Acute transcutaneous electrical stimulation (TES) augments esophageal contractility in patients with weak peristalsis
Sravanthi Nandavaram and Andres Pelaez contributed equally to this study.
Abstract
Background
Lung transplantation (LTx) remains controversial in patients with absent peristalsis (AP) given the increased risk for gastroesophageal reflux (GER), and chronic lung allograft dysfunction. Furthermore, specific treatments to facilitate LTx in those with AP have not been widely described. Transcutaneous Electrical Stimulation (TES) has been reported to improve foregut contractility in LTx patients and therefore we hypothesize that TES may augment the esophageal motility of patients with ineffective esophageal motility (IEM).
Methods
We included 49 patients, 14 with IEM, 5 with AP, and 30 with normal motility. All subjects underwent standard high-resolution manometry and intraluminal impedance (HRIM) with additional swallows as TES was delivered.
Results
TES induced a universal impedance change observable in real-time by a characteristic spike activity. TES significantly augmented the contractile vigor of the esophagus measured by the distal contractile integral (DCI) in patients with IEM [median DCI (IQR) 0 (238) mmHg-cm-s off TES vs. 333 (858) mmHg-cm-s on TES; p = .01] and normal peristalsis [median DCI (IQR) 1545 (1840) mmHg-cm-s off TES vs. 2109 (2082) mmHg-cm-s on TES; p = .01]. Interestingly, TES induced measurable contractile activity (DCI > 100 mmHg-cm-s) in three out of five patients with AP [median DCI (IQR) 0 (0) mmHg-cm-s off TES vs. 0 (182) mmHg-cm-s on TES; p < .001].
Conclusion
TES acutely augmented contractile vigor in patients with normal and weak/ AP. The use of TES may positively impact LTx candidacy, and outcomes for patients with IEM/AP. Nevertheless, further studies are needed to determine the long-term effects of TES in this patient population.
1 INTRODUCTION
Esophageal motility disorders are prevalent in patients with advanced lung disease1-3 and after lung transplantation (LTx).4-6 Ineffective esophageal motility (IEM) (>70% ineffective or >50% failed swallows) and absent peristalsis (AP) are good predictors of impaired bolus clearance and increased reflux burden.7, 8 Substantial experimental9 and clinical10 data suggests that gastroesophageal reflux (GER) induced micro-aspiration is a major non-alloimmune culprit involved in the development of chronic lung allograft dysfunction (CLAD), which accounts for 20%–30% of the deaths that occur in LTx after the first year.11-14
Chronic recurrent micro-aspiration of gastroduodenal contents has been shown to induce airway epithelial and endothelial injury that triggers an inflammatory cascade that results in dysregulated repair and fibrosis, which ultimately manifests as Bronchiolitis Obliterans Syndrome (BOS) and CLAD.9, 15, 16 Scleroderma-associated interstitial lung disease (SSc-ILD) has been considered historically as a relative contraindication for LTx given the higher proportion of AP, poor esophageal clearance, higher risk of CLAD, and poor outcomes with antireflux surgery (ARS).17, 18 No specific pharmacologic intervention is currently available that reliably restores esophageal peristalsis.7, 19 The effect of prokinetics is inconsistent and their use can be limited by their side effect profile. The current management of IEM/AP focuses on symptom relief and reflux control by either antisecretory therapy or ARS.7, 19 However, the presence of IEM/AP often dissuades surgeons from performing ARS.
Transcutaneous electrical stimulation (TES) applied to the torso has been reported to improve severe gastroparesis (GP) symptoms after LTx.20 Based on this report20 our multidisciplinary team conducted a real-time trial to determine if TES induced acute changes in esophageal peristalsis during manometry. Our index manometry case involved a 63-year-old male who progressed to end stage pulmonary arterial hypertension despite optimal medical therapy. The patient was declined for transplant at multiple transplant centers due to associated severe esophageal dysmotility. The patient denied any heartburn, dysphagia, hoarseness, or dyspepsia. The patient was referred to our center for a second opinion. At the time of evaluation, he required 6 L of oxygen at rest and desaturation was down to 77%, with activity consistent with WHO functional class IV, despite therapy.
Due to the severe respiratory distress, we performed seven swallows in supine position showing ineffective peristalsis in 100% of the swallows with 4 failed (57%), 2 weak (29%), and 1 (14%) fragmented peristalsis. Following routine manometry, we conducted a pilot study of five swallows while on TES at previously described settings20 with electrodes placed in the autonomic projection of the distal esophagus (T7–T9) over the spine. Surprisingly, the contractile vigor of the esophagus improved to normal parameters [mean DCI 923 mmHg-cm-s, max DCI 2209, intact 3 (60%), failed 1 (20%), and weak 1 (20%) peristalsis while on TES. Based on this response, the patient was listed and ultimately transplanted in our institution. A surveillance manometry performed 5 months following bilateral transplant, displayed normal esophageal motility. The explant showed features consistent with capillary hemagiomatosis-like foci and severe arterialization of the pulmonary arteries. Additionally, features of superimposed aspiration. Post-transplant, no acute rejection episodes occurred in the first year post transplant, and further no evidence of allograft dysfunction were present at the 4-year mark. We have reported our preliminary experience with TES applied between T6–T10 dermatomal levels (adrenergic autonomic control of distal esophagus and stomach) in esophageal motility21 and gastric emptying in a small cohort of LTx patients.22
In the pilot study presented here, we evaluated the acute effect of TES on the (i) contractile vigor of the esophagus, (ii) the upper esophageal sphincter resting pressure (UESP), (iii) the lower esophageal sphincter resting pressure (LESP), and the integrated relaxation pressure (IRP) in LTx patients.
2 MATERIALS AND METHODS
2.1 Subjects
53 adult patients undergoing High-resolution manometry and intraluminal impedance (HRIM) as part of our institutional LTx protocol were eligible for inclusion. Exclusion criteria included incomplete manometry study, intolerance to TES, and/or unwillingness to consent for the study. Data from the 49 patients was collected. Index HRIM/TES was done after transplant in most patients (41, 84%). Patients were recruited between December 2017 and March 2020. The four patients excluded did not consent to participate. This study was approved by the University of Florida Institutional Review Board.
2.2 HRIM
HRIM was performed using a solid-state catheter with 36 circumferential sensors spaced 1 cm apart and 12 impedance-sensing segments (Medtronic, Duluth, Georgia, USA). In brief, the HRM/MII catheter was placed via trans nasal approach under topical anesthesia into the esophagus and stomach. Pressure bands of the upper esophageal sphincter, the lower esophageal sphincter, and the diaphragmatic pinch were noted. Once proper position was confirmed, the catheter was secured and taped to the nose. A 30-s baseline pressure was obtained in supine position to identify the upper esophageal sphincter and lower esophageal sphincter followed by a series of wet swallows using 5 mL room temperature Gatorade to assess esophageal motility. One additional 30-s base line and additional 5 mL swallows were done while TES was applied.
The manometry studies were recorded and analyzed using the manufacturer's software (ManoView, Medtronic). The esophageal motility phenotypes were defined as follows: Normal peristalsis [normal IRP and ≥ 20% intact peristalsis], IEM [normal IRP and > 70% ineffective or ≥ 50% failed swallows], and AP [normal IRP and no scorable contractions (DCI < 100)]. These cut-offs were selected based on the concept of clinically significant ineffective peristalsis.7, 8 Following data collection, >70% ineffective peristalsis became the threshold for IEM based on the v4.0 Chicago classification.23 Once the manometry analysis was completed, the display mode was changed to impedance tracings to evaluate any TES-induced changes from the baseline to confirm its real-time effect.
2.3 TES
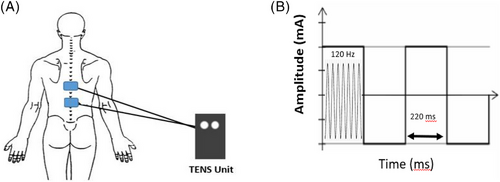
The skin over the torso was cleaned and prepped to optimize impedance and electrical conductivity. Two cutaneous electrodes were placed along longitudinal axis of the spine between T7 and T10 levels (distal esophagus autonomic segment, Figure 1) and electrical stimulation was applied in continuous square wave mode, with pulse rate 120 Hz, width 220 μs, and intensity to the maximum tolerated level without eliciting pain (Figure 2). Following the routine 10 wet swallows, TES stimulation was applied 1 min for accommodation and then kept firing for additional five wet swallows while on TES. The total stimulation time was 10 min or less. After completing the swallows on TES, stimulation was turned off, the study terminated, and the electrodes detached from the skin.
2.4 Data analysis
Categorical data was reported as proportions. Continuous data was reported as median (25%–75% IQR). The Mann-Whitney U-test was used to compare the nonparametric variables and box and whisker plots used for graphics. Statistical significance was defined at the p < .05 level. All statistics were done using Stats Direct 3.
3 RESULTS
A total of 49 patients (age 49 ± 12, 51% female, BMI 25 ± 5) completed the study. Indications for LTx included fibrotic lung disease (49%), obstructive lung disease (24%), cystic fibrosis/bronchiectasis (14%), and other advanced lung disease causes (13%). Most patients had pathologic reflux (overall DeMeester score 34 ± 30), and low GER (6 ± 2 for GERD-Q) and laryngopharyngeal reflux (7 ± 8 for RSI) symptom scores. Other relevant clinical details are listed in Table 1.
| All patients (n = 49) | |
|---|---|
| Age | 49 ± 12 |
| Female gender | 51% |
| BMI (mean ± SD) | 25 ± 5 |
| GERD-Q (mean ± SD) | 6 ± 2 |
| RSI (mean ± SD) | 7 ± 8 |
| DeMeester score | 34 ± 30 |
| Post-transplant | 84% |
| Transplant indication | |
| Fibrotic | 49% |
| Obstructive | 24% |
| Cystic fibrosis/bronchiectasis | 14% |
| Other | 13% |
| Type of transplant | |
| Bilateral | 87% |
| Unilateral | 13% |
| Proton pump inhibitor | 33% |
| H2-receptor Antagonist | 36% |
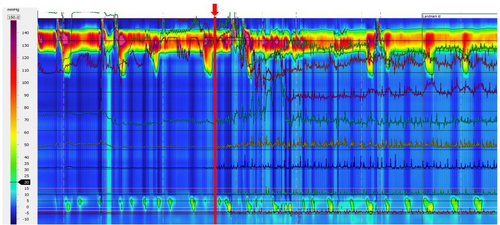
TES induced a universal change (100%) from baseline impedance to a characteristic spike activity in line tracings (TES signature, Figure 2). Spike impedance activity returned to baseline as the TES was turned off.
None of the subjects reported any undue discomfort, local reactions to the electrodes, or side effects during the acute study.
3.1 TES effect on the esophageal contractile vigor
A total of 449 swallows off TES were compared to 205 swallows on TES. Overall, TES induced a significant real-time increase in contractile vigor [DCI median (IQR) off TES 832 (1840) mmHg-cm-s vs. 1131 (2082) mmHg-cm-s on TES; p < .01, Table 2].
| UESP (mmHg) | LESP (mmHg) | DCI (mmHg-cm-s) | IRP (mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |||||||||
| TES off | TES on | p-value | TES off | TES on | p-value | TES off | TES on | p-value | TES off | TES on | p-value | |
| All Patients (n = 49) | 54 (35) | 42 (32) | ns | 23 (14) | 24 (15) | ns | 823 (1840) | 1131 (2082) | <.001 | 7.7 (7.3) | 7.6 (6.4) | ns |
| Normal motility (n = 30) | 50 (39) | 38 (18) | ns | 26 (14) | 26 (15) | ns | 1545 (1731) | 2109 (2260) | .01 | 8.7 (6.9) | 7.5 (5.8) | ns |
| Ineffective Motility (n = 14) | 62 (27) | 50 (32) | ns | 22 (13) | 24 (13) | ns | 0 (238) | 333 (858) | < .001 | 5.5 (6.7) | 8.3 (6.4) | <.001 |
| Absent Peristalsis (n = 5) | 104 (61) | 77 (32) | ns | 18 (28) | 16 (9) | ns | 0 (0-0) | 0 (182) | <.0001 | 8 (6.1) | 7 (5.7) | ns |
- Abbreviations: DCI, distal contractile integral; IQR, interquartile range; IRP, integrated relaxation pressure; LESP, lower esophageal sphincter resting pressure; ns, not significant; TES, transcutaneous electrical stimulation; UESP, upper esophageal sphincter resting pressure.
Subgroup analysis showed that TES induced a significant increase in contractile vigor for subjects with normal peristalsis [DCI median (IQR) off TES 1545 (1731) mmHg-cm-s vs. 2109 (2082) mmHg-cm-s on TES; p = .01, Figure 3A], and IEM [DCI median (IQR) off TES 0 (238) mmHg-cm-s vs. 333 (858) mmHg-cm-s on TES; p < .001, Figure 3B].
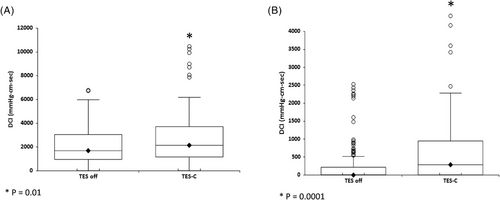
Of significant note, TES induced measurable contractile activity in three out of five patients with AP [DCI median (IQR) off TES 0 (0–0) mmHg-cm-s vs. 0 (0–182) mmHg-cm-s on TES; p < .0001, (Figure 4)]. TES decreased the proportion of failed peristalsis in the patients with either IEM and AP from a median of 75% off TES to a median of 33% on TES (p = .002).
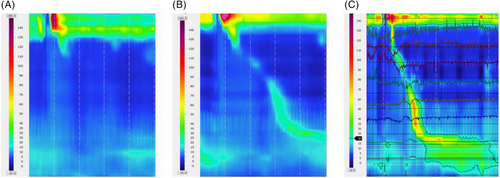
3.2 TES effect in the LESP and relaxation
TES did not result in any significant change in LESP in the overall analysis [median (IQR) off TES 23 (14) mmHg vs. 24 (15) mmHg on TES].
Analysis of the lower esophageal sphincter relaxation dynamics showed no significant effect of TES in the overall analysis [IRP median (IQR) off TES 7.7 (7.3) mmHg vs. 7.6 (6.4) mmHg post-TES] (Table 2).
3.3 TES effect in the upper esophageal resting pressure
There was a trend for lower UESP on TES [median (IQR) 42 (32) mmHg vs. 54 (35 mmHg) off TES. However, the change in UESP did not reach statistical significance in the overall or subgroup analysis (Table 2).
4 DISCUSSION
LTx remains controversial in patients with IEM-AP given the concern for GERD related BOS/CLAD and the absence of definitive treatment for such conditions. Hence such motility disorders pose a significant challenge for patients with advanced lung disease before and after LTx. Moreover, a diagnosis of IEM-AP is a clinical dilemma for surgeons involved in post-LTx management since poor peristaltic reserve has been linked to dysphagia following ARS.24
This study showed that TES significantly increases the vigor of esophageal contractions in patients with ESLD being referred for Tx and Ltx patients with IEM-AP. Furthermore, we describe the TES impedance signature and its real time effect on esophageal contractility using HRIM. Notably, TES induced a series of spike fluctuations in baseline impedance online tracings. This spike impedance activity returns to baseline once TES is discontinued. The TES approach could positively impact those patients whose IEM/AP does not improve after LTx, potentially minimizing micro-aspiration and progression to CLAD. Furthermore, TES can help patients awaiting LTx as the IEM-AP prevalence is very high in patients with SSc-ILD17, 18 and interstitial lung disease.1
The rational for enhancement of esophageal motility in LTx patients is supported by the findings described by Pellegrini et al.25 In their study cohort, patients who aspirated (defined by respiratory symptoms during or within 3 min of a reflux episode) had panesophageal weakness with lower proximal and distal amplitude of peristalsis and delayed bolus clearance.25 Furthermore, patients with AP that recovered peristalsis following LTx had improved long-term outcomes compared to those who did not.26
Neuromodulation emerges as an appealing alternative to treat esophageal hypomotility disorders given the disappointing results from pharmacologic interventions. Both invasive and non-invasive neuromodulation techniques have been shown to improve upper gastrointestinal symptoms. Gastric electrical stimulation (GES) with implanted electrodes is reserved for patients with severe GP. The mechanism of action remains unclear, with significant limitations to predict individual response and inconsistent acceleration of gastric emptying.27-29 Due to the limited effect of GES on gastric emptying and the potential for spontaneous resolution of GP after LTx,30 the use of GES as a prokinetic in LTx patients is controversial. In contrast, we have previously used TES as rescue therapy for moderate to severe GP (gastric retention > 25% at 4 h) that failed medical therapy and have seen a significant improvement in the percentage gastric retention at 4 h; 49% (25%–98%) median (min-max) before TES, 14% (1%–97%) median (min-max) after TES, p < .001.22
In the clinical arena, transcutaneous stimulation techniques have revealed promising results for GP. McNearny et al. reported in scleroderma patients that needleless transcutaneous electrical acupuncture (TEA) modulated gastric slow waves, improved GP symptoms, and changed plasma levels of Vasoactive Intestinal Peptide, Motilin, and Interleukin-6.31 McCallum's group from Texas Tech evaluated the central and peripheral effects of TEA in patients with Diabetic GP. TEA improved gastric dysrhythmias, gastroparesis symptoms and induced electroencephalography shift from dominant right to left inferior frontal activity during TEA.32
Data for electrical stimulation of the esophagus is scarce. In a double-blind sham-controlled study, Vigneri et al. evaluated the effect of transcranial direct current stimulation (tDCS) on the esophageal motility in GER patients. They recorded a significant increase in the contractile vigor of the esophagus during tDCS and confirmed the importance of cortico-esophageal circuits, particularly in patients with erosive GER.33
This study shows the acute effect of TES in LTx patients. We provided compelling evidence that TES augments the vigor of esophageal contractility, and that the technique could be easily applied in the LTx setting. None of the subjects reported side effects during the study. This is relevant since the settings are below pain threshold and for that reason TES could be use while eating and for 30 min after each meal and 15 min at bedtime to improve esophageal/gastric motility in lung transplant recipients.
Whether esophageal dysmotility improves after lung transplant is still up for debate. In 2019, a retrospective cohort of 76 patients showed that the overall contractile vigor improved post-transplant. However, this improvement was skewed by patients developing hypercontractile esophagus. Most patients had overall abnormal motility (43%) and jackhammer esophagus (25%) after transplant, compared to pre-transplant (31.6% and 7%) respectively.34 Masuda and colleagues showed that nearly absent contractility may improve after transplant.26 However, they used a definition that overlaps with the current definition of IEM based on the v4.0 Chicago classification.23 A follow-up study showed that those with restrictive lung disease and scleroderma are likely to have persistent aperistalsis.35
Taken together, we ambition the clinical application of TES be targeted to patients with restrictive lung disease and scleroderma, which represents the group less likely to recover peristaltic activity. Our goal for future studies is to treat patients with TES indefinitely for 30 min after meals and at bedtime with objective follow-up of symptoms, esophageal manometry, esophageal acid exposure, and gastric emptying. More importantly, we envision minimize the negative consequences IEM-AP could have on the allograft.
We recognize the potential limitations of this pilot study. First, there is a small number of patients. However, we described a universal TES impedance effect in over 200 individual swallows supporting the need of further studies to evaluate the long-term therapeutic effects of this technology in esophageal contractility. Second, there is the lack of a sham TES arm. Nonetheless, sham stimulation would have been challenging to include in the protocol due to the sensorial component required to titrate the current used for TES. Third, we avoided cross-over between on and off TES stimulation. Due to lessons learned from previous neurostimulation controlled trials, we avoided stimulation with TES at the beginning of the protocol or cross-over between on and off TES phases owing to the inherent “carry over effect” induced by continuous electrical stimulation that has confounded findings in previous trials.36 Fourth, the mechanism of action by which TES augments esophageal vigor remains unclear, but we hypothesize that our TES protocol acts through three potential mechanisms: (1) Activation of centrally mediated somato-visceral reflexes, (2) Spinal cord mediated sympatho-vagal balance modulation, and (3) Cortico-esophageal circuit activation from TES induced somato-sensorial afferents. Fifth, the possible long-term effects of TES remain speculative. In this regard, repeated sessions of chronic stimulation during prandial and postprandial times will be required in future studies. Sixth, our motility/impedance protocol did not include challenge tests to evaluate peristaltic reserve. However, we based our ineffective peristalsis definition based on clinically significant tresholds7, 8 associated to pathologic reflux and symptoms. Furthermore, the challenge tests were not included in the standard manometry protocols at the time of data collection. Bolus clearance was not analyzed as our goal was to evaluate esophageal contractility and the lack of impedance normative data or demonstrated association to symptoms.
In conclusion, TES augmented in real-time the esophageal contractility, a pathophysiological relevant mechanism linked to lower GER burden and better outcomes after LTx and ARS. The results of this study suggest TES as a potential therapeutic option for patients with IEM-AP as means to mitigate the non-alloimune impacts of GER and micro-aspiration on the allograft, thus ameliorating BOS/CLAD, and improving long-term outcomes in LTx. Additional studies with pH impedance and using challenge swallows are required to evaluate the true long-term clinical impact of this novel inexpensive, non-invasive, non-pharmacologic approach with prandial and post-prandial stimulation.
AUTHOR CONTRIBUTIONS
Manuel A. Amaris, Tiago Machuca, and Andres Pelaez designed the study. Manuel A. Amaris, Tiago Machuca, Carl Atkinson, and Andres Pelaez contributed to the analysis plan and data analysis. Manuel A. Amaris, Candice Rogers, David Estores, and Eyad Alakrad performed the interpretation of the manometry studies. Carl Atkinson designed and coordinated the review. Manuel A. Amaris and Andres Pelaez drafted the manuscript. All other authors contributed to the writing and reviewing of the article and approved the final version for submission.
ACKNOWLEDGMENTS
The authors have nothing to report. This work is supported by discretionary funds of the University of Florida Internal Medicine Department.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/.