Endoscopic full-thickness resection of a colonic schwannoma
Abstract
Watch a video of this article
Brief Explanation
We report the case of a 61-year-old woman with a submucosal tumor (SMT) located in the proximal transverse colon incidentally diagnosed on screening colonoscopy. Contrast-enhanced abdominopelvic computed tomography showed a well-circumscribed hyperenhancing mass 20 mm in size, compatible with a gastrointestinal stromal tumor.
After submucosal injection of indigo carmine and saline, a semi-circumferential incision was carried out using the HookKnife (Olympus, Tokyo, Japan). Initial dissection of the submucosal layer exposed a yellowish-white and firm SMT (Fig. 1). Stepwise dissection of the lesion from the surrounding tissues was carried on with the HookKnife. Finally, the insulated-tip knife (ITknife2; Olympus) was used to further resect the tumor from its origin deep in the muscularis propria layer, hence completing endoscopic full-thickness resection (EFTR). As the lesion was located in the mesenteric side of the transverse colon, no free perforation was observed. After adequate hemostasis of the wound, the wall defect was closed using several hemostatic clips (Microtech, Nanjing, China). Antibiotic prophylaxis was started 30 min before the endoscopic procedure and continued for 3 days. Pathology was consistent with a schwannoma (immunostaining was positive for S-100, CD34, SOX-10 and EMA and negative for CD117, DOG-1, SMA and DES; Ki-67 was 1%; Fig. 2). There were no complications.
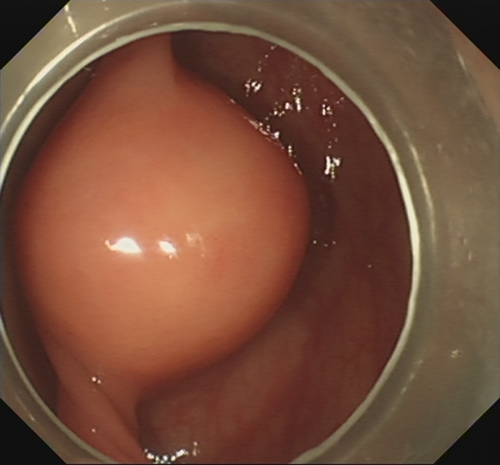
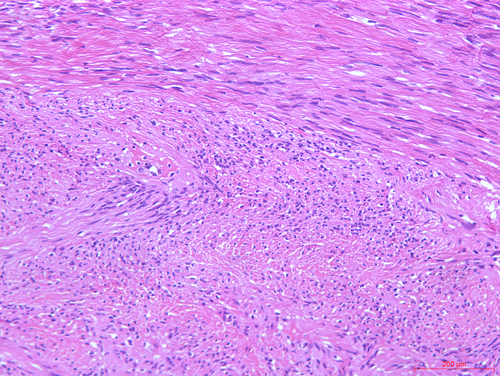
Colonic schwannomas are exceedingly rare tumors, with very few cases reported in the literature. Although generally regarded as benign lesions, malignant transformation has occasionally been described.1 Endoscopic resection of these and other colonic SMT originating from the muscularis propria layer remains challenging.2-4 We have previously published our initial experience with EFTR for tumors ≤3 cm, reporting a high rate of complete resection (18/19) and endoscopic closure (16/18).5 Therefore, we have proposed EFTR as a valid treatment option when carried out by experienced endoscopists in high-volume tertiary centers. Larger prospective studies are needed to further evaluate its efficacy and safety.
Informed consent was obtained from the patient for publication of this video case (Video S1).
Authors declare no conflicts of interest for this article.