NBI has been reported to be useful for making such a diagnosis.5, 6
Our literature review for studies on the diagnosis of lateral cancer extent identified 359 articles from the PubMed, 91 from the Cochrane, and 46 from the JAMAS databases. These 496 articles underwent primary screening and 34 were included in secondary screening. After applying stringent selection criteria, no articles investigating the lateral extent of esophageal adenocarcinoma were extracted. However, we considered that the diagnosis of lateral extent of esophageal adenocarcinoma was conducted in the same manner as cancer detection, whereby differential cancer/noncancer diagnosis is performed continuously to determine the cancer border. We therefore focused our search on articles related to the endoscopic detection of HGD and adenocarcinoma using image-enhanced magnifying endoscopy. Ten articles were extracted after primary and secondary screenings and a further four articles were extracted by manual searching of the literature. We finally conducted a qualitative systematic review of 14 articles.
Random biopsy (i.e., Seattle protocol) is the recommended screening method for superficial adenocarcinoma or dysplasia in Barrett's esophagus in Western countries. However, target biopsy using image-enhanced endoscopy is concurrently performed at advanced care facilities. A meta-analysis conducted by the technical committee of the American Society for Gastrointestinal Endoscopy (ASGE) showed that the diagnostic accuracy of acetic acid and NBI exceeded the performance thresholds (sensitivity ≥90%, negative predictive value ≥98%, and specificity ≥80% for each endoscopy patient). During surveillance, the concurrent use of acetic acid and NBI at the time of target biopsy is therefore recommended. On the other hand, autofluorescence endoscopy, chromoendoscopy using indigo carmine and methylene blue stain, and probe-based confocal laser endoscopy have poor sensitivities and specificities and are therefore not recommended.7 Several NBI studies included in the aforementioned analysis included concurrent magnifying endoscopy, and the diagnostic performance of magnifying endoscopy combined with NBI exhibited superior results to NBI alone, with a sensitivity of 94% (95% CI: 83–98%) and specificity of 94% (95% CI: 81–99%).8-12 Although no reports on the use of magnifying endoscopy combined with acetic acid application were included in the analysis, the usefulness of this combination has been demonstrated in other studies.13-16 When limited to reports from Japan, the diagnostic performances of NBI combined with magnifying observation and acetic acid combined with magnifying observation were also favorite10, 16-19 suggesting that the concurrent use of magnifying endoscopy can improve the qualitative diagnosis (cancer/noncancer differential diagnosis) of lesions (Table 16).
| Target lesion (n) | Sensitivity (%) | Specificity (%) | Accuracy (%) | Reference |
|---|---|---|---|---|
| HGD (41) Mucosal pattern diagnosis | 100 | 98.8 | 99.0 | 8 |
| HGD (36) Vascular pattern diagnosis | 100 | 97.4 | 98.2 | 8 |
| HGD (6) | 90.0 | 100 | ― | 9 |
| Adenocarcinoma (6) | 100 | 100 | 100 | 10 |
| HGD (52) | 96.2 | 98.7 | 98.5 | 11 |
| LGD (21) HGD (1) Adenocarcinoma (4) | 100 | 97.4 | 97.7 | 12 |
| HGD (25) Adenocarcinoma (20) | 91.1 | 92.9 | 92.2 | 20 |
| HGD (6) Adenocarcinoma (66) | 93.1 | 96.2 | 95.3 | 17 |
- HGD, high-grade dysplasia; LGD, low-grade dysplasia; NBI, narrow-band imaging.
A working group of the Barrett's International Group recently created a new simplified international classification of findings on NBI combined with magnifying endoscopy. An international multicenter collaborative study found that the classification had high diagnostic accuracy and good inter-observer agreement for diagnosing HGD/superficial adenocarcinoma.20 Although there is no direct evidence to support the use of image-enhanced magnifying endoscopic examination for diagnosing lateral extent of esophageal adenocarcinoma, numerous reports have indicated its usefulness for detecting cancer (Table 16).
CQ13: Are biopsies from the surrounding mucosa recommended for diagnosing lateral extent of superficial esophageal adenocarcinoma arising from Barrett's esophagus with a maximum length of ≥3 cm (LSBE) prior to endoscopic resection?
Recommendation statement: Biopsies from the surrounding mucosa are weakly recommended for diagnosing lateral extent of superficial esophageal adenocarcinoma arising from Barrett's esophagus with a maximum length of ≥3 cm (LSBE) prior to endoscopic resection.
Modified Delphi scores: median = 7, lowest = 4, highest 8Strength of evidence: D
Commentary: Barrett's esophagus with a maximum length of ≥ 3 cm is referred to in various countries as LSBE. However, LSBE is extremely rare in Japan. It is difficult to diagnose the lateral extent of superficial esophageal adenocarcinoma arising from LSBE, and the need for biopsies of the surrounding tissue is an important question. A literature review regarding CQ13 identified 1921 articles from the PubMed, 107 from the Cochrane, and 239 from the JAMAS databases. These 2267 articles underwent primary screening and 12 were included in secondary screening. After applying stringent selection criteria, no articles directly related to CQ13 were identified, but manual searching for articles that examined conditions similar to the above-mentioned ones identified six relevant articles.
In LSBE, LGD sometimes spreads flatly and extensively around the cancer and HGD,21 making it extremely difficult to diagnose the lateral extent of the tumor. Studies from Europe regarding the endoscopic treatment of superficial adenocarcinoma in Barrett's esophagus performed ESD in 87 patients. The rate of R0 resection (for HGD) was 85% in short-segment Barrett's esophagus (SSBE) compared with only 48% in LSBE.2 Moreover, after enquiries to the authors for data, the rates of R0 resection among 193 patients in Japan who underwent ESD for superficial adenocarcinoma in Barrett's esophagus were 91% in SSBE and 70% in LSBE3 (the definitions of LSBE and SSBE were based on circumferential segments of 3 cm in this report), suggesting the difficulty involved in diagnosing the lateral extent of lesions in LSBE.
It is therefore necessary to examine the usefulness of taking biopsies from the surrounding mucosa for the diagnosis of lesion extent. However, we failed to find any articles directly indicating that biopsies from the surrounding mucosa were useful in diagnosing the lateral extent of superficial adenocarcinoma in Barrett's esophagus, irrespective of the length of the Barrett's esophagus, and we were therefore unable to examine this point.
On the other hand, there is some concern regarding the accuracy of making a pathological diagnosis for Barrett's neoplasia, particularly for LGD, based on small biopsy specimens. Poor diagnostic agreement among pathologists regarding LGD is a problem in Western countries,22 and many Western guidelines recommend collaboration between at least two pathologists (including at least one pathologist specialized in gastroenterology) for making a biopsy-based diagnosis.23-26 Other reports have indicated that agreement among pathologists regarding a diagnosis of LGD can be improved by additional p53 immunostaining of the biopsy specimen,27, 28 and UK guidelines recommend supplementary p53 immunostaining of biopsy specimens.25
CQ14: Is EUS recommended for diagnosing the invasion depth of superficial esophageal adenocarcinoma prior to endoscopic resection?
Recommendation statement: It is weakly recommended that EUS should not be performed for diagnosing the invasion depth of superficial esophageal adenocarcinoma prior to endoscopic resection.
Modified Delphi scores: median = 8, lowest = 6, highest = 9 Strength of evidence: C
Commentary: The indications of ER for superficial adenocarcinoma are primarily determined based on the preoperative diagnosis of cancer invasion depth. However, the modalities useful for diagnosing invasion depth have not been elucidated. Clarification of the recommendations regarding the need to perform EUS for diagnosing invasion depth would help clinical decision making. A literature review regarding this CQ identified 243 articles from the PubMed, 20 from the Cochrane, and 28 from the JAMAS databases. These 291 articles underwent primary screening and 21 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of six articles was performed.29-34
Regarding the EUS specifications used in these six articles, a radial probe was used in three articles,29, 31, 32 a linear probe in one,30 a convex probe in one,33 and a miniature probe in one.34 The diagnostic accuracies for invasion depth, divided into pathological T1a and T1b cancers, were 76.4–85.4%.29-34
May et al.34 conducted a prospective study of 94 patients with superficial esophageal cancer, including 77 patients with adenocarcinoma, and reported that the accuracies of invasion-depth diagnosis based on macroscopic type were 83.4% using white-light observation and 79.6% for EUS, with no significant difference between the two modalities. However, we were unable to find any other studies directly stating that EUS improved the outcomes for white-light observation.
Based on a literature review pertaining to CQ14, we found no studies with a high level of evidence such as RCTs or meta-analyses. Furthermore, all the included studies were from Western countries, which mostly analyzed esophageal cancers in LSBE,29, 30, 33 while the incidence of SSBE is high among Japanese individuals.35 Two studies examining the diagnostic accuracy of invasion depth by EUS showed accuracies for cancers of the esophagogastric junction compared with cancers of other part of the esophagus of 68.6% vs. 92.9% (P < 0.001)34 and 47.6% vs. 87.1% (P < 0.001),36 respectively, indicating significantly lower accuracy for the esophagogastric junction. It is therefore likely that it is more difficult to diagnose invasion depth by EUS for superficial esophageal adenocarcinoma arising from SSBE, which is usually located in the esophagogastric junction, compared with cancers in other areas. Arima et al.37 examined the use of EUS for diagnosing superficial adenocarcinoma from Barrett's esophagus in Japanese individuals and reported a diagnostic accuracy of 73%, low sensitivity for T1a lesions (56%), and noted that superficial lesions tended to be over-interpreted as deeper cancers.
The present review did not consider the patient burden, increased cost, and procedural adverse events related to EUS in detail. However, based on the benefit-to-harm balance for invasion-depth diagnosis in patients with superficial esophageal adenocarcinoma, EUS cannot be generally recommended. We therefore weakly recommended that EUS should not be performed as standard practice for diagnosing invasion depth prior to ER of superficial esophageal adenocarcinoma.
References
Chapter 2: ER of esophageal adenocarcinoma
Introduction
The Esophageal Cancer Practice Guidelines 20171 strongly recommend ER for preoperatively diagnosed M cancer, i.e., cM cancer, in patients with superficial adenocarcinoma in Barrett's esophagus, and there is a worldwide consensus on this point. However, the resection methods differ, with ESD being common in Japan and EMR more common in Western countries.
This difference may depend on whether or not the institutions concerned can conduct ESD as a routine practice, whether they can conduct accurate endoscopic diagnosis prior to ER, and the availability of RFA. In Japan, where ESD is popular and RFA is not approved, ESD is primarily conducted because it is expected to lead to more reliable complete removal of the lesion. In contrast, in Western countries where EMR is primarily conducted, ESD is not popular and additional ablation of residual lesion after EMR can be performed by RFA. Considering these differing backgrounds, we addressed the CQ regarding the effectiveness and safety of the two procedures and established a recommendation based on a systematic literature review.
Endoscopic resection can be expected to be curative if histological examination following resection reveals pEP (limited to the epithelium)/SMM (limited to the superficial muscularis mucosae)/LPM (limited to the lamina propria mucosa) cancer.2, 3 However, there is no clear recommendation on how to treat patients with pDMM (invasion into the deep muscularis mucosa) cancer after ER. In these guidelines, we therefore addressed the CQ regarding the assessment of pDMM treatment and established a recommendation following a systematic review of the latest evidence on lymph node metastasis risk and superficial esophageal adenocarcinoma prognosis. The recommendation summary is presented in Figure 8.
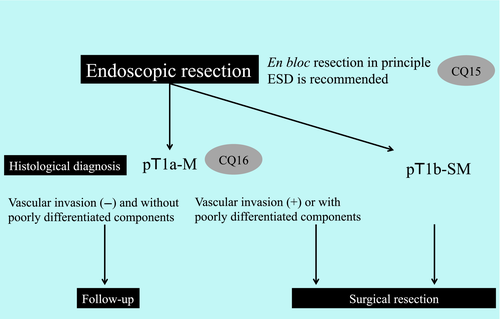
CQ15: Is ESD recommended over EMR for the treatment of superficial esophageal adenocarcinoma suitable for endoscopic resection?
Recommendation statement: ESD is strongly recommended over EMR for the radical treatment of superficial esophageal adenocarcinoma suitable for endoscopic resection.
Modified Delphi scores: median = 9, lowest = 7, highest = 9 Strength of evidence: B
Commentary: ER is performed for superficial esophageal adenocarcinoma with a low risk of metastasis. In Japan, R0 resection is attempted by ESD, whereas resection in other countries is primarily performed by EMR, sometimes resulting in piecemeal resection. A literature review regarding the CQ of whether or not ESD is recommended over EMR for the resection of superficial esophageal adenocarcinoma suitable for endoscopic treatment identified 523 articles from the PubMed, 151 from the Cochrane, and 26 from the JAMAS databases. These 700 articles and one manually searched article underwent primary screening and 74 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of 26 articles was performed.
The rates of en bloc resection for superficial esophageal adenocarcinoma with EMR and ESD were 50.0% (95% CI: 44.9–55.1%)2, 4-8 and 96.4% (95% CI: 95.2–97.7%),2, 8-20 respectively, and the rates of R0 resection were 39.7% (95% CI: 28.4–51.0%)2, 8 and 81.9% (95% CI: 79.3–84.5%),2, 8, 9, 11-19 respectively. The en bloc and R0 resection rates were both higher with ESD than with EMR.
The local recurrence rates of superficial esophageal adenocarcinoma following EMR and ESD were 12.4% (95% CI: 10.7–14.1%)2, 4-6, 8, 21-24 and 2.5% (95% CI: 1.3–3.6%),2, 8-16, 18-20 respectively, and the mean observation periods of EMR and ESD were 4.22, 4-6, 8, 21-24 and 2.8 years,2, 8-11, 13-16, 18-20 respectively. Thus although the observation period was shorter for ESD, the local recurrence rate was also lower for ESD than for EMR.
The rates of procedural adverse events (post-procedural bleeding, perforation, and stenosis) associated with ER of superficial esophageal adenocarcinoma were 9.3% (95% CI: 8.1–10.4%) for EMR4-8, 21, 23-28 and 10.5% (95% CI: 7.5–13.5%) for ESD.8-11, 13, 15, 16, 18, 20 To summarize each report according to post-procedural bleeding, perforation, and stenosis, the post-procedural bleeding rate was 3.1% (95% CI: 2.4–3.8%),4-8, 21, 23, 25-28 the perforation rate was 0.4% (95% CI: 0.1–0.6%),4-8, 21, 23-28 and the stenosis rate was 6.4% (95% CI: 5.5–7.5%) for EMR,4-8, 21, 23, 24, 27, 28 and the equivalent rates for ESD were 2.8% (95% CI: 1.0–4.5%), 1.5% (95% CI: 0.15–2.9%), and 6.3% (95% CI: 3.8–8.7%), respectively.8-11, 13, 15, 16, 20
Based on the articles extracted in the systematic review for this CQ, we were unable to evaluate length of hospital stay and ER procedure time.
CQ16: Is additional surgical resection recommended more than follow-up observation for differentiated pDMM esophageal adenocarcinoma without vascular invasion in which R0 resection is achieved by endoscopic resection?
Recommendation statement: It is weakly recommended that additional surgical resection should not be performed for differentiated pDMM esophageal adenocarcinoma without vascular invasion in which R0 resection is achieved by endoscopic resection.
Modified Delphi scores: median = 9, lowest = 8, highest = 9 Strength of evidence: C
Commentary: Histological findings of pEP/SMM/LPM esophageal adenocarcinoma after ER are associated with an extremely low risk of metastasis, and additional treatment is therefore considered unnecessary. However, the need for additional treatment (e.g., surgical resection) of pDMM cancer has not yet been elucidated. We therefore conducted a literature review regarding the CQ of the need for additional treatment for patients (excluding patients with poorly differentiated components intermixed) with differentiated pDMM cancer without vascular invasion, in which R0 resection is achieved. We extracted 77 articles from the PubMed, six from the Cochrane, and three from the JAMAS databases. These 86 articles plus six manually searched articles underwent primary screening and 35 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of 17 articles was performed.
The rate of lymph node metastasis in surgical specimens from 105 patients with pathologically diagnosed differentiated pDMM esophageal adenocarcinoma without vascular invasion following surgical resection for esophageal adenocarcinoma was 0.0% (95% CI: 0–2.5%),3, 29-32 compared with 0.5% (95% CI: 0–2.1%) in 200 patients diagnosed with differentiated pDMM esophageal adenocarcinoma without vascular invasion following ER,3, 4, 14, 23, 32-39 with lymph node metastasis reported in only one patient.32
The 5-year survival rate following surgical resection primarily for pT1a esophageal adenocarcinoma was 80.2–89.3%,14, 29, 30, 34, 35 and the 5-year disease-specific survival rate was 94.4–98.4%,30, 31, 37, 38 while the 5-year survival rate following ER primarily for pT1a esophageal adenocarcinoma was 91.5–100%,2, 4, 16, 23, 39 and the 5-year disease-specific survival rate was 96.2–100%.2, 14, 16 The 5-year disease-specific survival rates were high following both surgical and ERs for esophageal adenocarcinoma, whereas the 5-year survival rate following surgical resection tended to be slightly lower than that following ER. However, data regarding surgical outcomes were collected from Western countries and we believe that caution should be exercised when comparing the surgical outcomes between Western countries and Japan, given that different surgical procedures tend be chosen for cancers derived from LSBE, which is common in Western countries, and cancers derived from SSBE, which is common in Japan.
Furthermore, in this systematic review related to CQ16, we were unable to evaluate perioperative mortality, change in QOL, and length of hospital stay adequately for patients undergoing surgical resection.
In summary, the risk of metastasis is very low in patients with differentiated pDMM esophageal adenocarcinoma without vascular invasion in which R0 resection is achieved, and additional surgical resection is therefore not expected to reduce the risk further. Accordingly, we weakly recommended not performing additional surgical resection in patients with differentiated pDMM esophageal adenocarcinoma without vascular invasion in which R0 resection is achieved by ER.
References
Chapter 3: Surveillance following ER for esophageal adenocarcinoma
Introduction
Patients with Barrett's esophagus undergo surveillance to achieve early detection of HGD and metachronous cancer of the esophagus. The Esophageal Cancer Practice Guidelines 20171 acknowledge that surveillance of Barrett's esophagus is weakly recommended, despite insufficient supporting evidence. As expected, surveillance of Barrett's esophagus is also necessary after ER of superficial esophageal adenocarcinoma. We addressed two CQs to determine the optimal interval and methods of surveillance following ER of esophageal adenocarcinoma and established recommendations.
When conducting a systematic review in this area, it is important to understand the differences in endoscopic treatment and surveillance methods between Japan and Western countries. Western countries commonly treat all areas of Barrett's esophagus by ablation during the endoscopic treatment of superficial esophageal adenocarcinoma. This differs from the procedure in Japan, where only ER of superficial adenocarcinoma is performed. To answer a CQ regarding surveillance following ER in these guidelines, we therefore conducted a systematic review limited to articles specifically considering follow-up observation after ER without ablation of Barrett's esophagus, and excluded articles from Western countries addressing follow-up observation after endoscopic treatment of esophageal adenocarcinoma with ablation of Barrett's esophagus.
Regarding surveillance methods, random biopsies according to the Seattle protocol are standard practice in Western countries and Western studies evaluating the diagnostic accuracy of endoscopic examination for esophageal adenocarcinoma are based on the practice of performing random biopsies. It is accordingly difficult to evaluate the performance of white-light observation alone without random biopsies.
Taking these backgrounds into consideration, we conducted a systematic review of the diagnostic accuracy of each type of image-enhanced endoscopic examination for esophageal adenocarcinoma and dysplasia and examined the modalities that can be recommended for surveillance in Japan. A summary of the recommendation is presented in Figure 9.
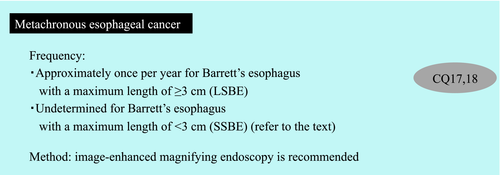
CQ17: At what interval is endoscopic examination recommended for the surveillance of metachronous cancer following endoscopic resection of superficial esophageal adenocarcinoma?
Recommendation statement: Endoscopic examination approximately once a year is weakly recommended for surveillance following endoscopic resection of superficial esophageal adenocarcinoma in Barrett's esophagus with a maximum length of ≥3 cm.
Modified Delphi scores: median = 8, lowest = 8, highest = 9 Strength of evidence: C
Commentary: A literature search to examine the evidence for surveillance following ER of superficial esophageal adenocarcinoma revealed 184 articles from PubMed, 91 from the Cochrane database, and two from the JAMAS database. These 277 articles and one manually searched article underwent primary screening and 67 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of three articles was performed.
When examining the frequency of surveillance, we considered “increased rate of early detection of metachronous cancer of the esophagus and dysplasia”, “reduced burden to patients”, and “reduced medical costs” as advantages, and “reduced detection rate of metachronous cancer in the esophagus in surveillance at prolonged intervals” and “adverse events caused by additional examinations in surveillance at short intervals” as disadvantages. We were unable to identify any articles that examined “reduced burden to patients” and “reduced medical costs.” Regarding adverse events, the sixth national survey of procedural accidents related to gastrointestinal endoscopy2 reported the rate of procedural adverse events in upper gastrointestinal endoscopic examination as 0.005% and the mortality rate as 0.00013%, indicating that the impacts of these events were low.
We were unable to find any RCT that compared surveillance intervals following ER of superficial esophageal adenocarcinoma. We therefore conducted an investigation based on the detection rate of metachronous cancer in the esophagus following ER of superficial esophageal adenocarcinoma. We extracted three Japanese observational studies following ER of superficial esophageal adenocarcinoma without ablation for Barrett's esophagus, because most Western reports included ablation for Barrett's esophagus.
Moreover, LSBE is defined in Japan as Barrett's mucosa circumferentially extending for ≥3 cm according to the Japanese Classification of Esophageal Carcinoma,3 which differs from the Western criteria of segments of Barrett's mucosa with a maximum length of ≥3 cm. These definitions are frequently mentioned in this chapter, and LSBE and SSBE based on circumferential segments of 3 cm in the Japanese criteria are therefore denoted as LSBE-C and SSBE-C, whereas LSBE and SSBE based on the Western criteria of a maximum length of 3 cm are denoted as LSBE-M and SSBE-M.
Incidence of metachronous cancer in patients with Barrett's esophagus following ER
Abe et al.4 examined the long-term prognosis of 238 patients with superficial adenocarcinoma in Barrett's esophagus (204 cases arising from SSBE-C and 34 from LSBE-C) and reported that metachronous cancers within the esophagus were identified with a yearly incidence of 0.26% for SSBE-C (three patients/1161 person-years) and 0.62% for LSBE-C (one patient/164 person-years). We pooled the data from this and two other articles5, 6 using a fixed-effects model and inverse-dispersion calculation technique and estimated that the integrated values for the yearly incidence of metachronous cancer within the esophagus were 0.29% for SSBE-C (95% CI: −0.35% to 0.97%, three patients/1262 person-years) and 0.70% for LSBE-C (95% CI: −1.90% to 3.30%, two patients/176 person-years).4, 5
Incidence of adenocarcinoma in patients with Barrett's esophagus
The long-term prognosis following ER of superficial endoscopic adenocarcinoma was only examined in a few patients with LSBE and we therefore supplemented the data in the literature with statistics on LSBE in patients without a history of cancer. In the surveillance of LSBE-M (132 patients) from Japan, esophageal cancer was reported with an annual incidence of 1.2% (three patients/251 person-years).7 Furthermore, the incidence of HGD/adenocarcinoma arising from Barrett's esophagus without dysplasia and cancer was examined in Western countries according to SSBE-M and LSBE-M, with incidences of esophageal adenocarcinoma arising from SSBE-M and LSBE-M (882 and 1061 patients, respectively) of 0.29% (14 patients/4767 person-years) and 0.91% (67 patients/7321 person-years), respectively, indicating a significantly higher incidence of LSBE-M.8 The incidence of esophageal adenocarcinoma for LSBE-M without a history of esophageal cancer was approximately 1%.
The annual incidence of metachronous gastric cancer following ER of early-stage gastric cancer was 2.3%,9 and surveillance was performed at least once a year. As mentioned above, the incidence of metachronous esophageal adenocarcinoma in LSBE-C was 0.7% following ER of superficial esophageal adenocarcinoma, which was slightly lower than that of gastric cancer.
However, one report indicated that the annual incidence of esophageal adenocarcinoma in LSBE-M was approximately 1% and patients who have undergone ER for superficial adenocarcinoma may have had a higher risk. Together with the difficulty in detecting esophageal adenocarcinoma, we believe that a surveillance interval similar to that for gastric cancer is warranted. We therefore recommend performing endoscopic examination approximately once a year following ER of LSBE-M. In contrast, the annual incidence of metachronous esophageal adenocarcinoma in patients who have undergone ER of SSBE is 0.2–0.3%, and a frequency of endoscopic examination of once every 2–3 years is considered suitable in these patients. However, in the present systematic review, we were unable to draw a conclusion about the appropriate frequency of endoscopic examination.
CQ18: Are image-enhanced endoscopy and magnifying endoscopy recommended for surveillance following endoscopic resection of superficial esophageal adenocarcinoma?
Recommendation statement: Image-enhanced endoscopy and magnifying endoscopy are weakly recommended for surveillance following endoscopic resection of superficial esophageal adenocarcinoma.
Modified Delphi scores: median = 7.5, lowest = 7, highest = 9 Strength of evidence: C
Commentary: Image-enhanced endoscopy using optical digital methods such as NBI is widely used in Japan, and staining, such as with indigo carmine or acetic acid, can be performed at any institution. The use of NBI in combination with magnifying endoscopy enables detailed observation of the vascular architecture and surface structure. Spraying Barrett's esophagus with 1–3% acetic acid creates contrast by temporarily whitening the non-neoplastic columnar epithelium of the esophagus, leaving the cancer or dysplasia more reddish than the surrounding mucosa. In addition, the use of magnifying endoscopy after spraying with acetic acid enables detailed observation of the surface structure.
As noted in the introduction, Barrett's esophagus is generally treated by ablation in Western countries, and we were unable to identify any articles evaluating the effectiveness of image-enhanced endoscopy and magnifying endoscopy in surveillance for Barrett's esophagus without ablation following ER. Furthermore, the capacity to diagnose esophageal adenocarcinoma using white-light observation in Western reports was based on random biopsy, and it was difficult to evaluate the capacity of white-light observation alone as a control group. We therefore searched the literature focusing on articles that evaluated the effectiveness (sensitivity, specificity, and negative predictive value) of image-enhanced endoscopy and magnifying endoscopy for the surveillance of Barrett's esophagus in patients who had not undergone endoscopic treatment. We extracted 295 articles from PubMed, 33 from the Cochrane database, and 16 from the JAMAS database. These 344 articles underwent primary screening and 29 articles were selected for secondary screening. After applying stringent selection criteria, a qualitative systematic review of 23 articles was performed, including one report on a meta-analysis of surveillance by image-enhanced endoscopy10 and nine cited references from the meta-analysis.11-19 There were two articles on NBI,20, 21 one on blue-laser imaging (BLI),22 and one on a meta-analysis of acetic acid,23 with nine cited references from the meta-analysis.24-32
To examine the effectiveness of each modality, “detection of superficial cancer and dysplasia” and “the effect on decreasing the number of biopsy specimens” were defined as advantages and “prolonged endoscopic observation” as a disadvantage. However, the “effect on reducing the number of biopsy specimens” was only reported in articles based on random biopsy. No articles examined the effect on reducing the number of target biopsies and few articles examined “prolonged endoscopic observation”. We therefore examined the effectiveness based solely on the “detection of superficial cancer and dysplasia” for each modality.
Regarding the diagnostic performance in terms of the sensitivity and specificity for detecting superficial cancer and dysplasia, NBI had a sensitivity of 94% (95% CI: 83–98%) and specificity of 94% (95% CI: 81–99%)10 (Table 17), whereas the acetic acid method had a sensitivity of 92% (95% CI: 83–97%) and specificity of 96% (95% CI: 85–99%)23 (Table 18), indicating excellent outcomes for both modalities. The ASGE proposed the criteria known as preservation and incorporation of valuable endoscopic innovations (PIVI) and reported that, for modalities with targeted biopsies, those with a sensitivity ≥90%, specificity ≥80%, and negative predictive value ≥98% were useful.33 The acetic acid method and NBI results were above the PIVI threshold and were therefore recognized as useful modalities by the ASGE.10 Furthermore, the sensitivity and specificity of NBI magnifying observation11-15, 19-21 were higher than those of NBI non-magnifying observation,16-19 suggesting that NBI combined with magnifying observation was more effective (Table 17).
| Observation method | Target | Analysis unit† | Total number | Sensitivity (%) | Specificity (%) | Diagnostic accuracy (%) | Reference |
|---|---|---|---|---|---|---|---|
| Magnifying NBI (Mucosal pattern) | HGD | Per patient | 50 | 100 | 97.7 | 98.0 | 12 |
| Per lesion | 204 | 100 | 98.8 | 99.0 | |||
| Magnifying NBI (Vascular pattern) | HGD | Per patient | 35 | 100 | 96.4 | 97.1 | 12 |
| Per lesion | 113 | 100 | 97.4 | 98.2 | |||
| Magnifying NBI | HGD | Per patient | 50 | 83.3 | 97.7 | 96.0 | 13 |
| Magnifying NBI | Cancer | Per lesion | 217 | 100 | 100 | 100 | 14 |
| Magnifying NBI | HGD | Per lesion | 1021 | 96.2 | 98.7 | 98.5 | 15 |
| Per patient | 111 | 92.9 | 96.9 | 96.4 | |||
| Magnifying NBI | LGD/HGD/cancer | Per patient | 40 | 100 | 86.2 | 90.0 | 16 |
| Per lesion | 221 | 100 | 97.4 | 97.7 | |||
| Magnifying NBI | HGD/cancer | Per lesion | 120 | 91.1 | 92.9 | 92.2 | 20 |
| Magnifying NBI | HGD/cancer | Per lesion | 254 | 93.1 | 96.2 | 95.3 | 21 |
| Non-magnifying NBI | HGD/cancer | Per patient | 65 | 100 | 100 | 100 | 16 |
| Non-magnifying NBI | HGD/cancer | Per patient | 101 | 96.8 | 55.7 | 68.3 | 17 |
| Per lesion | 874 | 45.0 | 88.2 | 82.3 | |||
| Non-magnifying NBI | LGD/HGD/cancer | Per lesion | 90 | 52.7 | 100 | 78.9 | 18 |
| Non-magnifying NBI | LGD/HGD/cancer | Per lesion | 221 | 100 | 93.8 | 94.6 | 19 |
- †“Per lesion” includes Barrett's esophagus without neoplasia.
- HGD, high-grade dysplasia; LGD, low-grade dysplasia; NBI, narrow-band imaging.
| Observation method | Target | Analysis unit† | Total number | Sensitivity (%) | Specificity (%) | Diagnostic accuracy (%) | Reference |
|---|---|---|---|---|---|---|---|
| Acetic acid method (with magnification) | HGD/cancer | Per patient | 62 | 100 | 100 | 100 | 24 |
| Acetic acid method (with magnification) | HGD/cancer | Per lesion | 223 | 64.7 | 89.1 | 87.0 | 25 |
| Acetic acid method (with magnification) | HGD | Per lesion | 72 | 50.0 | 98.5 | 94.4 | 26 |
| Acetic acid method (with magnification) | Cancer | Per lesion | 115 | 100 | 100 | 100 | 27 |
| Acetic acid method (without magnification) | HGD/cancer | Per patient | 57 | 83.3 | 100 | 93.0 | 28 |
| Acetic acid method (without magnification) | LGD/HGD/cancer | Per patient | 100 | 100 | 97.7 | 98.0 | 29 |
| Acetic acid method (without magnification) | LGD/HGD/cancer | Per patient (including overlapping cases) | 190 | 95.5 | 82.4 | 88.4 | 30 |
| Acetic acid method (without magnification) | HGD/cancer | Per patient | 701 | 96.7 | 66.5 | 70.5 | 31 |
| Per lesion | 5944 | 81.8 | 94.0 | 93.7 | |||
| Acetic acid method (without magnification) | HGD/cancer | Per patient | 263 | 97.0 | 75.3 | 89.0 | 32 |
- †“Per lesion” includes Barrett's esophagus without neoplasia.
- HGD, high-grade dysplasia; LGD, low-grade dysplasia.
We extracted one article comparing white-light observation and BLI from an international group, which reported that BLI was superior to white-light observation in terms of evaluating the macroscopic type of the tumor and diagnosis of lateral extent.22 Comparable outcomes can therefore be expected with BLI and NBI. Moreover, autofluorescence, indigo carmine, methylene blue, and probe-based confocal laser endoscopy had very low values for sensitivity and/or specificity (Table 19).
| Observation method | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| AFI | 81% (62–91%) | 46% (32–61%) |
| Indigo carmine | 67% (53–78%) | 99% (87–99%) |
| Methylene blue | 64% (36–85%) | 95% (77–99%) |
| pCLE | 90% (72–99%) | 77% (54–91%) |
- AFI, autofluorescence imaging; pCLE, probe-based confocal laser endoscopy.
There were no well-designed comparative trials examining the effectiveness of the acetic acid method, NBI, and magnifying observation. However, the diagnostic outcomes of these modalities were extremely good, although they may prolong endoscopic observation leading to increased patient stress.
Considering the benefit-to-harm balance and strength of the evidence for surveillance following ER of superficial esophageal adenocarcinoma, we therefore weakly recommend the use of image-enhanced endoscopy with NBI and acetic acid and magnifying endoscopy.
Conflicts of Interest
Regarding conflicts of interest among members of the guidelines working, review, and Japan Gastroenterological Endoscopy Society Guidelines committees, we requested declarations on the following details from each committee member:
Remuneration (≥1 million yen), stock profits (≥1 million yen, or ≥5%), patent fees (≥1 million yen), lecture fees (≥500,000 yen), manuscript fees (≥500,000 yen), research funds and grants (≥1 million yen), scholarship (premium) donations (≥1 million yen), courses financially maintained by enterprises (≥1 million yen), and contributions (≥50,000 yen) not directly related to the study.
Tsuneo Oyama (lecturer's fee: Takeda Chemical Industries, Ltd.), Tomonori Yano (lecturer's fee: Olympus), Manabu Mato (research funds and grant: Olympus), Mitsuhiro Fujishiro (lecturer's fees: Takeda Chemical Industries, Ltd., EA Pharma, and Nihon Pharmaceutical Co., Ltd.; research funds and grant: HOYA; scholarships: EA Pharma, Eisai, Taiho Pharmaceutical, AbbVie Inc., Nippon Kayaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., Gilead Sciences Inc., Kyourin Pharmaceutical Co., Ltd., and Mitsubishi Tanabe Pharma), Kazuma Fujimoto (lecturer's fees: Tsumura, EA Pharma, AstraZeneca, and Daiichi Sankyo; scholarships: AstraZeneca, Daiichi Sankyo, Astellas Pharma, Takeda Chemical Industries, Ltd., EA Pharma, and Asahi Kasei Medical). Other authors have no conflicts of interest to disclose.