Inflammatory cytokines induce caveolin-1/β-catenin signalling in rat nucleus pulposus cell apoptosis through the p38 MAPK pathway
Abstract
Objectives
Apoptosis of nucleus pulposus (NP) cells is a major cause of intervertebral disc degeneration. To elucidate relationships between caveolin-1 and cytokine-induced apoptosis, we investigated the role of caveolin-1 in cytokine-induced apoptosis in rat NP cells and the related signalling pathway.
Materials and methods
Rat NP cells were treated with interleukin (IL)-1β or tumour necrosis factor alpha (TNF-α), and knockdown of caveolin-1 and β-catenin was achieved using specific siRNAs. Then, apoptotic level of rat NP cells and expression and activation of caveolin-1/β-catenin signalling were assessed by flow cytometric analysis, qRT-PCR, western blotting and luciferase assays. The relationship between the mitogen-activated protein kinase (MAPK) pathway and caveolin-1 promoter activity was also determined by luciferase assays.
Results
IL-1β and TNF-α induced apoptosis, upregulated caveolin-1 expression and activated Wnt/β-catenin signalling in rat NP cells, while the induction effect of cytokines was reversed by caveolin-1 siRNA and β-catenin siRNA. Promotion of rat NP cell apoptosis and nuclear translocation of β-catenin induced by caveolin-1 overexpression were abolished by β-catenin siRNA. Furthermore, pretreatment with a p38 MAPK inhibitor or dominant negative-p38, blocked cytokine-dependent induction of caveolin-1/β-catenin expression and activity.
Conclusions
The results revealed the role of p38/caveolin-1/β-catenin in inflammatory cytokine-induced apoptosis in rat NP cells. Thus, controlling p38/caveolin-1/β-catenin activity seemed to regulate IL-1β- and TNF-α-induced apoptosis in the NP during intervertebral disc degeneration.
Introduction
Chronic lower back pain is the most common musculoskeletal complaint for middle-aged and older people, and the healthcare and socioeconomic costs associated with this condition are considerable 1. A relationship between chronic lower back pain and degenerative disc disease (DDD) has been established. The pathophysiology of DDD has been extensively studied in recent years, and various factors have been suggested as influencing its aetiology, including ageing, genetics, nutrition, metabolic factors, infection and mechanical factors 2. However, the potential contribution of each of these factors remains to be elucidated, and a causative relationship between DDD and lower back pain is yet to be confirmed.
DDD results from the development and progression of intervertebral disc (IVD) degeneration. During this degenerative process, cellular loss from the nucleus pulposus (NP) resulting from apoptosis has been demonstrated 3, 4. Apoptosis of NP cells has also been reported as one of the initial triggers of IVD degeneration 5-7. Additionally, inflammatory cytokines, including interleukin (IL)-1β and tumour necrosis factor alpha (TNF-α), were increased significantly in degenerative IVD. Through a series of signalling networks, inflammatory cytokines induce NP cell apoptosis, which results in progressive IVD degeneration 8. However, the exact signalling pathways involved in triggering NP cell apoptosis remain to be determined.
The cytokine-mediated induction of caveolin-1 has been reported in many cell types. Research has shown that caveolin-1 induces premature cellular senescence in response to various stress conditions, such as IL-1β and oxidative stress, in articular chondrocytes 9. These results suggest that caveolin-1 expression may play a role in common stress-induced and age-related diseases. Heathfield et al. demonstrated the expression of caveolin-1 in NP cells of human IVD 10, and more recently, caveolin-1 has been found to be a crucial factor in the initiation of IVD degeneration 11. NP cells of degenerate discs express high levels of caveolin-1 and show evidence of cellular senescence 12. As a marker of caveolae, caveolin-1 can be expressed in multiple tissues and regulates lipid transport, membrane trafficking and intracellular signalling pathways 13, 14. The regulatory role of caveolin-1 on apoptosis in bronchial epithelial cells, vascular smooth muscle cells and the human leukaemia cell line HL-60 has been reported previously 13, 15, 16. However, there is little information concerning the relationship between caveolin-1 and apoptosis in NP cells.
It has been reported that dysregulation of canonical Wnt signalling and caveolin-1 expression in early IVD degeneration appears to be essential to the physiology and preservation of notochordal cells 11. Caveolin-1 may act differently according to the cellular context, for example, the cell type and the stage of IVD degeneration. Caveolin-1 can activate the Wnt/β-catenin signalling pathway and regulate downstream gene expression in osteocyte and HepG2 cells 17, 18. Increased Wnt/β-catenin signalling activity was found in early IVD degeneration 19. Researchers have suggested that Wnt/β-catenin signalling plays an important role in IVD degeneration 20, 21. However, the role of Wnt/β-catenin signalling and caveolin-1 in NP cell apoptosis is not yet well understood.
In this study, we examined the expression and regulatory role of caveolin-1 and Wnt/β-catenin in rat NP cell apoptosis following inflammatory cytokine treatment. The aim of our study was to examine whether caveolin-1 and β-catenin signalling meditates cytokine-induced apoptosis in rat NP cells, and the mechanism by which IL-1β and TNF-α induce caveolin-1/β-catenin signalling. Our results showed that in rat NP cells, inflammatory cytokine-induced apoptosis is regulated by caveolin-1/β-catenin signalling and that cytokines control caveolin-1/β-catenin signalling through the mitogen-activated protein kinase (MAPK) pathway.
Materials and methods
Isolation and primary culture of rat NP cells
Animal experiments were performed according to a protocol approved by the Animal Experimentation Committee of our institution (The Institutional Animal Care and Use Committee of Second Military Medical University, Shanghai, China).
Rat NP cells were isolated using methods reported previously 22. NP cells were isolated from the lumbar discs of 12-week-old Sprague-Dawley rats (female, n = 30). In brief, rats were euthanized by a lethal dose of CO2 and then disinfected in 75% ethanol for 3–5 min. Gelatinous NP was separated from the discs and then washed twice with PBS. NP cells were released from the NP tissues by incubation with 0.25 mg/ml type II collagenase (Invitrogen, Carlsbad, CA, USA) for 2 h at 37 °C in Dulbecco's modified Eagle's medium (DMEM; GIBCO, Grand Island, NY, USA). After isolation, NP cells were resuspended in DMEM containing 15% FBS (GIBCO), 100 μg/ml streptomycin and 100 U/ml penicillin and then incubated at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The medium was charged every 3 days. NP cells were cultured in vitro for 20 days. Because no significant changes in morphology of cells between primary cells (passage 0, P0) and later passage cells (P2) were noticed, the low-passage (<3) cells cultured in monolayers were used for subsequent experiments, and the rat NP cell phenotype was confirmed by using immunohistochemistry for type II collagen and aggrecan (Fig. S1).
Inflammatory cytokine treatment of rat NP cells
Rat NP cells were plated at 3 × 105 cells/well in 1 ml of culture medium in 24-well plates. After 24 h, cells were divided into two groups and cultured with either IL-1β (10 ng/ml; PeproTech, Rocky Hill, NJ, USA) or TNF-α (50 ng/ml; PeproTech) for the indicated times. The concentration of inflammatory cytokines was determined according to the previously reported studies 23, 24.
Small-interfering RNA transfection
A single-stranded siRNA construct corresponding to rat caveolin-1 (GenBank accession number NM_031556.2) was designed: 5ʹ-CGCGCACACCAAGGAGATT-3ʹ. Another sequence, 5ʹ-CGAUCCUCAAAGCACUACUTT-3ʹ, was used as a control construct. Rat-specific β-catenin siRNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). NP cells were plated in 12-well plates at a density of 6 × 104 cells/well and transfected the next day with caveolin-1, β-catenin or scrambled-siRNA duplexes at a final concentration of 100 nM in DMEM using Lipofectamine 2000 reagent (Invitrogen). The transfection efficacy was determined by fluorescent microscopy using the fluorescein-conjugated control siRNA (Santa Cruz Biotechnology, sc-36869) and was approximately 70% at 24 h in four separate experiments (Fig. S2). After 24 h, the medium was aspirated and cells were cultured along with IL-1β (10 ng/ml) or TNF-α (50 ng/ml). After 12 h, the apoptotic rate of rat NP cells was determined by a FITC-annexin V/PI flow cytometry assay or a Caspase-Glo 3/7, 8, 9 activity assay (as described below).
Overexpression of caveolin-1
Caveolin-1 rat cDNA clone (OriGene, Rockville, MD, USA) was inserted into the pcDNA3.1+ vector between the corresponding restriction sites to construct the recombinant plasmid pcDNA-Caveolin-1. NP cells were plated at 3 × 105 cells/well in 1 ml culture medium in 24-well plates. After 24 h, cells were co-transfected with pcDNA-Caveolin-1 or the corresponding backbone vector as a control using Lipofectamine 2000 transfection reagent. Caveolin-1 overexpression was confirmed by Western blotting and qRT-PCR.
Western blot analysis
After treatment with inflammatory cytokines, protein expression of caveolin-1 and β-catenin in rat NP cells was analysed. Western blot analysis was conducted using standard methods. Protein quantification using a BCA assay (Beyotime, Jiangsu, China) was performed according to the manufacturer's instructions. Nuclear or total cellular proteins were separated on 10% SDS-PAGE gels and then transferred to PVDF membranes (Amersham, Buckinghamshire, UK). The membranes were blocked with 5% non-fat-dried milk for 2 h and then incubated for 12 h with an anti-caveolin-1 antibody (Abcam, Cambridge, UK; 1:1000), anti-β-catenin antibody (Cell Signaling Technology, Beverly, MA, USA; 1:1000), anti-p-p38, p38, p-ERK1/2 or ERK1/2 (Santa Cruz Biotechnology; 1:500), anti-histone or anti-β-actin antibody (Abcam; 1:1000). After washing with TBST (10 mm Tris, pH 8.0, 150 mm NaCl and 0.1% Tween20), the membranes were incubated for 2 h with a goat anti-rabbit antibody. After detection, band densitometric analysis was performed using NIH ImageJ software (Bethesda, MD, USA), and data were normalized to β-actin expression.
RNA isolation and real-time PCR
Expression of caveolin-1 and β-catenin in rat NP cells at the mRNA level was also analysed after inflammatory cytokine treatment. Total RNA from rat NP cells was isolated using TRIzol reagent (Invitrogen) and a mirVana™ PARIS™ Kit (Applied Biosystems) according to the manufacturer's instructions. RNA integrity was monitored by electrophoresis in 8% denaturing polyacrylamide gels. RNAs were reverse transcribed to cDNAs, using a RevertAid™ First Strand cDNA Synthesis Kit (MBI; Fermentas, Newington, NH, USA). The primers were designed based on cDNA sequences from the NCBI sequence database (Table 1). qRT-PCR was performed on a GeneAmp PCR 9700 Thermocycler (ABI; Applied Biosystems) with SYBR Green detection reagent according to the manufacturer's instructions. The cycle threshold (Ct) values were collected and normalized to the housekeeping gene β-actin. The ∆∆Ct method was used to calculate the relative mRNA levels of each target gene. All RT reactions, including β-actin controls, were run in triplicate. The relative amounts of target gene were calculated using the relative expression, RQ.
| Gene | Primer | Product length |
|---|---|---|
| Caveolin-1 | F: 5′-GCAGACGAGGTGAATGAGAAG-3′ | 219 bp |
| R: 5′-GGTAGACAGCAAGCGGTAAAA-3′ | ||
| β-catenin | F: 5′-ATCTGTGCTCTTCGTCATCT-3′ | 326 bp |
| R: 5′-CACCCTTCAACTATCTCCTC-3′ | ||
| β-actin | F: 5′-GTGGGAATGGGTCAGAAGGA-3′ | 262 bp |
| R: 5′-TGGCTGGGGTGTTGAAGGTC-3′ |
Flow cytometric (FCM) analysis
Cells were detached from tissue culture plates using 0.25% trypsin/EDTA (Invitrogen). Apoptotic NP cells were identified by staining with FITC-annexin V/PI (BD Biosciences, San Diego, CA, USA) and then analysed by FCM. In brief, after washing twice with PBS, 1 × 106 cells were resuspended in binding buffer (10 mm HEPES, pH 7.4; 140 mm NaCl; 2.5 mm CaCl2). PI and FITC-annexin V were added, and the cells were incubated at room temperature for 10 min before analysis.
Caspase-Glo 3/7, 8, 9 activity assay
Caspase-3/7, 8, 9 activity assay was conducted using the Caspase-Glo 3/7, 8, 9 activity assay kit (Promega, Madison, WI, USA) according to the manufacturer's protocols. Data were collected using a Wallac Victor 3 microplate reader (Perkin Elmer, Waltham, MA, USA).
Dual luciferase assays
Dual luciferase assays (TOPflash/FOPflash) were performed to assess the activation of Wnt/β-catenin signalling in rat NP cells after inflammatory cytokine treatment using a TCF reporter plasmid kit (Upstate Biotechnology, Lake Placid, NY, USA). Rat NP cells were plated at 3 × 105 cells/well in 1 ml culture medium in 24-well plates. After 24 h, cells were co-transfected with TOP Flash/pRL vectors (DNA ratio 20:1) or FOP Flash/pRL vectors (DNA ratio 20:1) using Lipofectamine 2000 transfection reagent (Invitrogen). After 6 h, the medium was aspirated and cells were cultured along with IL-1β (10 ng/ml) or TNF-α (50 ng/ml). After 6, 12 or 24 h, dual luciferase assays were performed using the Promega Dual Luciferase Assay System (Promega) according to the manufacturer's protocols. Renilla luciferase activity was used to normalize the measured firefly luciferase activity.
Caveolin-1 promoter deletion mutants (Cav-1-W1, −1296/−1, and Cav-1-W2, −222/−1) were generated by PCR and cloned into the luciferase reporter vector (pTA-luc; Clontech, Mountain View, CA, USA) as previously described 25. To measure the effect of cytokine treatment on caveolin-1/β-catenin signalling, cells were transfected with 2 μg of reporter plasmids and 1 μg of a β-galactosidase-expressing construct. To investigate the effect of p38 MAPK on caveolin-1, cells were co-transfected with 1 μg of wild-type (WT) or dominant negative (DN)-p38 (Addgene, Cambridge, MA, USA), with or without appropriate backbone vector(s) and 1.5 μg Cav-1-W1 reporter and 1.5 μg β-galactosidase-expressing construct using Lipofectamine 2000. Then, 24 h after transfection, some cells were treated with IL-1β (10 ng/ml) or TNF-α (50 ng/ml). In other experiments, cells were treated with the p38 MAPK inhibitor SB203580 (10 μm) (Calbiochem, San Diego, CA, USA) 1 h prior to treatment with cytokines. Then, 48 h after transfection, the luciferase activity was measured. At least three independent transfections were performed, and all analyses were carried out in triplicate.
Statistical analysis
Data normality was assessed by the Shapiro–Wilk test. Data analysis was performed using analysis of variance (ANOVA) followed by Dunnette's post hoc multiple comparison test. Differences with P < 0.05 were considered statistically significant. All statistical tests were performed using the SPSS 17.0 statistical package (SPSS, Chicago, IL, USA).
Results
IL-1β and TNF-α induced caveolin-1/β-catenin signalling in rat NP cells
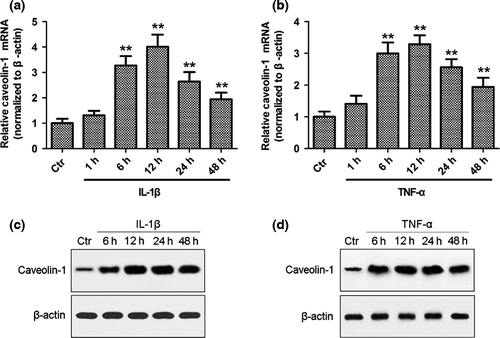
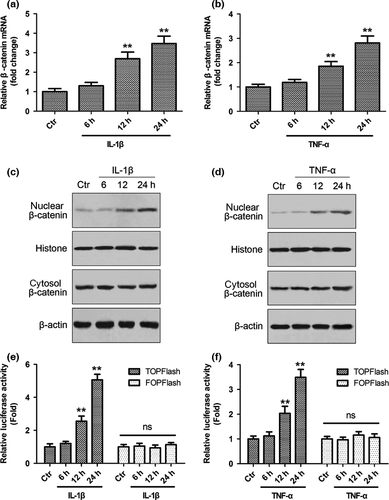
Inflammation is one of the critical factors leading to disc degeneration, which in turn can affect the phenotype of NP cells and the integrity of the extracellular matrix. To investigate the effect of inflammatory cytokines on caveolin-1/β-catenin signalling, rat NP cells were treated with IL-1β or TNF-α, and the expression of caveolin-1/β-catenin and the activation of β-catenin signalling were analysed. As assessed by qRT-PCR and Western blot analysis, both IL-1β and TNF-α significantly enhanced caveolin-1 mRNA and protein expression after 6 h of inflammatory cytokine treatment (Fig. 1, P < 0.01). The mRNA expression of caveolin-1 increased by 4.0-fold (IL-1β group) or 3.3-fold (TNF-α group) after 12 h of treatment (Fig. 1a,b). The mRNA expression of β-catenin increased in a time-dependent manner after treatment, reaching 3.5-fold (IL-1β group) and 2.8-fold (TNF-α group) that of the baseline value at 24 h (Fig. 2a,b). Nuclear translocation of β-catenin was increased after inflammatory cytokine treatment (Fig. 2c,d). To further test the activation of Wnt/β-catenin signalling, the TCF4-dependent luciferase reporter vector TOP Flash was transfected into NP cells. As a control, cells were also co-transfected with FOP Flash, which is identical to TOP Flash except that the TCF4 sites had been mutated. After inflammatory cytokine treatment, transactivation of TOP Flash was significantly higher, whereas FOP Flash was not activated (Fig. 2e,f).
Caveolin-1/β-catenin signalling regulated IL-1β and TNF-α-induced apoptosis in rat NP cells
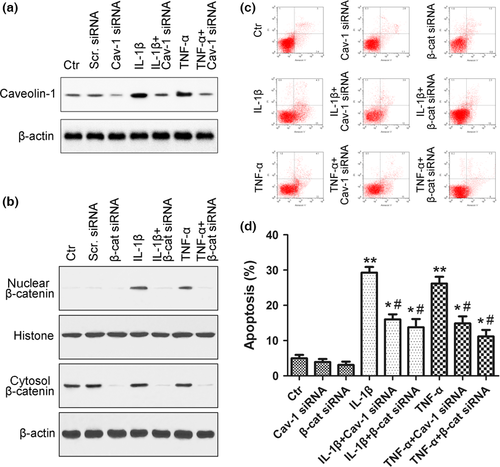
NP cell apoptosis has been suggested to be a major cause of DDD 3. To investigate the effect of caveolin-1/β-catenin signalling on NP cell apoptosis, rat NP cells were treated with IL-1β or TNF-α, and the expression of caveolin-1 and β-catenin was silenced using siRNA. An FITC-annexin V/PI FCM assay was performed to assess the rate of rat NP cell apoptosis. Silencing by caveolin-1 and β-catenin siRNAs was verified by decreased expression of caveolin-1 and β-catenin protein compared with the control (Fig. 3a,b). Increased caveolin-1 expression and nuclear translocation of β-catenin in rat NP cells induced by inflammatory cytokines were also inhibited by siRNA (Fig. 3a,b). After inflammatory cytokine treatment, the apoptotic rate increased from 5.0% (control group) to 29.3% (IL-1β group, P < 0.01) and 26.2% (TNF-α group, P < 0.01) (Fig. 3c,d). These increases were reversed in the presence of caveolin-1 siRNA, with the apoptotic rate being decreased to 16.0% (IL-1β + caveolin-1 siRNA group, P < 0.01) and 14.9% (TNF-α + caveolin-1 siRNA group, P < 0.01). β-catenin siRNA also reversed this effect (P < 0.01), with the apoptotic rate being decreased to 13.8% (IL-1β + β-catenin siRNA group, P < 0.01) and 11.2% (TNF-α +β-catenin siRNA group, P < 0.01) after β-catenin siRNA treatment (Fig. 3c,d).
To investigate the role of caveolin-1/β-catenin signalling in NP cell apoptosis, caveolin-1 silencing and overexpression were performed in rat NP cells. Activation of Wnt/β-catenin signalling was tested as above. Apoptosis of NP cells was evaluated by both FITC-annexin V/PI FCM and caspase-3, caspase-8 and caspase-9 assays (representing the common, extrinsic and intrinsic apoptotic pathways, respectively). Increased nuclear translocation of β-catenin was detected after inflammatory cytokine treatment, which could be reversed in the presence of caveolin-1 siRNA (Fig. 4a). The activity of caspase-3 and caspase-9 increased significantly after inflammatory cytokine treatment, and this increase was inhibited by both caveolin-1 and β-catenin siRNA (Fig. 4b,d). No significant difference in the activity of caspase-8 was detected (Fig. 4c).
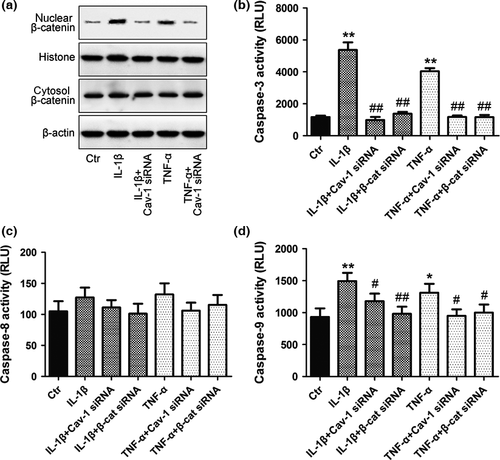
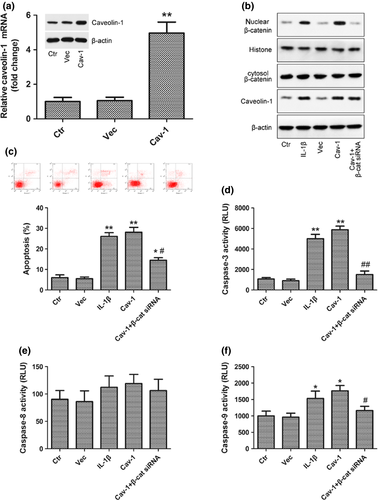
To confirm caveolin-1 overexpression, caveolin-1 protein and mRNA levels were determined following transfection with pcDNA-Caveolin-1 and were found to be significantly increased compared with the control and empty vector (Vec) group (Fig. 5a). Nuclear translocation of β-catenin (Fig. 5b), the activity of caspase-3 and caspase-9 (Fig. 5d,f) and the rate of apoptosis of rat NP cells (Fig. 5c) were increased after caveolin-1 overexpression, and these increases were abolished by transfection of β-catenin siRNA into caveolin-1 overexpressed rat NP cells.
IL-1β and TNF-α promoted caveolin-1/β-catenin signalling and induced rat NP cell apoptosis through the MAPK pathway
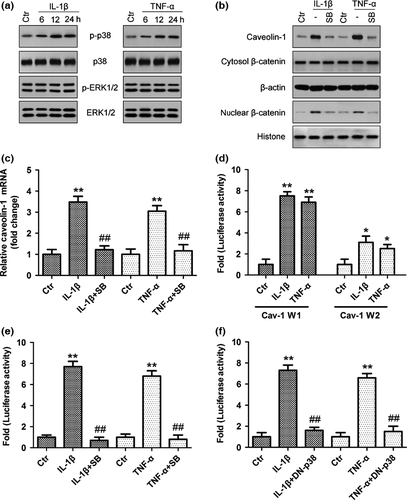
To further investigate how cytokines enhance caveolin-1/β-catenin signalling in rat NP cells, we determined whether MAPK signalling controls cytokine-dependent induction of caveolin-1/β-catenin expression and activity. Expression levels of phospho-p38 MAPK were markedly increased after IL-1β and TNF-α stimulation, peaking at 12 h and remaining high for more than 24 h, but IL-1β and TNF-α stimulation did not have a significant effect on ERK activation (Fig. 6a). Furthermore, pretreatment with the specific p38 MAPK inhibitor SB203580 blocked the increase in caveolin-1 mRNA and protein expression (Fig. 6b,c) and then inhibited nuclear translocation of β-catenin induced by IL-1β and TNF-α (Fig. 6b). We then investigated the mechanism of cytokine action on caveolin-1 expression by measuring the activity of two promoter fragments: Cav-1-W1 (−1296/−1) and Cav-1-W2 (−222/−1). The activity of the Cav-1-W1 reporter is significantly induced by cytokines (~7.2-fold), whereas Cav-1-W2 showed only a ~2.8-fold induction upon cytokine treatment (Fig. 6d). Due to robust activation of the Cav-1-W1 reporter, it was chosen for subsequent mechanistic studies to investigate cytokine regulation of Cav-1 expression. To elucidate the role of p38 MAPK in controlling promoter activity in rat NP cells, we measured Cav-W1 activity following IL-1β and TNF-α treatment with or without p38 MAPK inhibitor SB203580 or dominant negative (DN)-p38 transfection. The results showed that cytokine-mediated induction of Cav-W1 was completely blocked by the p38 MAPK pathway inhibitor (Fig. 6e) and DN-p38 (Fig. 6f). The results suggested that the p38 MAPK pathway regulated cytokine-mediated induction of Cav-1 promoter activity and controlled cytokine-induced caveolin-1/β-catenin signalling in rat NP cells.
Discussion
Reduction of the NP cell population is one of the major alterations detected during disc degeneration. Cellular loss due to cell death has been believed to play an important role in the process of IVD degeneration 3, but the mechanisms triggering cell apoptosis remained unclear. There is accumulating evidence that inflammatory cytokines, including IL-1β and TNF-α, contribute significantly to this NP cell loss 26. In this study, we demonstrated the promotion of NP cell apoptosis by treatment with IL-1β and TNF-α. Similarly, Zhang and colleagues indicated that IL-1β-induced NP cell apoptosis was involved in disc degeneration and that cells could be rescued by treatment with insulin-like growth factor-1 27. TNF-α was also thought to be acting as an initiator and regulator of multiple inflammatory cytokines in IVD. It was reported that TNF-α can initiate matrix breakdown and an inflammatory cascade in NP cells 28. However, the mechanism of cytokine-induced NP cell apoptosis in IVD degeneration remained to be determined.
Caveolin-1 is a regulator of signalling events originating from plasma membrane microdomains termed caveolae 13. In our study, we demonstrated that caveolin-1 expression increased significantly in NP cells after treatment with inflammatory cytokines. It has previously been reported that the expression of caveolin-1 can be induced by IL-1 in human NP cells 29, and evidence has been provided that caveolin-1 is a crucial factor in the initiation of IVD degeneration 11, 12. Gene silencing and overexpression of caveolin-1 proved that enhanced expression of caveolin-1 mediated cytokine-induced NP cell apoptosis. Our findings demonstrate for the first time the regulatory role of caveolin-1 on cytokine-induced NP cell apoptosis. However, there are some conflicting reports regarding the role of caveolin-1 in IVD cell loss. The downregulation of caveolin-1 expression was associated with the loss of notochordal cells (NCs) from the NP in chondrodystrophic dogs during the early stage of disc degeneration 11. However, this discrepancy may simply result from species differences. It is important to note that like dogs, at least two distinct cell types have been demonstrated to reside in the nucleus pulposus in rat, chondrocyte-like cells and NCs. The primary rat NP cells were round at the moment of isolation (Fig. S1a), and they had attached to the culture dish after 5–7 days of culture. The cells gradually became elongated and triangular or polygonal in shape, similar to chondrocyte-like cells. The passaged rat NP cells (<3) exhibited an appearance similar to the primary cells (Fig. S1b), and the rat NP cell phenotype was confirmed by using immunohistochemistry for type II collagen and aggrecan (Fig. S1c and d). However, increasing evidence has shown that cells isolated from NP, anulus fibrosus (AF), and chondrocytic end plate also express most of the phenotype markers that are characteristic of mesenchymal stromal cells (MSCs), thus supporting the existence of resident stem cells within IVD 30, 31. To determine if progenitor cells are present in cultured rat NP cells in our study, the cells were subjected to FACS analysis to evaluate MSC marker expression. Results showed that rat NP cells expressed CD29, CD90 and CD44 markers, indicating that rat NP cells contained populations of MSC-like cells (Fig. S1E). The NP-derived cells could be a combination of stem cells, NCs and NP cells together. Caveolin-1 may act differently according to the cellular context, for example, in the cartilage-like cells present in human degenerated discs, and at different stages of IVD degeneration. Therefore, the role of caveolin-1 in IVD cell loss and degeneration may differ between species, and our findings need to be confirmed in human cells and in vivo studies.
On investigating the signalling pathway involved in cytokine-induced NP cell apoptosis, we detected the activation of the Wnt/β-catenin signalling pathway in rat NP cells after inflammatory cytokine treatment. Involvement of this signalling pathway in NP cell apoptosis was confirmed by the finding that β-catenin siRNA abrogated cytokine-induced NP cell apoptosis, validating the findings of a previous study 21. Wnts are a family of secreted glycoproteins involved in multiple cellular processes, including proliferation, differentiation and viability 18. β-catenin is a key signal molecule for the activation of the Wnt/β-catenin signalling pathway. Activation of the Wnt canonical pathway causes β-catenin stabilization and translocation to the nucleus where it binds to the T-cell factor/lymphoid enhancer factor family of transcription factors and induces gene transcription. Wnt/β-catenin signalling in NP cells induces the expression of matrix metalloproteinases (MMPs) that are involved in the breakdown of the extracellular matrix and the progression of disc disease. In addition, Wnt/β-catenin signalling accelerates the senescence of NP cells, leading to compromised cell survival due to the induction of NP cell apoptosis 22. TNF-α induced NP cell apoptosis through Wnt/β-catenin signalling has been reported previously 8. Previous evidence has shown that caveolin-1 interacts with β-catenin through its scaffolding domain 32. Our results showed that caveolin-1 controlled nuclear translocation of β-catenin, suggesting that caveolin-1 plays an important role in regulating cytokine-induced NP cell apoptosis by facilitating Wnt/β-catenin signalling. The regulation of β-catenin activity by caveolin-1 has been reported in previous studies 33, 34. However, the role of caveolin-1 and Wnt/β-catenin signalling in the complex network of biological communication that regulates NP cell apoptosis remains to be fully elucidated.
Cellular stress factors, including IL-1β and TNF-α, interact with and inactivate several signalling molecules along cell death/proliferation pathways, such as the activation of nuclear factor-κB and MAPK signalling pathways 35, 36. The signalling machinery that links inflammatory cytokines to the upregulation of caveolin-1 expression also remains to be determined. In this study, we found that the expression levels of phospho-p38 MAPK were markedly increased after IL-1β and TNF-α stimulation, but did not have a significant effect on ERK activation. Using a specific p38 MAPK inhibitor SB203580, we demonstrated that MAPK signalling controls the cytokine-dependent induction of caveolin-1/β-catenin expression and activity in rat NP cells. We also observed that cytokine-mediated induction of Cav-1 promoter activity was completely blocked by the p38 MAPK pathway inhibitor and DN-p38. These results suggested that the p38 MAPK pathway regulated cytokine-mediated induction of Cav-1 promoter activity and controlled cytokine-induced caveolin-1/β-catenin signalling in rat NP cells.
The limitations of our study were as follows: first, our experiments were performed under conditions of normoxia. Apoptosis was not monitored under conditions of hypoxia, which may have been physiologically relevant and may have affected the rate of apoptotic cell death. We also did not investigate other factors that may induce NP cell apoptosis, such as reactive oxygen species and mechanical stress. These factors may be relevant in vivo and should be considered in further studies on the promotion of NP cell apoptosis. Second, our experiments were performed using cell monolayers as opposed to 3D suspensions of cells in a matrix that may be more physiologically relevant and more representative of the in vivo environment. The potential impact of our culturing method on the behaviour of the cells must be considered when interpreting the results, and it would be prudent to confirm these findings in a more biologically relevant context. Third, Wnt/β-catenin signalling and caveolin-1 may mediate multiple complex functions in cells, potentially controlling body axis patterning, cell fate specification, cell proliferation and cell migration. Therefore, silencing of these molecules may affect other physiological functions within the cell that were unrelated to focus of our study and this should be considered on interpretation of the results.
In summary, our results provide evidence for the role of p38/caveolin-1/β-catenin in inflammatory cytokine-induced apoptosis in NP cells, and caveolin-1 was found to regulate cytokine-induced NP cell apoptosis in IVD degeneration through Wnt/β-catenin signalling. Thus, controlling caveolin-1/β-catenin activity may regulate IL-1β- and TNF-α-induced apoptosis in the NP during IVD degeneration.
Acknowledgement
We are grateful for the financial support provided by the National Natural Science Foundation of China (grant no. 81171752).