Endoscopic ultrasound-guided hepaticogastrostomy using a novel covered metallic stent with a fine-diameter delivery system
Abstract
Watch a video of this article
Brief Explanation
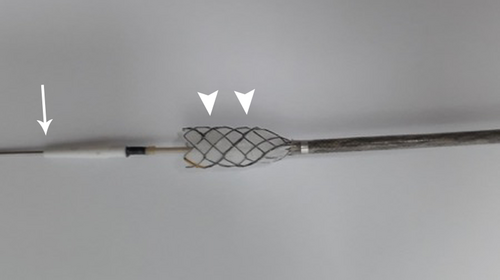
Endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS) is a useful bile drainage method for the treatment of malignant biliary obstruction.1, 2 However, its complication rate is high, and therefore various improvements in both devices and techniques have been made to improve its safety.3-5 Some of the most frequent complications include bile peritonitis due to intraperitoneal bile leakage while exchanging devices during the procedure, and excessive fistula dilation when inserting the stent delivery system. Herein, we report a technique for EUS-HGS without balloon-based fistula dilation, using a novel fully-covered metallic stent (Braided 6, S&G Biotech, Seongnam, Korea) mounted within a fine 6-Fr diameter delivery sheath. The tip of the sheath is tapered to 5 Fr allowing advancement into the fistula coaxially along a 0.025-inch guidewire (Fig. 1). This also allows injection of contrast medium via the sheath for bile duct imaging.
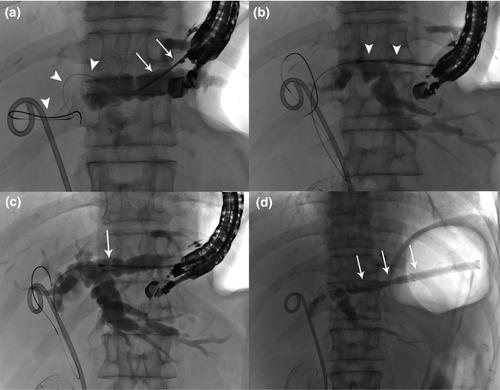
A 63-year-woman with gallbladder cancer was referred to our hospital with jaundice due to occlusion of a previously placed biliary stent. As a duodenal stent had been placed because of cancer invasion, treatment through the transpapillary route was difficult and EUS-HGS was selected as the biliary drainage method. First, the bile duct of segment 2 (B2) was punctured with a 19-G needle. Then, a guidewire was inserted and coiled into the common bile duct. The fistula was dilated with a 7-Fr dilator (ES Dilator; ZEON Medical, Tokyo, Japan), and the stent system was advanced coaxially along the guidewire. Contrast medium was injected through the sheath, and the stent was deployed at the appropriate position (Fig. 2, Video S1). No procedure-related adverse events occurred, and the entire procedure took 25 min. Use of this stent system obviates the need for balloon-based fistula dilation and reduces the number of device exchanges, thus minimizing any intraperitoneal bile leakage. In this way, the stent system can reduce the risk of complications during EUS-HGS.
Authors declare no conflict of interests for this article. No funding was received.