Modelling the spatial abundance of a migratory predator: A call for transboundary marine protected areas
Abstract
Aim
During their migration, highly mobile species cross multiple jurisdictional boundaries and multiple not-specific marine protected areas (MPAs). When identifying the critical habitats where individuals aggregate, these areas can be ideal candidates for MPAs. This study was focused on the endangered fin whale (Balaenoptera physalus) for which there is little knowledge on its distribution and abundance in non-breeding temperate latitudes.
Location
Bay of Biscay (BoB).
Methods
Firstly, we modelled the relative abundance of fin whales by means of generalized additive models (GAMs) using data collected on the PELACUS (2007–2008) and JUVENA (2013–2016) oceanographic surveys during late summer. Secondly, we evaluated the reliability of the predictions by distinguishing environmental extrapolations and interpolations. Finally, we identified critical areas of highest predicted abundance and we assessed whether existing MPAs comprised within the Natura 2000 network and designated for other species offer protection to fin whales in the BoB.
Results
Fin whales were especially abundant in deep off-shore waters, mainly associated with intermediate temperature water values in the inner part of the BoB. The years with the highest relative predicted abundances (an average of 1,500 whales) matched with years when warmer sea surface temperature extended into larger areas. In colder years, the average predicted abundance dropped to 400 whales. The main critical area for fin whales (defined by the highest 40% of abundance) was common for both surveys, and it was located in the south-eastern part of the BoB.
Main conclusions
Our study contributes to the identification of important concentration areas of fin whales during late summer, based on reliable spatial predictions. The assessment of the current Natura 2000 network highlights the fact that only three MPAs marginally covered the critical area we have identified for fin whales. We propose a transboundary potential MPA to aid the conservation of the species in the BoB.
1 INTRODUCTION
Wide-ranging animals perform annual migratory movements in search of foraging areas to overcome energetic limitation during certain periods of the year (Edwards, Hall, Moore, Sheredy, & Redfern, 2015; Silva, Prieto, Jonsen, Baumgartner, & Santos, 2013). Migratory species use environmental cues to locate prey fields, and inter-annual differences in distribution and abundance patterns are a consequence of environmental variability (Stern, 2009). The marine environment is a highly dynamic system where different oceanographic processes influence the distribution of prey and their predators (Mann & Lazier, 2013; Sims et al., 2008). Specifically, mesoscale oceanographic features such as fronts, eddies and upwelling events are important processes that can drive the foraging locations of highly migratory oceanic species (Bost et al., 2009).
The management and conservation of highly migratory species face particular challenges since animals cross multiple jurisdictional boundaries (Lascelles et al., 2014). One initial step to advance in their conservation and management would be to delineate candidate protected marine areas for highly migratory species, by identifying high abundance areas that are visited every year (Lascelles et al., 2014). Statistical tools such as habitat modelling can help identify areas with the highest presence probability or higher abundance of a species during critical periods (Louzao et al., 2011; Pérez-Jorge et al., 2015). The dynamic nature of the marine environment needs to be considered when designing and implementing marine protected areas (MPAs): understanding how oceanographic processes influence marine vertebrate distribution is essential for effective conservation (Hooker et al., 2011). MPAs delimited by fixed geographical boundaries might not have the capacity to cover the habitat requirements of the species (Lascelles, Langham, Ronconi, & Reid, 2012), and consequently, a flexible design approach adapted to overlap the life history traits of the species and the pelagic environment will require implementing dynamic MPAs (Hooker et al., 2011).
Among highly migratory species, baleen whales are of special conservation and management interest since they were commercially hunted until almost forty years ago (Stoett, 2011). For instance, fin whale Balaenoptera physalus populations were reduced by 70% during the commercial whaling era (Brownell & Yablokov, 2009; Buckland, Cattanach, & Lens, 1992). Since the International Whaling Commission's (IWC) moratorium on commercial whaling (Stoett, 2011), fin whale populations have increased (Víkingsson et al., 2009). However, they remain classified as Endangered (IUCN, 2016) in need of appropriate management measures to ensure the recovery of the populations (Edwards et al., 2015).
In Europe, the Habitats Directive (HD, Council Directive 92/43/EEC) requires that each Member State set up Special Areas of Conservation (SACs) for those species listed under Annex II which for cetaceans includes only harbour porpoise, Phoconea phocoena and the bottlenose dolphin Tursiops truncatus. Together, these areas will constitute a network of protected sites across the European Union (EU), called the Natura 2000 network (Trouwborst, 2011). However, the HD Annex II is insufficiently representative of marine species in need of conservation (Trouwborst & Dotinga, 2011). The Marine Strategy Framework Directive (MSFD, 2008/56/EC), which aims at achieving and maintaining the Good Environmental Status (GES) of EU marine ecosystems by 2020, addresses some shortcomings of the HD (Trouwborst & Dotinga, 2011). MSFD requires Member States to monitor progress towards GES and set up appropriate measures to restore GES if needed. Such measures may include setting up MPAs for species not listed under the HD Annex II (Trouwborst & Dotinga, 2011), such as the fin whale which is endangered worldwide (IUCN, 2016) and have been listed in the Annex IV of the HD as species of Community interest. Besides, fin whales are listed in the Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES; https://www.cites.org/eng/app/index.php), Appendix I and II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS; https://www.cms.int/en/page/appendix-i-ii-cms), Appendix II of the Bern Convention (https://www.coe.int/en/web/conventions/full-list/-/conventions/treaty/104), and they are recognized by the Ecologically or Biologically Significant Marine Areas (EBSAs; https://www.cbd.int/ebsa) under the criteria of threatened, endangered or declining species. Since fin whales require strict protection, the designation of MPAs that include appropriate management measures to minimize/eliminate the anthropogenic threats whales are facing is required although the species is not specifically listed in the Annex II of the HD.
Fin whales are widely distributed in the North Atlantic (NA), spending the summer in high-latitude feeding grounds and breeding in middle and low latitudes during winter (Edwards et al., 2015). Their migratory patterns remain unclear and not all individuals migrate seasonally: some individuals remain in higher latitudes during colder months and in lower latitudes during warmer months (Edwards et al., 2015). Lack of detailed knowledge about their migratory patterns partly stems from most research taking place in non-breeding high latitudes areas, while temperate latitudes have been less studied (Mizroch, Rice, Zwiefelhofer, Waite, & Perryman, 2009). Fin whale population structure in the NA is similarly poorly known, though recent studies suggest the existence of two subpopulations (Vighi, Borrell, & Aguilar, 2015). Fin whales present in the Bay of Biscay (BoB) are part of the British Isles–Spain–Portugal subpopulation, with an estimated abundance of 17,400 individuals in Aguilar & García-Vernet (2017). Presumably, fin whales occupy the BoB during the spring–autumn period, but only sparse information is available on their distribution and abundance in this temperate biogeographical area.
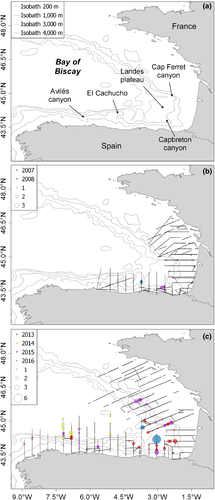
Given the oceanic habitat of the species, dedicated surveys are costly and logistically difficult to organize; thus, their periodicity to date has been decadal (e.g., SCANS; Hammond et al., 2017 and CODA; Hammond et al., 2009). Marine mammal sightings can also be obtained from other non-dedicated monitoring schemes, such as oceanographic surveys directed at assessing the status of the stocks of commercial fish species, which cover the same geographical area every year with standardized methodology (Lambert et al., 2018; Saavedra et al., 2018). Within this framework, we took advantage of JUVENA (Boyra et al., 2013) and PELACUS (ICES, 2009) annual multidisciplinary oceanographic surveys that every September monitor the pelagic environment of the BoB.
The main objective of the present work was to obtain relative spatial abundance estimates of the endangered fin whale to assess critical conservation areas in the BoB. Specifically, (a) we explored the oceanographic and physiographic features explaining the observed patterns of fin whale abundance, (b) we obtained spatial predictions of fin whale density, (c) we identified critical areas within the 6-years study period, and (d) we assessed the relevance of the Natura 2000 network for fin whales of both Spanish and French waters. Our spatial modelling approach relies on generalized additive models to predict marine mammal spatial abundance and on model averaging to account for model uncertainty, a rarely used method to model marine mammal spatial abundance. In addition, we assessed the reliability of predictions by carefully distinguishing environmental extrapolations and interpolations. The present study exemplifies a methodological approach to obtain spatial abundance estimates of marine animals sampled following non-dedicated line-transect surveys and provides an assessment of the importance of existing MPAs for the protection of an endangered highly migratory predator.
2 METHODS
2.1 Data collection
At-sea observations were collected during PELACUS and JUVENA multidisciplinary surveys that took place in the BoB yearly during late summer (September) in 2007–2008 on board R/V Thalassa (TH) and 2013–2016 on board R/V Ramón Margalef (RM), respectively (Figure 1). Visual line-transect protocols (Buckland et al., 2001) were followed during all surveys. For each sighting, top predator observers recorded detection distance using a stick based on the Heinemann (1981) method and the angle based on an angle metre and measured with respect to the track line. Additional data recorded for each sighting were the time, species, group size, animal heading relative to the ship, behaviour and the presence of calves. The study area, surveys’ protocol (see Supporting information Appendices S1 and S2), and a schematic workflow of the entire analytical process (Supporting information Appendix S3: Figure S3.3) are included in the Supporting Information.
2.2 Environmental data
Environmental covariates were selected based on biological relevance and data availability (Supporting information Appendix S4). We selected the same physiographic covariates selected by previous studies examining baleen whale distribution (Breen, Brown, Reid, & Rogan, 2016; Laran & Gannier, 2008). We included depth, slope, the closest distance to the coast, to the shelf-break and to the 1,000- and 2,000-m isobaths. For oceanographic variables, we were especially interested in those variables explaining mesoscale features (Scales et al., 2014). Oceanographic covariates included sea surface temperature (SST) and its spatial gradient (SSTg), sea level anomalies (SLA), the modulus of the geostrophic currents (w) and eddy kinetic energy (EKE). As biologically relevant oceanographic variables, we included chlorophyll a concentration (Chl-a) mean and its spatial gradient (Chl-ag). Chl-a values were used as a proxy for phytoplankton biomass. SST and Chl-a gradients were calculated following Louzao et al. (2009). All oceanographic predictors were integrated over 7 days prior to each sampling day. All environmental datasets were either provided in, or modified so they were in, the geographical coordinate system WGS 1984, with a regular cell size of 0.04°.
2.3 Detection function modelling
Detection functions were estimated pooling fin whale sightings from the six years. Only sightings with a Beaufort Sea-state ≤5, wave height ≤2 m and overall medium and good visibility conditions were used. Perpendicular distances were truncated to exclude sightings beyond 4,000 m (around 5% of the individuals detected at the longest distances; Buckland et al., 2001). We used conventional distance sampling (CDS) and multiple-covariate distance sampling (MCDS; Marques & Buckland, 2004) using the mrds R-package (Laake, Borchers, Thomas, Miller, & Bishop, 2015). Covariates tested in the MCDS analyses included group size as continuous variable and Beaufort Sea-state, cloud cover, year, wave height and type of vessel as factor variables. The long period that fin whales remain at the surface and the high visibility of their blows make them easily detectable. This fact justifies the assumption that detection on the track line is close to 100%, that is, g(0) = 1 (Hammond et al., 2017); however, we have not formally corrected for availability and perception bias and consequently the abundance estimates should be considered relative. We selected the model specification that resulted in the smallest value of the Akaike information criterion (AIC) and by comparing the p-value of the Cramér-von Mises goodness-of-fit test statistics (Thomas et al., 2010). Detection function selection was made on parsimony grounds (i.e., similar explicative power but less parameters; Arnold, 2010) when the two best detection functions remained within a difference of AIC of 2 (∆AIC < 2). Once the best detection function was selected, the effective strip half-width (ESW) was calculated for each level of the covariate as the perpendicular distance in which the missing detections at lower distances were equal to the recorded detections at higher distances.
2.4 Data processing
Surveyed transects were split into legs of identical detection conditions, and then, each leg was subdivided into 10-km-long segments, so the variability in environmental characteristics was limited within segments (Mannocci et al., 2014; Virgili et al., 2017). To fit the models on the best quality data, we kept only segments with a Beaufort Sea-state ≤5, wave height ≤2 m and overall medium and good visibility conditions. For every segment, we summed up the group size of each fin whale sightings (Table 1). The mid-point of each segment was used to assign the environmental data to the segments.
| Survey | Year | Effort (km) | Filtered effort (km) | Sightings | Individuals | Mean group size (CV) | Encounter rate (ind/km) | Segments | Sightings’ segments |
|---|---|---|---|---|---|---|---|---|---|
| PELACUS | 2007 | 3,315 | 2,310 | 11 | 12 | 1.09 (27%) | 0.005 | 396 | 8 |
| 2008 | 2,265 | 1,560 | 4 | 6 | 1.50 (66%) | 0.004 | |||
| JUVENA | 2013 | 2,166 | 1,555 | 4 | 11 | 2.75 (86%) | 0.007 | 962 | 78 |
| 2014 | 2,630 | 1,845 | 19 | 25 | 1.32 (44%) | 0.010 | |||
| 2015 | 2,550 | 2,261 | 44 | 59 | 1.34 (40%) | 0.020 | |||
| 2016 | 2,286 | 2,170 | 55 | 74 | 1.37 (47%) | 0.025 |
2.5 Density surface models
Density surface models were fitted using generalized additive models (GAMs) to identify the most important environmental variables explaining fin whale abundance patterns (i.e., to relate the number of fin whales per segment to environmental covariates). To account for overdispersion in the data, we selected a negative binomial distribution. The logarithm of the effective sampled area (L*2*ESW where L is the length of the segment in kilometres) was included as an offset. To limit the scope for over-fitting the data, smoothers in the models were constrained to a maximum of 3 degrees of freedom (k = 4) and a maximum number of four covariates was used (Lambert, Pettex, et al., 2017). Prior to modelling, all variables were standardized to have a mean of 0 and a standard deviation of 1 due to differing ranges of variables (Zuur, Ieno, & Smith, 2007). To avoid co-linearity problems, we calculated pairwise Spearman correlation coefficients (r) between all pairs of variables and did not include variables with |r| > |0.7| (Dormann, Elith, & Bacher, 2013). We selected the “non-correlated” predictors using the lowest Akaike information criteria (AIC) from univariate models of the two predictor variables. This analysis led to the removal of the closest distance to the 1,000-m isobath and the modulus of the geostrophic currents (w), correlated to 2,000-m isobath and EKE, respectively (Supporting information Appendix S5: Table S5.2).
GAMs were implemented following the information-theoretic framework using the dredge command of the MuMIn R-package (Barton, 2016). We then ranked the models using their AIC value corrected for small sample sizes (AICc), and we calculated the Akaike weight (ωi) for each model (Burnham & Anderson, 2002). Incorporating all possible explanatory variables produce a large number of models: if no clear top model was identified (i.e., ωi > 0.90), a model averaging procedure was used instead to account for all models and parameters uncertainty (Burnham & Anderson, 2002). Therefore, to obtain averaged coefficients and variance estimator, we used a model averaging approach from the top set of models where the cumulative sum of ωi was ≥0.95, starting with the model with the highest ωi (Johnson & Omland, 2004). Finally, we measured the relative variable importance as the sum of the ωi of the models in which the predictor was included (Burnham & Anderson, 2002). We used the resulting averaged model to compute the spatial predictions for each year on a 0.04°×0.04° resolution grid of covariates. This procedure provided maps of fin whale density per year.
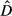 ) by the cell area (A) over the whole study area (Equation 1). Furthermore, 95% confidence interval was calculated assuming a positively skewed distribution of
) by the cell area (A) over the whole study area (Equation 1). Furthermore, 95% confidence interval was calculated assuming a positively skewed distribution of 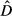 following the Equations 2, 3 and
following the Equations 2, 3 and
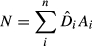 (1)
(1)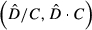 (2)
(2)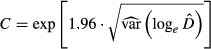 (3)
(3)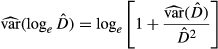 (4)
(4)2.6 Spatial prediction reliability
We assessed spatial prediction reliability by quantifying whether a prediction was an inter- or an extrapolation in environmental space, which amounts to testing whether the combination of environmental variable values associated with the prediction lies inside or outside the smallest convex hull defined by the environmental variables used when calibrating (i.e., estimating) the model (Authier, Saraux, & Péron, 2017; King & Zeng, 2006). Importantly, assessing convex hulls does not require any model fitting: the definition of an environmental extrapolation is thus model independent. In the simple case of two environmental variables, a convex hull is the polygon with vertices at the extreme points of the data (see Supporting information Appendix S6). When a prediction falls outside the convex hull, it is an extrapolation stricto sensu, but can be still informed by any data point lying within a given radius of the prediction here chosen as one geometric mean Gower's distance of all pairs of available data points (King & Zeng, 2007).
Since we used model averaging, extrapolation was estimated as the average frequency across models included the 95% confidence set with which each prediction was an extrapolation stricto sensu. For each prediction, its neighbourhood (in percentage) was estimated as the average proportion of calibration data points in environmental space lying within one geometric mean Gower's distance (King & Zeng, 2007). Reliable predictions (less model-dependent) were defined by a low percentage of extrapolation and a high percentage of neighbourhood (defined as the percentage of calibration data used to inform neighbouring cells), while a high percentage of extrapolation and a low percentage of neighbourhood indicate that predictions are less trustworthy. Reliability thus defined reflects how much a prediction is informed by actual data versus modelled inferences. Thus, place more confidence in a prediction that is informed by a lot of data than in a prediction that is not, although both may turn out to be correct if the model used for the prediction captures accurately the underlying relationships between the covariates and the response variable (whale abundance in our case). We used the R-package WhatIf (Stoll, King, & Zeng, 2014) to calculate the convex hull and Gower`s distances.
2.7 Critical areas of fin whales in the Bay of Biscay
Since this species requires strict protection, we assessed whether existing MPAs within the Natura 2000 network could be relevant to aid in their conservation in the BoB. MPA designation should be accompanied by the implementation of appropriate management measures that minimize/eliminate the anthropogenic threats faced by the species in the area, but to carry out this exercise, we followed the thresholds proposed by the European Commission (Hab. 97/2 rev. 4 18/11/97): a site was considered relevant if encompasses more than 1% of the national population. Whether a national protected area network contains less than 20% of the national population (i.e., individuals belonging to the focal species present within the national territory) is considered inadequate, while a network which covers more than 60% of the national population would be considered sufficient. Lastly, a network that covers between 20% and 60% of the national population is considered adequate, although they are to be subjected to further expert judgement (European Commission, 2007). Although our study area only covers the Northern Spanish and Western French waters of the Bay of Biscay, we still used the term “national population” because it is the one used by the European Commission to apply the thresholds to assess the Natura 2000 network. Furthermore, this term does not consider the importance of biogeographical populations or conservations units as it is the case of our study area which includes the British Isles–Spain–Portugal fin whale subpopulation (IWC, 2007). Therefore, the term “national population” of the present study refers separately to Spanish and French sectors of the Bay of Biscay.
We first identified marine areas of highest predicted abundance estimates as critical areas. Areas of highest abundances were calculated based on the averaged abundance values predicted for each survey, and therefore, different years were considered (PELACUS: 2007–2008 and JUVENA 2013–2016). For each survey, we sorted all grid cells by their decreasing estimate of mean abundance. We then transformed the numerical estimated averaged abundance in a relative index of abundance in terms of percentage by steps of 10%, ranging from the minimum value (0) to the maximum value over the study period (100%) over the whole study area (Cañadas & Vázquez, 2014). Critical areas comprising the highest abundance were defined by the highest 40% of predicted abundance. Secondly, we overlapped the average fin whale abundance per survey with the location of existing MPAs included in both Spanish and French Special Areas of Conservation. The location of current MPAs was obtained from the World Database on Protected Areas (UNEP-WCMC and IUCN, 2017). We identified an additional Large off-shore Sector belonging to the French Economic Exclusive Zone (EEZ), which is currently under designation process (http://www.aires-marines.fr/Les-aires-marines-protegees).
3 RESULTS
3.1 Detection function modelling
We estimated detection functions based on 137 fin whale sightings after filtering for weather conditions. The hazard-rate function with no adjustment terms and vessel identity as covariate was selected as the best-fitting detection function (Supporting information Appendix S7: Figure S7.4). Although this was not the model with the lowest AIC value (all the models tested are shown in Supporting information Appendix S7: Table S7.3), it remained within a difference of AIC of 2 (∆AIC <2) and it was preferred on parsimony grounds. From the detection function selected, we calculated the corresponding effective strip half-widths (ESW) for fin whales as 2,050 m (CV = 7%) for the RM (JUVENA) and 2,680 m (CV = 13%) for the TH (PELACUS).
3.2 Density surface models
After excluding segments with missing environmental predictors, a total of 1,252 segments of which 85 segments included 183 individual fin whales were used to fit density surface models (Table 1). The number of models combined to achieve the 95% confidence set was 26 out of a total of 561 (Supporting information Appendix S8: Table S8.4). Explained deviances ranged between 32.3% and 37.4%. Effective degrees of freedom of smooth terms in the 95% confidence set of models show that the relationship between the explanatory variables and the fin whale density were nonlinear (estimated degrees of freedom > 1). BAT, SST, SLA and Chl-ag were the most important variables describing the spatial abundance of fin whales and those included in the top ranked GAM as explanatory variables (Figure 2a). Densities of fin whale increased at depths (BAT) higher than 1,000 m with maximum values around 2,000 m depth, that is beyond the continental shelf, limited by the 200-m isobath (Figure 2b). The SST ranged between 16°C and 24°C, showing the highest densities around 20°C, whereas densities increased within a positive SLA range (Figure 2b). Chl-ag values showed a negative effect, since fin whale densities decreased as the Chl-ag values increased (Figure 2b).
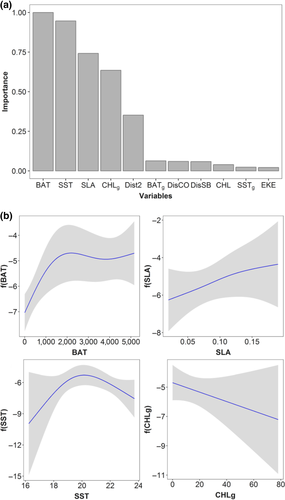
Estimated relative density predictions were higher in the south-eastern part of the BoB (SE-BoB) within the Capbreton and Cap Ferret canyons, and off the French and Spanish continental slopes associated with deeper waters (Figure 3). While in 2013, 2014 and 2016 the highest relative densities of fin whales were found in the abyssal plain, in 2007, 2008 and 2015 the highest densities were concentrated in the SE-BoB. The lowest predicted relative densities were identified recurrently every year over both the French and Spanish continental shelves.
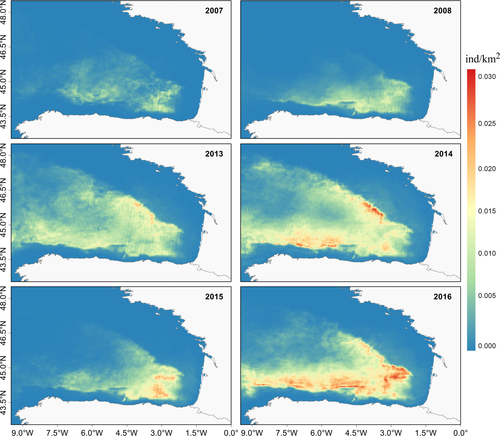
The averaged relative estimated density varied from 7.10−4 to 4.10−3 fin whales/km2, with total predicted relative abundances ranging from 291 to 1,735 fin whales. Annual density and abundance estimates are shown in Table 2. When warmer sea surface temperature extended into larger areas (2013, 2014 and 2016 (Supporting information Appendix S4: Figure S4.3)), relative abundance values were higher than 1,200 whales (1,241–1,600). The remaining years the abundance values were much lower, ranging between 291 and 610 animals.
| Year | D | CVD | N | 95% CIN |
|---|---|---|---|---|
| 2007 | 0.0007 | 0.89 | 291 | 65–1,310 |
| 2008 | 0.0010 | 0.66 | 387 | 118–1,267 |
| 2013 | 0.0030 | 0.63 | 1,241 | 395–3,901 |
| 2014 | 0.0045 | 0.54 | 1,735 | 638–4,718 |
| 2015 | 0.0010 | 0.61 | 610 | 201–1,853 |
| 2016 | 0.0040 | 0.58 | 1,600 | 551–4,641 |
- Averaged animal density (D, individuals/km2) and its coefficient of variation (CVD), estimated abundance (N) and its 95% confidence interval (95% CIN).
3.3 Spatial predictions reliability
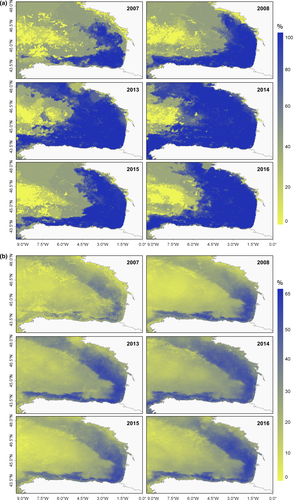
Environmental extrapolation and neighbourhood maps showed that across years, predictions were reliable (that it is less model-dependent) on the continental shelf, and partially covering the oceanic area. Predicted fin whale abundances on the shelf and shelf-break areas of the BoB were informed by at least 30% of the calibration data (Figure 4b): these areas of high neighbourhood percentages were located mainly over Spanish and French continental shelves and extended to deeper waters in the former case.
3.4 Suitability of Marine Protected Areas in the Bay of Biscay for fin whales
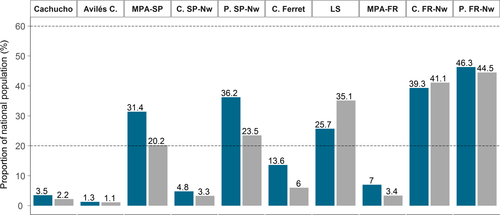
By overlapping the spatial location of existing and projected MPAs under the Natura 2000 network with the averaged abundance for each survey (Figures 5 and 6), we observed that only the French MPAs were adequate (i.e., covering >20% but less than 60% of the national population) by covering the 39.3% and 41.1% of the French population for PELACUS and JUVENA surveys. When considering only the Large off-shore Sector, the 25.7% and 35.1% of the French fin whale population for PELACUS and JUVENA surveys, respectively, would be protected. However, the Spanish MPAs did not achieve the threshold to consider a network as adequate covering only the 4.8% and 3.3% of the Spanish fin whale population (Figure 6).
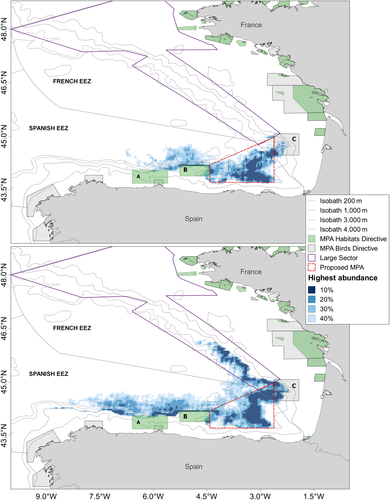
The area comprising the highest 40% was selected to incorporate most of the sightings and it was based on the approximate limit of the 4,000-m isobath. The critical areas had an average density of 0.006 animals/km2 (CV = 0.21) and 0.010 animals/km2 (CV = 0.13) for the PELACUS and JUVENA survey, respectively. These critical areas (Figure 5) included Capbreton and Cap Ferret canyons in both surveys. Furthermore, the critical area was smaller and located off the central Spanish continental slope for PELACUS, whereas the JUVENA critical area covered also the area off the southern French continental slope.
By delimiting the common critical area for fin whales based on both surveys, we identified an important marine area for the conservation of a highly migratory predator in the BoB (Figure 5). This area, located in the SE-BoB, was delimited following the 1,000-m isobath in the south and eastern part and the 4,000-m isobath to define the northern boundary and represents an area of 4,090 nmi2. In late summer, this marine area encompasses 31.4% and 20.2% of Spanish national population of fin whales based on the PELACUS and JUVENA estimations, respectively. Regarding the French EEZ, this marine area would comprise 7% and 3.4% of the French fin whale population estimated based on PELACUS and JUVENA surveys, respectively. Regarding the whole network and this important marine area, 36.2% and 23.5% of the Spanish fin whale population for PELACUS and JUVENA, respectively, would be protected implying that the Spanish network would be adequate, while in the case of French population the network would cover 46.3% and 44.5% and would continue to be adequate (Figure 6).
4 DISCUSSION
The protection of highly migratory species faces multiple challenges due to the difficulty of data collection and the implementation of effective conservation measures, among others, mainly due to the range of pressures they encounter during their extensive movements (Lascelles et al., 2014). Despite the recovery of baleen whale populations after the IWC's moratorium on commercial whaling, fin whales are still classified as Endangered by the IUCN (2016). The present study advances the conservation of fin whales by providing relative abundance estimates in a temperate non-breeding area. We also assessed the adequacy of the existing and projected network of MPAs for fin whale conservation and concluded the need to include an additional marine area to encompass the identified fin whale critical areas in the Bay of Biscay.
4.1 Spatial abundance of fin whales in the Bay of Biscay
Within their annual migratory journey, North Atlantic fin whales visit high-latitude areas for foraging (Edwards et al., 2015). The BoB is presumably one of the foraging grounds exploited by the British Isles–Spain–Portugal subpopulation outside polar and subpolar feeding grounds, as it is the case of other areas (e.g., Azores; Silva et al., 2013). Here, our effective strip half-width results were consistent to those previously reported for fin whales (Barlow & Forney, 2007; Moore & Barlow, 2011). Within the BoB, fin whale abundance was driven by both physiography and oceanography. Depth was the most important physiographic parameter as fin whales were found predominantly in deep off-shore waters of the BoB at depths beyond 1,000 m, in accordance with previous studies (Laran et al., 2017). Additionally, SST had an important role with preferred fin whale habitat occupying intermediate temperature waters (20°C). Despite the inter-annual variability in SST spatial patterns, fin whale abundance was better explained by the spatial distribution of SST values, which were intermediate and concentrated over the abyssal plain of the SE-BoB, shaping fin whale abundance patterns. Densities increased with SLA, being highest around 15–20 cm: these values coincide with those near the core of anticyclonic eddies typical of off-shore areas of the SE-BoB during this period (Caballero et al., 2014). As has been reported by other studies, we found a negative relationship between the spatial patterns of fin whales abundance and Chl-ag (Cotté, Guinet, Taupier-Letage, Mate, & Petiau, 2009; Panigada et al., 2008). In the BoB, the highest values of Chl-a were found near the coast (Supporting information Appendix S4: Figure S4.3-f) due to the presence of local upwelling events (Koutsikopoulos & Le Cann, 1996) which explains why the highest Chl-ag was also located in these areas (Supporting information Appendix S4: Figure S4.3-g). This oceanographic feature would explain the negative relationship between the whale predicted densities and the Chl-ag as the highest whale densities were found predominantly in off-shore waters where the lowest Chl-ag values are found.
This marked fin whale off-shore distribution and abundance may be driven by food resources. There are no studies on fin whale diet in the inner part of the BoB, but their diet off north-western Spanish waters is constituted by krill (mainly euphausiids) and small pelagic fish (Aguilar, 2009). Fin whales are reported to rely on krill when available and to prey on small pelagic fish otherwise (Vighi et al., 2015). In the BoB, plankton blooms take place in spring and late autumn, whereas in summer and winter this area reaches minimum plankton biomass (Pingree & Garcia-Soto, 2014). In this area, higher fin whale abundances could occur with a time-lag of some months after the onset of the spring phytoplankton bloom as suggest by Visser, Hartman, Pierce, Valavanis, and Huisman (2011). These authors found that baleen whale peak abundance in the Azores archipelago occurred three months later than the onset of the spring phytoplankton bloom. Although a study conducted during our survey period confirmed that the zooplankton detected in the area was predominantly composed by euphausiids (Lezama-Ochoa, Boyra, Goñi, Arrizabalaga, & Bertrand, 2010), the absence of high biomass of zooplankton supports the hypothesis that fin whales feed mainly on small pelagic fish during the late summer in the BoB. In fact, from early August onwards, off-shore French waters support a high biomass of age 0 fish (i.e., juveniles) of the European anchovy Engraulis encrasicolus (Boyra et al., 2013, 2016). This small pelagic fish could constitute the main food resource for fin whales during this period in the BoB as has been demonstrated for other areas such as the Celtic Sea, where fin whales feed on age 0 sprat Sprattus sprattus and herring Clupea harengus (Vighi et al., 2015).
Abundance predictions produced by our spatial models should be carefully considered, since they are relative abundances due to the absence of available data to correct for perception and availability bias for ship-based surveys in the Bay of Biscay (Certain, Ridoux, Canneyt, & Bretagnolle, 2008). However, the environmental extrapolation analysis showed that the main predicted areas of the highest fin whale abundance were interpolations stricto sensu (inside the convex hull) and they were informed by at least (on average) 25% of the data. Relative abundance estimates for fin whales were shaped by SST patterns during the study period. Estimates were higher (>1,200 individuals) in those years when warmer SST extended over the oceanic areas of the BoB. On the contrary, in years when warmer SST values were restricted to the inner part of the BoB the predicted relative abundances were lower (<600 individuals). Our abundance estimates are consistent with previous studies carried out in summer in the BoB such as CODA (Hammond et al., 2009) and SAMM (Suivi Aérien de la Megafaune Marine 2012; Laran et al., 2017), despite the different extension of the sampling areas.
4.2 Critical areas for oceanic species
Under the United Nation Convention on Biological Diversity, the EU has committed to ensure the conservation of 10% of its coastal and marine areas by 2020 following the 11th Aichi Biodiversity Target of the Convention (CBD, 2010). Usually, marine zoning strategies are based on geographically fixed features to define the extent of the (MPAs) contours (Louzao et al., 2006). However, oceanographic habitats are highly dynamic and many pelagic species (e.g., cetaceans) are highly migratory covering extensive areas annually (Silva et al., 2013) with the location of their aggregations and migratory routes depending on oceanographic features. For these reasons, the efficiency of static MPAs to conserve highly mobile species is still discussed (Wilson, 2016) but the need to develop dynamic MPAs that can be adapted to deal with this variability within and between years is gaining momentum (Game et al., 2009). Despite the large-scale movements of many pelagic predators, the protection of specific zones where individuals aggregate either during breeding or foraging (Louzao et al., 2006) or during their migration routes (Víkingsson & Heide-Jørgensen, 2015) can help their conservation if appropriate management and conservation measures are in place to ensure that the anthropogenic threats are reduced/eliminated (i.e., overfishing causing food resources depletion or maritime traffic increasing the probability of collision of cetacean species; Avila, Kaschner, & Dormann, 2018). Detailed information on the distribution and abundance of cetaceans is paramount to assess critical areas of conservation interest. However, obtaining these data for oceanic cetaceans is a difficult task due to the costs and logistics involved in developing dedicated surveys. Additionally, platforms of opportunity such as ecosystem-based surveys usually do not cover oceanic areas (Authier et al., 2018). The oceanic sampling of JUVENA and PELACUS surveys is exceptional in this respect. It is therefore important to take advantage of the non-dedicated monitoring programs already in place to conduct long-term monitoring studies of cetacean populations.
In the BoB, multiple MPAs are designated under the provision of the HD and Birds Directive (Council Directive 79/409/EEC) either in the French or in the Spanish EEZs. Even though the Natura 2000 network was not designated specifically for most marine mammal species (which are not listed under HD Annex II), it is well known that the implementation of ecologically and economically sustainable management practices can benefit these species if accompanied by appropriate management measures. As Lambert, Virgili, et al. (2017) suggest for the French network in the case of most of the cetacean species inhabiting the BoB (bottlenose, common Delphinus delphis and striped Stenella coeruleoalba dolphins, long-finned pilot whale Globicephala melas and Risso's dolphin Grampus griseus), the current Spanish MPA network does not reach the less stringent threshold proposed by the European Commission (2007) and is inadequate (i.e., encompassing <20% of the national population of interest). The Large off-shore Sector (still in the process of designation) is the most important area for fin whales due to their location in off-shore waters where the predicted abundance is higher. This result was in line with Lambert, Virgili, et al. (2017) who demonstrated that this area is highly relevant for cetaceans in summer. Thus, even if fin whales and other cetacean species are not considered under the Annex II of the HD and therefore they are not candidates for SACs within the Natura 2000 network at the European level, additional legislation (e.g., CITES, CMS, EBSAs) specifically recognizes the need to account for threatened species in need of management measures to recover or maintain their population. Therefore, fin whales as an endangered species should be the focus of specific protection and management measures.
The main critical area for fin whales was common for both surveys, located in the SE-BoB. Previous studies showed that this area is also important for other cetacean species such as long-finned pilot whales, Risso's, common and striped dolphins (Lambert, Pettex, et al., 2017). Based on the critical areas defined by this study, we propose a potential MPA in the area of the Capbreton and Cap Ferret canyons and the Landes plateau. Although the French area covered by the potential MPA has a small contribution it is important to include this area inside the French EEZ to ensure that all the critical area for fin whales are included to ensure MPA connectivity, which improves the protection of such an important area for cetaceans in the BoB. This potential MPA would be similar in spirit to the Pelagos Sanctuary in the Mediterranean Sea (Notarbartolo-Di-Sciara, Agardy, Hyrenbach, Scovazzi, & Klaveren, 2008) in that it would involve transboundary collaboration between neighbouring countries to agree on conservation measures that would benefit the marine species inhabiting this MPA.
Furthermore, as many other cetacean species, fin whales are facing many threats which disregard for jurisdictional boundaries (di Sciara et al., 2016) such as climate change, underwater noise and other forms of pollution (e.g., litter consisting of plastics or derived from fishing activities; Butterworth, 2017). Besides, it is well known that the BoB supports a significantly high level of maritime traffic and fishing activity (OSPAR, 2000) increasing the possibility of ship strikes and entanglement in fishing gears which along with the vulnerability of this species to seismic surveys (ICES, 2015) contribute to the importance of taking appropriate management measures for the protection of this species, such as the creation of transboundary MPAs (Edwards et al., 2015; Lascelles et al., 2014). Thus, this potential MPA would not only cover critical areas for fin whales in summer, but also it could be useful for the conservation of many other cetacean species.
4.3 Futures perspectives
Not all fin whales perform seasonal migrations (Edwards et al., 2015) and the migration routes from the summer to the wintering grounds in the NA are poorly known (Vighi et al., 2015). Likewise, it is unknown whether the BoB corresponds to a stop-over site or hosts a resident population of fin whales. Tracking studies, such as those performed in middle latitudes of the NA (Silva et al., 2013), as well as the development of habitat and abundance models for the whole year are necessary to assess the importance of the BoB for fin whales. For that purpose, additional dedicated surveys as well as expanding the sampling area of the existing non-dedicated surveys are necessary to monitor cetacean oceanic species in the BoB; dedicating surveys covering off-shore waters are currently only carried out every ten years (Hammond et al., 2017).
The analysis of critical areas along with the assessment of the adequacy of existent MPAs reveals that further studies are necessary to improve the management and conservation of oceanic populations at their key areas. Furthermore, as we show in the present work, critical areas can encompass more than one EEZ; hence, transboundary collaboration and agreements between governments are necessary to implement and manage new high seas MPAs (Chin et al., 2017; Kark et al., 2015). In the case of the EU, the MSFD encourages transboundary initiatives through existing conservation instruments, such as Regional Sea Conventions, but leaves much discretion to Member States. Although it is the responsibility of individual EU Member States to develop and implement the MSFD in the waters under their jurisdiction, the success of monitoring strategies and conservation measures at broad scales will require policy coordination at a regional level to facilitate and guide the cooperation between EU Member States, a role led by OSPAR for the Northeast Atlantic through the “OSPAR Regional Implementation Framework for the MSFD”.
ACKNOWLEDGEMENTS
The authors would like to thank both JUVENA (AZTI-IEO) and PELACUS (IEO) crew and scientists for all their support during oceanographic surveys, especially to marine predator observers. Special thanks to Guillermo Boyra and Enrique Nogueira for facilitating this study. I.GB was funded by a PhD fellowship from the Spanish Ministry of Economy Competitiveness (BES-2014-070597). M.L. was funded by a Juan de la Cierva (JCI-2010-07639) and a Ramón y Cajal (RYC-2012-09897) postdoctoral contract. This study is a contribution to the CHALLENGES project (CTM2013-47032-R). Finally, we would like to thank two anonymous referees and AE for their constructive reviews. The contribution number is 884 from AZTI (Marine Research).
REFERENCES
BIOSKETCH
Isabel García-Barón (I.GB) is a PhD student in AZTI. Her research focuses on ecological spatial modelling, systematic conservation planning, the identification and study of anthropogenic threats and their applications in biodiversity and conservation management in marine ecosystems.
Author contributions: M.L., MB.S. and I.GB conceived the study; M.L., I.GB, MB.S., JA.V., A.C. and JL.M. compiled the data; I.GB and M.A. analysed the data; I.GB and M.L. wrote the manuscript; all authors commented on earlier versions of the manuscript.




