Oxalate Nephropathy After Kidney Transplantation: Risk Factors and Outcomes of Two Phenotypes
Funding: This study was supported by University of Wisconsin School of Medicine and Public Health Shapiro Summer Search Program.
ABSTRACT
Describing risk factors and outcomes in kidney transplant recipients with oxalate nephropathy (ON) may help elucidate the pathogenesis and guide treatment strategies. We used a large single-center database to identify patients with ON and categorized them into delayed graft function with ON (DGF-ON) and late ON. Incidence density sampling was used to select controls. A total of 37 ON cases were diagnosed between 1/2011 and 1/2021. DGF-ON (n = 13) was diagnosed in 1.05% of the DGF population. Pancreatic atrophy on imaging (36.4% vs. 2.9%, p = 0.002) and gastric bypass history (7.7% vs. 0%; p = 0.06) were more common in DGF-ON than with controls with DGF requiring biopsy but without evidence of ON. DGF-ON was not associated with worse graft survival (p = 0.98) or death-censored graft survival (p = 0.48). Late ON (n = 24) was diagnosed after a mean of 78.2 months. Late ON patients were older (mean age 55.1 vs. 48.4 years; p = 0.02), more likely to be women (61.7% vs. 37.5%; p = 0.03), have gastric bypass history (8.3% vs. 0.8%; p = 0.02) and pancreatic atrophy on imaging (38.9% vs. 13.3%; p = 0.02). Late ON was associated with an increased risk of graft failure (HR 2.0; p = 0.07) and death-censored graft loss (HR 2.5; p = 0.10). We describe two phenotypes of ON after kidney transplantation: DGF-ON and late ON. Our study is the first to our knowledge to evaluate DGF-ON with DGF controls without ON. Although limited by small sample size, DGF-ON was not associated with adverse outcomes when compared with controls. Late ON predicted worse allograft outcomes.
Abbreviations
-
- BMI
-
- body mass index
-
- cPRA
-
- calculated plasma reactive antibody
-
- CT
-
- computed tomography
-
- DCGS
-
- death-censored graft survival
-
- DGF
-
- delayed graft function
-
- eGFR
-
- estimated glomerular filtration rate
-
- ESKD
-
- end-stage kidney disease
-
- IBD
-
- inflammatory bowel disease
-
- KTR
-
- kidney transplant recipient
-
- NAFPD
-
- non-alcoholic fatty pancreas disease
-
- ON
-
- oxalate nephropathy
-
- WisARD
-
- Wisconsin Allograft Recipient Database
1 Introduction
Oxalate or oxalic acid is a dicarboxylic acid that is derived either from diet or from endogenous metabolism of ascorbic acid and some amino acids. There is no human enzyme that degrades oxalate further, and normally it is freely filtered and excreted by the kidneys [1]. Primary hyperoxaluria represents a set of rare genetic disorders in which oxalate production in the liver is increased. The resulting oxalate nephropathy (ON) can manifest as progressive chronic kidney disease and ultimately end-stage kidney disease (ESKD). Since high oxalate production continues after kidney transplantation, ON frequently recurs after kidney-alone transplantation, and simultaneous liver-kidney transplantation may be the best option for some patients [2].
Secondary hyperoxaluria, also referred to as enteric hyperoxaluria, is due to increased dietary consumption of oxalate, increased absorption in the gut, or decreased metabolism by gut bacteria. Plasma oxalate levels in secondary hyperoxaluria are usually not as high in primary hyperoxaluria. Levels do rise with declining kidney function, however [3]. The excess oxalate can form a salt with calcium, which is insoluble and tends to crystallize, leading to stones or ON. Oxalate accumulates with progressive decrease in estimated glomerular filtration rate (eGFR) [4]. In one study of persons with ESKD, 98% had plasma oxalate levels above the upper limit of normal at the time of transplantation, with a median of 35.0 μmol/L (reference: 3.0–11.0 μmol/L) [5]. Ten weeks after transplantation, levels dropped significantly to a median of 9.0 μmol/L; however, 37% were still above the upper limit of normal. A large amount of oxalate is excreted in a relatively short period of time following transplantation. Several studies have shown that oxalate deposition in the kidney allograft is not uncommon, especially early after transplantation, and that there is an association between oxalate deposition in the kidney allograft and worse function [6-8]. However, it remains to be established whether the oxalate deposition causes poor kidney allograft function, or whether poor function initiates or increases oxalate deposition, which might then worsen allograft injury. Determining the clinical significance of ON may help guide the role of dietary, pharmacologic, and dialysis management after transplantation. Additionally, there are limited data on traditional ON risk factors associated with fat malabsorption, such as inflammatory bowel disease (IBD), gastric bypass surgery, pancreatic atrophy and small bowel resection in ON after kidney transplantation [9-11].
In this retrospective case-control study, we used a large single-center database to evaluate risk factors for ON and outcomes after this diagnosis. Given the likely differences in pathophysiology underlying the diagnosis, the ON cases were categorized into early ON associated with delayed graft function (DGF) and late ON, which occurred after stable allograft function without the need for dialysis had initially been established.
2 Materials and Methods
2.1 Study Population and Design
We reviewed adult recipients of kidney-alone transplant or simultaneous pancreas and kidney (SPK) transplant at the University of Wisconsin between January 1, 1994 and January 31, 2021. Data were collected from the Wisconsin Allograft Recipient Database (WisARD) and the electronic medical record. Patients were included if they had evidence of ON on kidney allograft biopsy and were categorized into DGF-ON and late ON.
DGF-ON was defined as the need for dialysis within the first week after transplantation. At our center, an allograft biopsy is performed for patients with prolonged DGF. The timing of the biopsy is determined by their allograft function trajectory and clinical risk factors such as presence of pre-transplant donor-specific antibodies and cold ischemia time. Kidney transplant recipients with DGF requiring biopsy which revealed ON were included as cases (n = 13). Controls were randomly selected through incidence density sampling with a 5:1 ratio whenever possible, matched on number of previous transplants and history of DGF requiring biopsy but without oxalate deposition of their respective histopathology (n = 46). Of note, all cases and controls were kidney-alone transplants.
Late ON was defined as the finding of ON at least a month after transplantation and when patients were no longer dependent on dialysis (n = 24). If a patient had multiple biopsies with ON, the first biopsy documenting ON was used for inclusion in the study. Controls for late ON cases were also selected through incidence density sampling with a 5:1 ratio matched for organ(s) transplanted (kidney-alone vs. SPK), number of previous transplants, and living versus deceased donor transplants (n = 120).
This study was approved by the local institutional review board.
2.2 Covariates and Outcome Variables
The data recorded included recipient characteristics at time of transplantation, including variables relevant to oxalate metabolism such as duration of dialysis prior to transplantation, and histories of nephrolithiasis, IBD, gastric bypass surgery, small bowel resection, and pancreatic atrophy on computed tomography (CT) scan. Text of CT scan reports was reviewed to determine if atrophy in the pancreas was described. For DGF-ON, CT scans performed pre-transplant and within one year after transplant were reviewed. For late ON, CT scans performed prior to the diagnosis of late ON or the corresponding time point in controls were reviewed. Since an intact colon is necessary for oxalate absorption, for patients with IBD, we collected data on whether patients had had a colectomy. Additionally, since exocrine function is restored in pancreatic allograft recipients with enterically drained allografts, the analysis of pancreatic atrophy was limited to kidney-only transplant recipients. Data on donor characteristics, induction and maintenance immunosuppression, calculated plasma reactive antibody (cPRA), and rejection history were also collected. Outcome variables includes graft survival and death-censored graft survival (DCGS) for both DGF-ON and late ON cohorts.
2.3 Statistical Analysis
Differences in the demographic and clinical characteristics of cases versus controls in both DGF-ON and late ON cohorts were examined through use of t-tests or chi-square tests, as appropriate. Multivariate Cox regression analysis was used to identify risk factors for development of ON after adjusting for the potential confounding risk factors identified based on a univariate relationship with p-value of less than 0.1. Robust variance estimates were used to account for the clustering due to incidence density sampling. A p-value of 0.05 or less was used to define statistically significant relationships.
To compare clinical outcomes between DGF-ON cases and respective controls, the data were censored at the time of graft loss, last available follow up with a functioning graft, or 5 years after point of entry into survival analyses. The data for late ON and respective controls were censored at graft loss or last available follow-up with a functioning graft. Kaplan-Meier analyses were done to analyze graft survival and DCGS. All statistical analyses were conducted using Stata MP 13.0 (StataCorp, College Station, TX).
3 Results
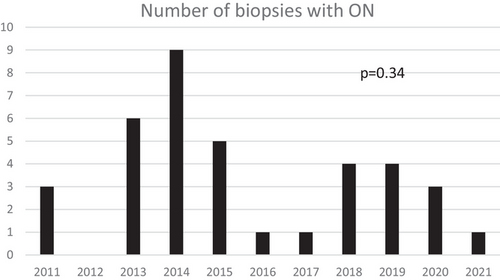
A total of 6619 kidney-alone and 1087 SPK transplants were performed at our institution during the study period. Of these, 37 (0.48%) were diagnosed with ON, of which 13 were in the DGF-ON cohort and 24 were in the late ON cohort. Notably, there were no biopsies with a diagnosis of ON prior to 2011; however, there was no statistically significant difference in the number of ON diagnoses per year between 2011 and 2021 (p = 0.34; Figure 1).
3.1 Baseline Characteristics and Outcomes of DGF-ON
Thirteen (0.2%) of 6619 kidney-alone transplant recipients were diagnosed with DGF-ON. The incidence of DGF-ON in kidney transplant recipients who developed DGF (n = 1,239) was 1.05%. We attempted to match five controls per case and were able to identify a total of 46 controls in our database via incidence density sampling methodology, matched for transplant number and history of DGF requiring biopsy, but without diagnosis of ON. Of note, DGF-ON was not diagnosed in any SPK recipient.
The differences in baseline characteristics between DGF-ON and the matched controls are summarized in Table 1. Overall, the groups were well matched for several recipient variables including age, sex, race, body mass index (BMI), cause of ESKD, and history of prior transplantation. Pancreatic atrophy identified on CT scan done pre-transplant or within the first year of transplant was significantly more common in DGF-ON patients (36.4% in DGF-ON vs. 2.9% in controls, p = 0.002). One (7.7%) patient with DGF-ON had history of gastric bypass surgery, as compared to none of the controls (p = 0.06). There was no statistically significant difference between the duration of dialysis prior to transplantation between the two groups (55.7 ± 33.3 in DGF-ON vs. 46.0 ± 31.0 months in controls, p = 0.41). None of the patients had a documented history of nephrolithiasis, IBD, or small bowel resection. There were no significant differences in donor characteristics, including type (living vs. deceased) and cold ischemia time. HLA mismatch, cPRA, induction immunosuppression, and maintenance immunosuppression were similar in the two groups. The control group had a much higher incidence of rejection (37.0% in controls versus 7.7% in DGF-ON controls, p = 0.06). The time to biopsy after transplantation in the whole cohort was 24.46 ± 13.5 days, with no significant difference between cases and controls.
| Variable | DGF-ON (n = 13) | DGF controls without oxalate (n = 46) | p-value |
|---|---|---|---|
| Recipient variables | |||
| Age at transplant, years, mean ± SD | 55.6 ± 12.6 | 52.4 ± 12.3 | 0.42 |
| Prior transplant, % | 15.4 | 13.0 | 0.83 |
| BMI at transplant, kg/m2, mean ± SD | 28.9 ± 4.4 | 27.2 ± 5.8 | 0.25 |
| Male, % | 69.2 | 60.9 | 0.58 |
| Caucasian, % | 53.8 | 67.4 | 0.37 |
| Black, % | 23.1 | 17.4 | 0.64 |
| Asian, % | 15.4 | 13.0 | 0.83 |
| Other, % | 7.7 | 2.2 | 0.94 |
| Cause of ESKD, % | 0.13 | ||
| Diabetes mellitus | 53.8 | 26.1 | |
| Hypertension | 7.7 | 6.5 | |
| Polycystic kidney disease | 7.7 | 15.2 | |
| Glomerulonephritis | 0 | 30.4 | |
| Other | 30.8 | 21.7 | |
| Pre-transplant dialysis duration, months, mean ± SD | 55.7 ± 33.3 | 46.0 ± 31.0 | 0.41 |
| History of nephrolithiasis, % | 0 | 0 | NA |
| History of IBD, % | 0 | 0 | NA |
| History of gastric bypass, % | 7.7 | 0 | 0.06 |
| History of small bowel resection, % | 0 | 0 | NA |
| History of pancreatic atrophy | 36.4 | 2.9 | 0.002 |
| History of parathyroidectomy, % | 0 | 13.0 | 0.17 |
| Donor variables | |||
| Deceased donor, % | 92.3 | 93.5 | 0.88 |
| Cold ischemia time, hours, mean ± SD | 13.8 ± 7.4 | 17.5 ± 8.0 | 0.14 |
| Donor age, years, mean ± SD | 50.5 ± 10.6 | 48.8 ± 14.1 | 0.64 |
| Transplant-related variables | |||
| Induction immunosuppression, % | 0.3 | ||
| Thymoglobulin | 23.0 | 37.0 | |
| Alemtuzumab | 61.5 | 58.7 | |
| Basiliximab | 15.4 | 4.4 | |
| Maintenance immunosuppression, % | |||
| Calcineurin inhibitors | 100 | 89.1 | 0.21 |
| Mycophenolic acid/mycophenolate mofetil | 84.6 | 95.6 | 0.16 |
| Steroid | 84.6 | 95.6 | 0.16 |
| cPRA, mean ± SD | 15.9 ± 2.0 | 17.4 ± 30.7 | 0.54 |
| HLA mismatch, mean ± SD | 4.2 ± 1.3 | 4.4 ± 1.1 | 0.72 |
| Rejection, % | 7.7 | 37.0 |
0.06 |
| Biopsy variables | |||
| Time to biopsy, days, mean ± SD | 25.7 ± 14.4 | 24.1 ± 13.4 | 0.73 |
| Calcium level at biopsy, mg/dL, mean ± SD | 8.4 ± 1.0 | 8.5 ± 1.1 | 0.84 |
| Phosphate level at biopsy, mg/dL, mean ± SD | 5.1 ± 1.6 | 4.6 ± 1.8 | 0.32 |
- Abbreviations: BMI, body mass index; cPRA, calculated panel reactive antibody; DGF-ON, delayed graft function - oxalate nephropathy; DGF, delayed graft function; ESKD, end-stage kidney disease; IBD, inflammatory bowel disease; Late ON, late oxalate nephropathy.
There was no difference in graft survival (Figure 2A; p = 0.66) or DCGS (Figure 2B; p = 0.85) at 5 years after transplantation between the two groups.
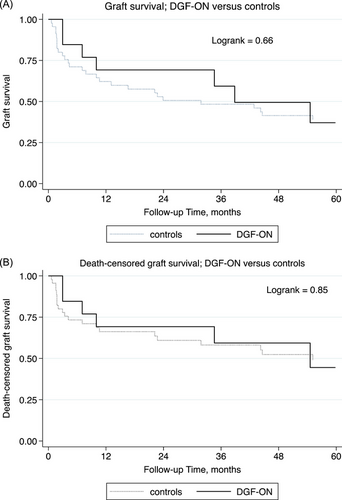
ON was not a significant predictor of graft failure (hazard ratio [HR] 1.0; p = 0.98) or death-censored graft failure (HR 1.4; p = 0.48) in multivariate models (Table 2). History of rejection by the time of DGF-ON or sampling among controls remained a significant predictor of graft failure and death-censored graft failure.
| Graft failure | Death-censored graft failure | |||
|---|---|---|---|---|
| Variables | HR | p-value | HR | p-value |
| Oxalate | 1.0 | 0.98 | 1.4 | 0.48 |
| History of gastric bypass | 1.8 | 0.10 | 1.6 | 0.21 |
| History of pancreatic atrophy | 0.6 | 0.45 | 1.6 | 1.0 |
| Rejection | 2.6 | 0.02 | 2.6 | 0.02 |
- Note: Baseline variables found to be significantly statistically different with p < 0.10 between the cases and controls were included in the model.
3.2 Baseline Characteristics and Outcomes of Late ON
Twenty-four (0.31%) kidney-alone and SPK recipients were diagnosed with late ON. Among these, 20.8% (5/24) were SPK recipients, 33.3% (8/24) were recipients of living kidney donor transplants, and 29.2% (7/24) had prior kidney transplants. Five controls were matched per case, via incidence density sampling methodology, matched for organ (kidney-alone vs. SPK), number of prior transplants, and living vs. deceased donor for the current transplant.
The differences in baseline characteristics between late ON and the respective matched controls are summarized in Table 3. The cases were older (55.1 ± 10.8 in late ON vs. 48.4 ± 12.9 years in controls, p = 0.02) and less often men (37.5% in late ON vs. 61.7% in controls, p = 0.03). Pancreatic atrophy identified on CT prior to diagnosis of ON or corresponding time point among controls was significantly more common in kidney-only transplant recipients with late ON as compared to controls (38.9% in late ON vs. 13.3% in controls; p = 0.02). History of gastric bypass surgery was also more common in late ON (8.3% in late ON vs. 0.8% in controls; p = 0.02). Two of the three late ON cases with gastric bypass surgery had surgery prior to transplant, and one had surgery after transplantation but 12.8 years prior to diagnosis of late ON. There were no differences in terms of history of nephrolithiasis or IBD with or without history of colectomy, or small bowel resections. There were also no differences in terms of the immunological parameters, including HLA mismatch, cPRA, induction as well as maintenance immunosuppression. Median time to biopsy in the late ON group was 78.2 months. We compared the renal function in the late ON group with the controls. The most recent eGFR prior to biopsy or matched time, as well as the mean of the eGFRs in the 1 to 6 months prior to biopsy or matched time were lower among late ON cases.
| Variable | Late ON (n = 24) | Controls (n = 120) | p-value |
|---|---|---|---|
| Recipient variables | |||
| Age at transplant, years, mean ± SD | 55.1 ± 10.8 | 48.4 ± 12.9 | 0.02 |
| BMI at transplant, kg/m2, mean ± SD | 27.8 ± 6.1 | 26.6 ± 4.8 | 0.45 |
| Male, % | 37.5 | 61.7 | 0.03 |
| Caucasian, % | 91.7 | 83.3 | 0.30 |
| Black, % | 4.2 | 10 | 0.36 |
| Asian, % | 0 | 5.8 | 0.22 |
| Other, % | 4.2 | 0.8 | 0.20 |
| Cause of ESKD, % | 0.28 | ||
| Diabetes mellitus | 36.8 | 20.0 | |
| Hypertension | 5.3 | 10.8 | |
| Polycystic kidney disease | 10.5 | 10.0 | |
| Glomerulonephritis | 10.5 | 19.2 | |
| Other | 36.8 | 40.0 | |
| Pre-transplant dialysis duration, months, mean ± SD | 9.8 ± 9.9 | 3.1 ± 5.8 | 0.2 |
| History of nephrolithiasis, % | 0 | 0 | NA |
| History of IBD without colectomy, % | 4.2 | 0.8 | 0.20 |
| History of gastric bypass, % | 8.3 | 0.8 | 0.02 |
| History of pancreatic atrophy in kidney-only transplants | 38.9 | 13.3 | 0.02 |
| History of small bowel resection | 4.2 | 4.2 | 1.00 |
| History of parathyroidectomy, % | 12.5 | 5.8 | 0.24 |
| Donor variables | |||
| Deceased donor, % | 66.7 | 66.7 | 1.0 |
| Donor age, years, mean ± SD | 49.0 ± 9.7 | 44.7 ± 15.2 | 0.18 |
| Transplant-related variables | |||
| DGF, % | 5.3 | 19.2 | 0.14 |
| Induction immunosuppression, % | 0.37 | ||
| Thymoglobulin | 25.0 | 21.6 | |
| Alemtuzumab | 50.0 | 47.7 | |
| Basiliximab | 8.3 | 5.0 | |
| Maintenance immunosuppression, % | |||
| Calcineurin inhibitors | 100 | 98.9 | 0.71 |
| Mycophenolic acid/ mycophenolate mofetil | 91.7 | 94.3 | 0.72 |
| Steroid | 100 | 100 | 1.00 |
| cPRA, mean ± SD | 25.3 ± 39.7 | 13.3 ± 27.8 | 0.33 |
| HLA mismatch, mean ± SD | 4.1 ± 1.4 | 3.8 ± 1.8 | 0.37 |
| Rejection, % | 20.8 | 26.7 |
0.55 |
| Biopsy variables | |||
| Most recent Cr prior to biopsy or matched time, mg/dL, mean ± SD | 2.3 ± 1.1 | 1.7 ± 1.2 | 0.04 |
| Most recent eGFR prior to biopsy or matched time, mL/min/1.73 m2, mean ± SD | 34.8 ± 20.8 | 54.5 ± 22.6 | <0.01 |
| Mean of serum Cr 1-6 months prior to biopsy or matched time, mg/dL, mean ± SD | 2.2 ± 0.8 | 2.0 ± 1.5 | 0.34 |
| Mean of eGFR Cr 1-6 months prior to biopsy or matched time, mL/min/1.73 m2, mean ± SD | 30.6 ± 16.2 | 49.8 ± 24.1 | <0.01 |
| Time to biopsy, months, mean ± SD | 78.2 ± 86.0 | NA | NA |
Kaplan- Meier analyses showed that graft survival (Figure 3A, p < 0.001), as well as death-censored graft survival (Figure 3B, p < 0.001), were significantly worse among recipients with late ON.
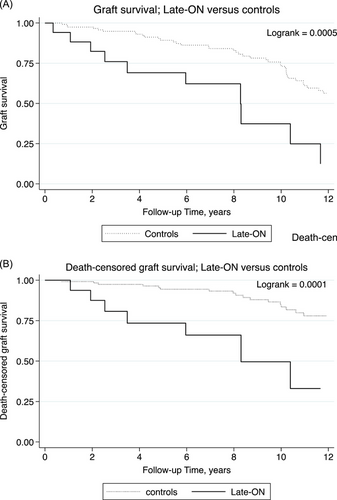
Late ON was associated with increased risks of subsequent graft failure (HR 2.0; p = 0.07) and death-censored graft failure (HR 2.5; p = 0.10) with p-values approaching significance in multivariate analyses (Table 4). In addition, history of gastric bypass was a strong predictor of graft failure with HR of 9.6, and of death-censored graft failure with HR 7.0 (both p < 0.001). Estimated GFR 1-6 months prior to biopsy also remained a strong predictor of adverse outcomes. Age, sex and history of pancreatic atrophy were not significantly associated with either outcome in multivariate analyses.
| Graft failure | Death-censored graft failure | |||
|---|---|---|---|---|
| Variables | HR | p-value | HR | p-value |
| Oxalate | 2.0 | 0.07 | 2.5 | 0.10 |
| Age, per year | 1.0 | 0.46 | 1.0 | 0.95 |
| Male | 0.7 | 0.53 | 0.5 | 0.12 |
| History of gastric bypass | 9.6 | <0.001 | 7.0 | <0.001 |
| History of pancreatic atrophy | 0.8 | 0.63 | 0.3 | 0.14 |
| Mean eGFR between 1 and 6 months prior to biopsy, per 1 mL/min/1.73 m2 increase | 0.98 | 0.001 | 0.95 | 0.002 |
- Note: Baseline variables found to be significantly statistically different with p < 0.10 between the cases and controls were included in the model.
4 Discussion
Oxalate accumulates in ESKD and is rapidly cleared after successful transplantation [6-8, 12]. Based on this known physiology, it is likely that the ON that is observed early after transplant in association with DGF is different from what is observed later in the post-transplant course, after the initial high burden of accumulated oxalate is expected to have cleared. With this background, we separately examined the risk factors and outcomes in kidney transplant recipients with early ON associated with DGF, and those with late ON. Additionally, it should be noted that there were no diagnoses of ON in our transplant recipient population until the year 2011. The exact reasons for this are not known, but we believe this may be related to reduced awareness of the impact of oxalate deposits on kidney function in the transplant population.
Slightly more than 1% of our DGF population was found to have oxalate deposits on kidney allograft biopsy performed due to prolonged DGF. An interesting finding of our study was that the significant association between pancreatic atrophy identified on CT scans performed pre-transplant or in the first year after transplantation. Over a third of the patients with DGF-ON with available CT scan data had pancreatic atrophy. Pancreatic atrophy, also known as non-alcoholic fatty pancreas disease, has been associated with several clinical entities including metabolic syndrome, diabetes mellitus and atherosclerosis. Data on this clinical entity are scarce, and further studies are needed to determine pathophysiology and sequelae [13, 14]. Our study suggests that the steatorrhea associated with pancreatic atrophy may play a role in the pathogenesis of ON, and consequently, intervention with pancrelipase may be protective against ON. Further studies are needed to understand the prevalence and risk factors for pancreatic atrophy in ESKD, which affected patients are at risk of DGF-ON, and effectiveness of mitigation strategies such as pancrelipase for prevention and treatment. In a prior study, bariatric surgery and IBD were associated with enteric hyperoxaluria in transplant candidates [15]. In our study, only one of the patients with DGF-ON had history of gastric bypass surgery. Other than this, we did not identify any pre- or peri-transplant variables that were associated with DGF-ON. Given that oxalate clearance is reduced in dialysis, we hypothesized that increased duration of dialysis could be a potential risk for DGF-ON though did not find any difference in pre-transplant dialysis duration between the two groups. Although limited by small sample size, when compared to transplant recipients with prolonged DGF but without evidence of oxalate deposition, we did not find any difference in graft outcomes. This relationship was unchanged even after adjustment for rejection diagnosis, which was more prevalent in the control group. While there are anecdotes of DGF-ON in the literature [16], ours is the first study to compare outcomes with controls also experiencing prolonged DGF necessitating biopsy but without ON. Prior studies evaluating early ON have shown variable frequency of ON and variable outcomes, depending on the control group selected. Palsson et al. described their findings in a population of kidney transplant recipients who underwent a biopsy within the first 3 months after transplantation and found a strong association between ON and DGF (odds ratio 11.3) [7]. They also documented worse outcomes in those with ON compared to those without. The difference in the findings of our study compared to Palsson's could be related to selection bias, as patients with DGF are more likely to have biopsies and worse outcomes, regardless of ON. In another study from Johns Hopkins comparing recipients with ON on biopsies done within the first year after transplant with controls without ON showed that the renal function was worse at 1-year after transplant, but not at 2 years [6]. In a study by Pinheiro, oxalate deposition was found in a surprisingly high percentage of the patients at 52.6%, and was found to be associated with worse long-term outcomes [8]. In summary, the data on impact of early ON and DGF-ON on outcomes in not clear, and it remains to be established whether ON causes graft dysfunction or DGF, or whether the oxalate deposition is a result of reduced clearance seen in DGF. Protocols to reduce plasma oxalate in order to be able to prevent ON after transplantation have been described [17]. Many transplant centers, including ours, intensify dialysis in the setting of DGF-ON; however, the benefits of this strategy are unclear.
Late ON, on the other hand, behaved similarly to ON in native kidneys. Roux-en-Y gastric bypass surgery and exocrine pancreatic insufficiency are associated with fat malabsorption: calcium binds the excess fat, thereby reducing its availability to bind oxalate in the gut lumen, leading to increased absorption and consequently increased urinary oxalate and calcium oxalate supersaturation. This, in turn, increases the risk of oxalate nephrolithiasis and ON [18, 19]. ON carries a poor prognosis, with high rates of kidney failure [20]. While prior data are limited to case reports [21, 22], our study showed that patients with late ON were more likely to have a history of gastric bypass surgery. Moreover, pancreatic atrophy on CT scans done prior to the diagnosis of late ON or corresponding time in controls was significantly more common in patients with late ON. Additionally, the risk of graft failure with late ON was 100% higher, and the risk of death-censored graft loss was 150% higher than controls, although these results did not reach statistical significance likely due to our small sample size. There are several case reports in the literature which document improvement in hyperoxaluria and renal function with reversal of gastric bypass, in both native and transplanted kidneys [23-25]. Another notable observation is that patients with late ON had reduced kidney function prior to diagnosis, as compared to controls. This may represent a decline in kidney function due to ON in the kidney that is not yet diagnosed. Alternatively, reduced kidney function may lead to decreased oxalate excretion, and consequently increased risk of oxalate deposition in the kidney.
Our study has the usual limitations of a small retrospective, case-control study. We did not have serum or urine oxalate levels available on patients and controls in our study. Serum oxalate level has been shown to be a risk factor for cardiovascular events and sudden cardiac death in patients on dialysis [26]. Further studies are needed to evaluate if and what degree of serum oxalate level elevation prior to transplantation correlates with rates of ON; these data would be helpful in developing screening and treatment algorithms for patients at risk for ON. In this context, it should be noted that several variables complicate measurement of blood oxalate levels, including timing in relation to dialysis treatment (i.e., performed just prior to dialysis vs. on non-dialysis day). Oxalate levels rise in the sample after a venous blood draw as ascorbate is converted to oxalate in vitro, and proper specimen handling is critical to obtaining useful data. In addition, there is a high degree of variation between measurement methods [27].
In summary, we describe two phenotypes of secondary ON in kidney transplantation. Pancreatic atrophy on imaging and history of gastric bypass surgery were more common in DGF-ON as compared to controls that also had DGF requiring biopsy but did not have any evidence of ON. In our limited sample, DGF-ON was not associated with worse outcomes compared to controls. Older age, female sex, pancreatic atrophy, and gastric bypass surgery history were associated with development of late ON, and this complication adversely impacts graft survival, similar to as seen in native kidneys. Future studies elucidating the underlying mechanisms could help devise preventative and treatment strategies for affected patients.
Author Contributions
Neetika Garg: research design, literature review, data acquisition, data analysis, drafting and revising the manuscript. Thanh Thanh Nguyen: data collection and analysis, literature review, drafting and revising the manuscript. Brad C. Astor: data analysis, drafting and revising the manuscript. Weixiong Zhong: data collection, drafting and revising the manuscript. Sandesh Parajuli: data analysis, drafting and revising the manuscript. Fahad Aziz: data analysis, drafting and revising the manuscript. Maha Mohamed: data analysis, drafting and revising the manuscript. Arjang Djamali: data analysis, drafting and revising the manuscript. Suzanne M. Norby: data analysis, literature review, drafting and revising the manuscript. Didier A. Mandelbrot: Research design, data acquisition, data analysis, literature review, drafting and revising the manuscript. Didier A. Mandelbrot is the recipient of an unrestricted research grant from the Virginia Lee Cook Foundation, which supported this study. Suzanne M. Norby is the recipient of an unrestricted research grant from the Flesch Family Foundation.
Acknowledgments
University of Wisconsin School of Medicine and Public Health Shapiro Summer Search Program for supporting Thanh Thanh Nyugen's efforts in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




