Improved visibility of colorectal flat tumors using image-enhanced endoscopy
Abstract
Colonoscopy is considered the gold standard for detecting colorectal tumors; however, conventional colonoscopy can miss flat tumors. We aimed to determine whether visualization of colorectal flat lesions was improved by autofluorescence imaging and narrow-band imaging image analysis in conjunction with a new endoscopy system. Eight physicians compared autofluorescent, narrow-band, and chromoendoscopy images to 30 corresponding white-light images of flat tumors. Physicians rated tumor visibility from each image set as follows: +2 (improved), +1 (somewhat improved), 0 (equivalent to white light), −1 (somewhat decreased), and −2 (decreased). The eight scores for each image were totalled and evaluated. Interobserver agreement was also examined. Autofluorescent, narrow-band, and chromoendoscopy images showed improvements of 63.3% (19/30), 6.7% (2/30), and 73.3% (22/30), respectively, with no instances of decreased visibility. Autofluorescence scores were generally greater than narrow-band scores. Interobserver agreement was 0.65 for autofluorescence, 0.80 for narrow-band imaging, and 0.70 for chromoendoscopy. In conclusion, using a new endoscopy system in conjunction with autofluorescent imaging improved visibility of colorectal flat tumors, equivalent to the visibility achieved using chromoendoscopy.
Introduction
Colonoscopy is considered the gold standard for detecting colorectal tumors. Previous research has indicated that endoscopic resection for colorectal tumors results in a 76–90% reduction in the risk of developing colorectal cancer;1, 2 however, factors such as endoscopist's insertion and withdrawal skill, tumor location, quality of bowel preparation, and tumor characterization can reduce the effectiveness of colonoscopy screenings. The adenoma miss rate varies from 6% to 27%.3, 4 Flat tumors, in particular, tend to be missed during colonoscopy, because they are difficult to detect using the standard white-light imaging (WLI) system.5 Although chromoendoscopy provides advantages over conventional colonoscopy,6, 7 the procedure is more complicated and time-consuming.
In recent years, image-enhanced endoscopy (IEE) has been proposed to enhance microvascular contrast and facilitate minute resolution of superficial patterns and color differences. Digital image enhancement involves signal- and image-processing algorithms. Various algorithms have been proposed depending on the objectives and modality of imaging, including one that enhances the contours or fine patterns in the constructed images, one emphasizing image contrast, one for color conversion etc. The use of IEE modalities such as autofluorescence imaging (AFI) and narrow-band imaging (NBI) may lead to improvements in colorectal tumor detection rates, although this notion is controversial.8-14 These mixed results were obtained using the endoscopy system one generation before the current one. At present, however, a new-generation endoscopy system with a dedicated xenon lamp has been developed.
We conducted a retrospective study comparing visibility of colorectal flat tumors on AFI and NBI images to those obtained with WLI. Specifically, we aimed to determine whether the diagnostic capabilities of AFI and NBI were superior to that of WLI for the visualization of colorectal flat tumors.
Methods
Endoscopic images obtained from 30 flat tumors of 30 patients at the Jikei University School of Medicine were used for the present study. Histopathological findings of the 30 tumors were as follows: 26 tubular adenocarcinomas, three tubular adenomas, and one sessile serrated adenoma. Endoscopic examinations were initially carried out using the white-light mode of the AFI videoendoscope system to identify colorectal flat lesions. Colonoscopists conducted AFI and NBI examinations by switching first to the AFI mode followed by the NBI mode, and lesions were examined by chromoendoscopy (CE) with 0.4% indigocarmine using the white-light mode. We used the following equipment in this study: AFI colonoscopes (EVIS CF-FH260AZI; Olympus Medical Systems, Tokyo, Japan), light sources (EVIS CLV-260SL; Olympus Medical Systems), and video processors (EVIS LUCERA ELITTE system; Olympus Medical Systems).
The AFI videoendoscope system is a novel illumination method that produces real-time pseudocolor images. The AFI system allows for immediate modality switching from WLI to AFI and NBI using a button on the control head of the endoscope. Images of the lesions using WLI, NBI, AFI, and CE without magnification were captured and electronically archived in electronic medical records. The images were selected by an experienced endoscopist (N.T.) blinded to the current study's methods. The WLI, NBI, AFI and CE images for each lesion were downloaded, randomly arranged, and a Microsoft PowerPoint (Microsoft Corp., Redmond, WA, USA) presentation was created. These images did not contain any information that could identify the patient or the lesion characteristics. The PowerPoint presentations were sent to the respective raters for their independent evaluation.
Eight experienced physicians (S.O., H.I., T.K., M.S., Y.T., T.U., T.M. and T.M.) participated in the image evaluation. The endoscopic images were presented to each of the physicians in random order for comparison with the WLI. Physicians scored each of the AFI, NBI and CE images for visibility of flat colorectal tumors according to the following scale: +2 (improved visibility), +1 (somewhat improved visibility), 0 (visibility equivalent to that of WLI), –1 (somewhat decreased visibility), and –2 (decreased visibility) as previously reported.15 The eight physicians’ scores for each image were tallied. The possible maximum score for any image was +16, and the possible minimum score was –16. If an image earned a total score of +10 or more, the image was considered improved, a score between +9 and –9 indicated no change, and a score of –10 or less indicated decreased visibility. We also examined interobserver agreement in relation to the evaluation of the AFI, NBI, and CE images (Fig. 1).
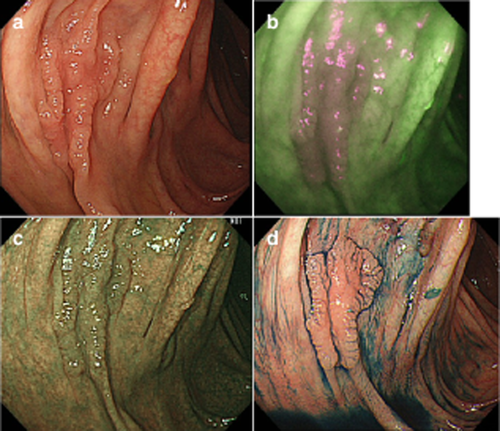
Endoscopic images using autofluorescence imaging (AFI), narrow-band imaging (NBI) and chromoendoscopy (CE). (a) White-light imaging (WLI). A flat lesion (laterally spreading tumor non-granular type). (b) AFI. The eight physicians awarded the AFI image +13 points as improved visibility. (c) NBI. The eight physicians awarded the NBI image +3 points as equivalent visibility. (d) CE. The eight physicians awarded the CE image +15 points as improved visibility.
Differences between each endoscopic image in the number of improved images were analyzed by Fisher's exact probability test or χ2-test. Interobserver agreement was measured with the kappa statistic. Statistical significance was defined as a P-value <0.05.
Results
Results for AFI, NBI and CE are shown in Table 1. With AFI, improved visibility was found in 63.3% (19/30) of cases, equivalent visibility was found in 36.7% (11/30) of cases, and there was no decreased visibility. With NBI, improved visibility was found in 6.7% (2/30) of cases, equivalent visibility was found in 93.3% (28/30), and there was no decreased visibility. With CE, improved visibility was found in 73.3% (22/30) of cases, equivalent visibility was found in 26.7% (8/30), and there was no decreased visibility. The incidence of improved visibility of AFI and CE was significantly higher than with CE images. Distribution of total visibility scores detailed by case number is shown in Figure 2. Average scores were 9.4 for AFI, 4.0 for NBI, and 12.3 for CE. The AFI scores tended to be higher, whereas NBI scores tended to be lower. There was no significant difference in total visibility scores among histopathological findings. Interobserver agreement was 0.65 for AFI, 0.80 for NBI, and 0.70 for CE. There were no significant differences in interobserver agreement between endoscopic images.
| Endoscopic image | Total (%) cases | No. (%) cases | ||
|---|---|---|---|---|
| Improved visibility | Equivalent visibility | Decreased visibility | ||
| AFI | 30 (100) | 19 (63.3) | 11 (36.7) | 0 (0) |
| NBI | 30 (100) | 2 (6.7) | 28 (93.3) | 0 (0) |
| CE | 30 (100) | 22 (73.3) | 8 (26.7) | 0 (0) |
- Kappa statistic: AFI 0.65, NBI 0.80, CE 0.70.
- *P < 0.01.
- AFI, autofluorescence imaging; CE, chromoendoscopy; NBI, narrow-band imaging.
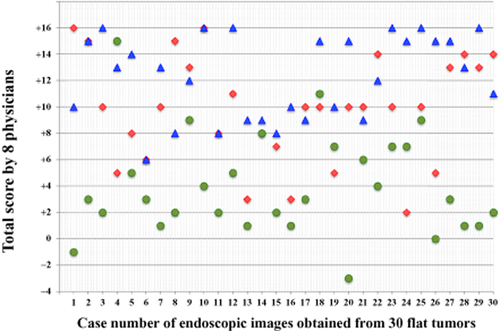
Distribution of total visibility scores using autofluorescence imaging (AFI), narrow-band imaging (NBI) and chromoendoscopy (CE) detailed in each case number of endoscopic images obtained from 30 flat tumors. The eight physicians’ scores for each image were tallied. Possible maximum score for any image was +16, and the possible minimum score was −16.  , AFI (9.4);
, AFI (9.4);  , NBI (4.0);
, NBI (4.0);  , CE (12.3) (average score).
, CE (12.3) (average score).
Discussion
Although colorectal flat tumors are less prevalent than colorectal polypoid lesions, flat lesions have greater malignant potential.16-19 Flat lesions tend, however, to be more difficult to detect using standard WLI than polypoid tumors, especially for less-experienced endoscopists. Using pan-colonic dye spray has been reported to improve the colorectal adenoma detection rate;6, 7 however, this procedure is complicated and time-consuming for use in routine examinations. In contrast, IEE colonoscopy does not require spray dye solution; a single push of a button on the endoscope allows IEE imaging during the colonoscopic examination.
A novel technique, AFI uses short-wavelength light to excite endogenous tissue fluorophores that emit longer wavelengths of fluorescent light.20, 21 Thus, neoplastic tissues are highlighted without requiring exogenous fluorophore administration. Using AFI colonoscopy, non-neoplastic lesions appear green, whereas neoplastic lesions appear magenta. We used the AFI system with the new-generation endoscopy system and found a high improvement rate for visualization of colorectal flat lesions. Our results revealed that AFI provided visibility comparable to that of CE. Tamai et al.22 reported that AFI provided high-quality visualization of laterally spreading tumors non-granular type. Several other studies have reported that AFI is quite useful for differential diagnosis between neoplastic and non-neoplastic lesions.23-27 However, whether AFI is truly useful for colorectal tumor detection remains controversial, with researchers reporting mixed results.8-11 Matsuda et al.8 reported that AFI detected more polyps in the right-side of the colon compared to WLI, and the miss rate for all polyps with AFI (30%) was significantly less than when using WLI (49%). Significantly more small and/or flat neoplastic lesions were detected by AFI than by WLI. Takeuchi et al.9 also reported that AFI colonoscopy with a transparent hood detected significantly more colorectal neoplasias than did WLI without a transparent hood, even when conducted by less-experienced endoscopists.
Although NBI is commonly used for managing colorectal lesions in Japan, meta-analyses have concluded that NBI in conjunction with the previous endoscopy system is not effective for detecting colorectal neoplasia.13, 14 The new generation of NBI was therefore developed with the goal of improved brightness, although prospective trials examining this claim have not been published. In the present study, there was no significant difference in the quality of visualization of flat tumors between WLI and NBI. Our results using NBI with a new-generation endoscopy system were not promising; however, there was no decreased visibility using NBI compared to WLI. This result indicated some potential for NBI colonoscopy. Further study is needed to reveal the usefulness of visibility and detectability of flat lesions using the new generation of NBI colonoscopy.
Our results showed that AFI colonoscopy using the new-generation endoscopy system may be superior to NBI colonoscopy for the detection of flat tumors. Suzuki et al.27 also reported that AFI may be more effective for the characterization of colorectal adenomas because of better visualization of lesions compared to NBI. This preliminary study had some limitations. First, only still images, rather than real-time images during a colonoscopy, were evaluated. Second, there were a small number of cases. Last, despite our best efforts at maintaining a complete blind and avoiding bias, a certain amount of error is invariably introduced when using human measurements. Further studies are needed to prove the clinical usefulness of IEE colonoscopy for improved detectability of flat tumors using the new-generation endoscopy system.
In conclusion, our data showed that AFI was useful for improving image quality in cases of colorectal flat tumors. A prospective multicenter trial for screening colonoscopy using the new-generation endoscopy system is needed to clarify clinical indications for AFI colonoscopy.
Conflicts of Interest
Authors declare no conflicts of interest for this article.




