Aortic valve type and calcification as assessed by transthoracic and transoesophageal echocardiography
Summary
Introduction
Aortic valve calcification (AVC) may predict poor outcome. Bicuspid aortic valve (BAV) leads to several haemodynamic changes accelerating the progress of aortic valve (AV) disease.
Aims
To compare the diagnostic accuracy of transoesophageal echocardiography (TEE) and transthoracic echocardiography (TTE) in the assessment of aortic valve phenotype and degree of AVC, with intra-operative evaluation as a reference.
Methods
We examined 169 patients (median age 65 years, 51 women) without significant coronary artery disease undergoing AV and/or aortic root surgery. TTE was performed within a week prior to surgery and TEE at the time of surgery.
Results
Compared with surgical AVC assessment, visual evaluation using a 5-grade scoring system and real-time images showed a higher correlation (TTE r = 0·83 and TEE r = 0·82) than visual (TTE r = 0·64 and TEE 0·63) or grey scale mean (GSMn) (TTE r = 0·63 and TEE r = 0·52) assessment of end-diastolic still frames. AVC assessment using real-time images showed high intraclass correlation coefficients (TTE 0·94 and TEE 0·93). With regard to BAV, TEE was superior to TTE with a higher interobserver agreement, sensitivity and specificity (0·86, 92% and 94% versus 0·57, 77% and 82%, respectively).
Conclusion
Semi-quantitative AVC assessment of real-time cine loops from both TEE and TTE correlated well with intra-operative evaluation of AVC. Applying a predefined scoring system for AVC evaluation assures a high interobserver correlation. TEE was superior to TTE for evaluation of valve phenotype and should be considered when a diagnosis of BAV is clinically important.
Introduction
Aortic valve calcification (AVC) is considered an active disease process of the aortic valve associated with risk factors and molecular pathways that lead to disease progression and, eventually, complications (Otto, 2008). Moreover, AVC is a powerful predictor of adverse clinical events for patients with cardiac (Pohle et al., 2001; Palmiero et al., 2007; Corciu et al., 2010; Pradelli et al., 2013) and vascular diseases (Rossi et al., 2013), and in the general population (Rosenhek et al., 2000, 2004; Pohle et al., 2001; Branch et al., 2002; Nemcsik et al., 2007; Palmiero et al., 2007; Ishii et al., 2009; Corciu et al., 2010). AVC has also been recognized as a risk factor for complications after transcatheter aortic valve implantations (Colli et al., 2011; Koos et al., 2011). Bicuspid aortic valve (BAV) is a congenital malformation especially prone to developing degeneration and calcification leading to premature aortic valve disease (Basso et al., 2004; Roberts & Ko, 2005).
Quantification of AVC using different imaging modalities, such as computed tomography (CT), transthoracic echocardiography (TTE) (Rosenhek et al., 2000, 2004; Corciu et al., 2010) and transoesophageal echocardiography (TEE) (Colli et al., 2011), has recently gained much scientific interest. Different scoring systems and quantitative measures have been proposed (Palmiero et al., 2007; Colli et al., 2011; Yousry et al., 2012). Although it is known that TEE is superior to TTE in diagnosing complications of infective endocarditis (Griffin et al., 2007) and evaluation of valvular regurgitation (Ward, 2000), left ventricular outflow tract dimensions (Shiran et al., 2009) and valve type (Espinal et al., 2000; Alegret et al., 2005), the accuracy of TEE for the assessment of AVC has so far not been compared with intra-operative evaluation.
In this study of patients undergoing surgery for aortic valve and/or ascending aorta disease, we compared the accuracy of TEE with TTE and with surgical evaluation for assessing AVC and BAV. Moreover, quantitation of AVC from TTE and TEE images, using grey scale measurement (GSM) software, was compared with a semi-quantitative AVC scoring system and intra-operative evaluation.
Methods
One hundred and sixty-nine patients (118 men, 51 women) were consecutively included in this study. Data on TTE findings with regard to this population have been described in a previous study from our group (Yousry et al., 2012). All patients underwent aortic valve and/or aortic surgery between February 2007 and December 2008 within the Advanced Study of Aortic Pathology (ASAP), a prospective single-centre trial performed at the Karolinska University Hospital, Stockholm, Sweden. Significant coronary artery disease diagnosed by a preoperative coronary angiography was considered an exclusion criterion. The local ethics committee approved the study protocol, and all patients gave their informed consent to participate.
Study design
All patients underwent a comprehensive TTE investigation using a Philips IE33 ultrasound scanner with an S5-1 transducer (1–5 MHz) (Philips Healthcare, Best, the Netherlands) within a week prior to the planned cardiac surgery. A comprehensive TEE study was performed in the operating room immediately before the heart surgery using a Sequoia c512 ultrasound scanner (Siemens Medical Systems, Mountain View, CA, USA) with a V5Ms transoesophageal transducer at a frequency of 6 or 7 MHz. All TTE and TEE studies were performed with focus on aortic valve and aortic root morphology and function by investigators with long experience in echocardiography. Ultrasound images were digitally stored and analysed offline on dedicated workstations. Short-axis view real-time cardiac cine loops and end-diastolic still frames obtained from both TTE and TEE studies were used for AVC scoring and valve type determination by an experienced investigator (MY). The still frames were also used for quantitative grey scale measurement (GSM). These visual interpretations and GSM were compared with intra-operative determinations. To test the accuracy in AVC scoring and valve type evaluation, two independent investigators (MY and AR) analysed the same TTE and TEE images a few days apart from each other and were unaware of each other's scores and of the surgical evaluation.
Aortic valve calcification scoring system
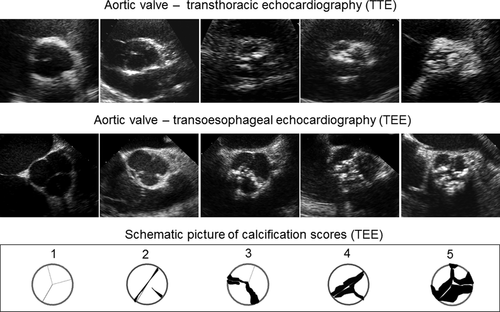
We used a previously proposed semi-quantitative 5-grade scoring system for visual assessment of the degree of AVC for echocardiographic images and for surgical scoring (Yousry et al., 2012). The system scale ranged from 1 to 5 where normal and non-thickened valves were classified as grade ‘1’; thickened non-calcified valves as ‘2’; mildly calcified valves (<1/3 of the valve leaflet area highly echogenic) as ‘3’; moderately calcified valves (1/3-2/3 of the area) as ‘4’ and severely calcified valves (>2/3 of the area) as ‘5’. A single summary score was given for the ultrasound still frames of the entire aortic valve, whereas the mean value of the score given for each single leaflet was calculated for aortic valves assessed by real-time heart cycle loops. The surgeon assessed the entire explanted valve using a corresponding 5-grade classification of the aortic valve tissue.
Grey scale measurement
The GSM methodology has been previously described in detail (Yousry et al., 2012). Briefly, grey scale mean (GSMn) values of the aortic valve leaflets were obtained by Artery Measurement System (AMS), a commercially available software (Image & Data Analysis, [email protected]) developed by Chalmers University of Technology in collaboration with the Physiology Group at the Wallenberg Laboratory (www.wlab.gu.se), Göteborg University. Parasternal short-axis end-diastolic still frames with the aortic valve leaflets fully closed were analysed from TTE and TEE studies. Software calibration was performed using a sample of the intracardiac blood pool as the black reference (i.e. grey scale value of 0), avoiding areas of noise, and the brightest part of the valve or its surroundings as the white reference (i.e. grey scale value of 255) (Griffin et al., 2007; Yousry et al., 2012). The region of interest containing aortic valve leaflets was manually traced, excluding the aortic annulus. The mean grey scale value of the valve leaflets area was automatically calculated by the program.
Determination of aortic valve phenotype
The morphology of the aortic valve was assessed using the following criteria. Tricuspid aortic valve (TAV) was defined by the presence of three cusps and three commissures. Criteria for BAV included presence of two cusps and two commissures with or without a raphe. BAV with a raphe, the result of fusion of commissures of two underdeveloped cusps, is usually characterized by asymmetric cusps. In this study, we did not carry out a detailed classification of the different conjoined cusp configurations in BAV (Brandenburg et al., 1983; Sievers & Schmidtke, 2007), and patients surgically classified as having unicuspid valves (n = 6) were included in the BAV group.
Intra-operative score
The surgeon in charge of the operation was asked to classify the aortic valve with regard to valve calcification and valve morphology. After gross visual assessment and palpation of the aortic valve in situ, and after explantation, the surgeon gave a single intra-operative score (IOS) for the whole aortic valve using the same AVC 5-grade scoring system as for echocardiographic assessment. The surgeon classified the aortic valve as TAV or BAV using the same criteria as those used for offline echocardiographic analyses. The surgeon was unaware of the ultrasound AVC or valve type scores assigned by independent investigators (MY and AR).
Statistical analysis
All statistical calculations were performed using commercially available statistics software (SPSS version 22; IBM, Chicago, IL, USA). Descriptive statistics were calculated and presented as means and standard deviation. Student's t-test and chi-square test were used for group comparisons according to the nature of variables compared. Relationships were determined by Pearson's correlation coefficients. Results were regarded significant at P<0·05. Interobserver variability was analysed using intraclass correlation coefficients (ICCs); values above 0·75 indicated good correlation and values between 0·4 and 0·75 fair reproducibility. Cohen's kappa statistics were used to assess agreement between different methods and observers in diagnosing valve type (Landis & Koch, 1977). Kappa values of 0·41–0·60 were consistent with moderate, 0·61–0·80 with good and values ≥0·81 with excellent agreement (Landis & Koch, 1977).
Results
Ninety-eight of the 169 patients in this study were diagnosed with BAV, and 71 with TAV by the surgeon. Patient characteristics are presented in Table 1. Patients with BAV were generally younger than those with TAV (61% versus 28% younger than 65 years of age). In the BAV group, aortic stenosis comprised the majority (65%) of the AV lesions, reaching up to 90% in older patients, while 90% of aortic regurgitation was found in patients younger than 65 years. Significant combined aortic stenosis/regurgitation was found in six patients in the entire study group, all of them with BAV. Among patients older than 65 years of age, there were more women than men. A subgroup of patients with BAV and aortic regurgitation showed a somewhat higher mean transvalvular pressure gradient compared with the TAV group, 12 mmHg versus 8 mmHg.
| All patients (n = 169) | Bicuspid (n = 98) | Tricuspid (n = 71) | P value | |
|---|---|---|---|---|
| General characteristics | ||||
| Mean age, years | 63·7 (11·6) | 61·2 (11·7) | 67·1 (10·8) | 0·001 |
| Females, n (%) | 51 (30·2%) | 27 (27·6%) | 24 (33·8%) | 0·493 |
| AS/AR/AS+AR/AAA, n | 99/59/6/5 | 64/25/6/3 | 35/34/0/2 | 0·007 |
| BSA, m2 | 1·98 (0·20) | 1·98 (0·19) | 1·98 (0·21) | 0·978 |
| AV peak gradient, mmHg | 55·4 (37·6) | 62·5 (38·1) | 45·7 (34·8) | 0·004 |
| AV mean gradient, mmHg | 34·6 (24·4) | 39·5 (24·5) | 27·8 (22·7) | 0·002 |
| Aortic valve calcification | ||||
| Mean intra-operative score, score 1–5 | 3·5 (1·5) | 4·0 (1·2) | 2·8 (1·7) | 0·000 |
| TTE grey scale mean, grey level 0–255 | 66·6 (28·8) | 68·9 (24·4) | 63·4 (33·9) | 0·217 |
| TEE grey scale mean, grey level 0–255 | 64·1 (21·1) | 64·9 (20·2) | 62·9 (22·3) | 0·529 |
| TTE real-time AVC score, score 1–5 | 3·6 (1·3) | 3·9 (1·1) | 3·1 (1·5) | 0·000 |
| TEE real-time AVC score, score 1–5 | 3·3 (1·3) | 3·6 (1·1) | 2·8 (1·3) | 0·000 |
| TTE still-frame AVC score, score 1–5 | 2·5 (1·2) | 2·7 (1·1) | 2·3 (1·3) | 0·037 |
| TEE still-frame AVC score, score 1–5 | 2·0 (1·1) | 2·1 (1·0) | 1·9 (1·1) | 0·118 |
- AAA, ascending aorta aneurysm; AS, aortic stenosis; AR, aortic regurgitation; BSA, body surface area.
- Values are shown as mean (standard deviation), numbers or % as applicable.
Aortic valve calcification
Aortic valve calcification results for all patients, and the BAV and TAV groups, obtained by semi-quantitative intra-operative and echocardiographic evaluation using the 5-grade scoring system and values from quantitative GSM analysis are shown in Table 1 and Figs 1 and 2. Table 2 presents Pearson's correlation coefficients for the intra-operative AVC reference scores and the results for GSMn, real-time loops and still frames for TTE and TEE. The highest correlation coefficients were for the surgical score with TTE and TEE (r = 0·83 and r = 0·82, both P<0·01) when real-time heart cycle loops were assessed visually. When analysing BAV and TAV patients separately, higher correlation coefficients were obtained in the TAV group. The interobserver agreement between the real-time AVC assessment scores of the two investigators was high, with ICC values of 0·93 and 0·94 for TTE and TEE, respectively.
| TTE | TEE | |||||
|---|---|---|---|---|---|---|
| GSMn | Still frames | Real-time loopsa | GSMn | Still frames | Real-time loopsa | |
| All | 0·63 | 0·64 | 0·83 | 0·52 | 0·63 | 0·82 |
| Bicuspid | 0·54 | 0·58 | 0·78 | 0·45 | 0·56 | 0·77 |
| Tricuspid | 0·72 | 0·69 | 0·83 | 0·63 | 0·72 | 0·84 |
- GSMn, grey scale mean value.
- a Interobserver agreement (ICC) for real-time assessment for TTE = 0·93; and for TEE = 0·94.
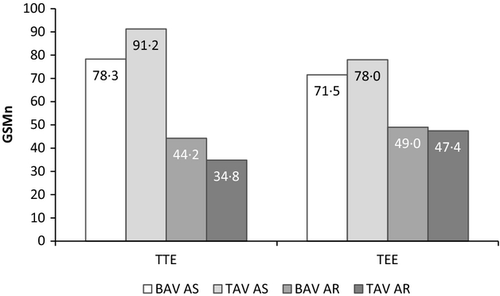
Visual assessment of AVC in echocardiographic still frames had less favourable correlation coefficient values than did real-time images: r = 0·64 versus 0·83 (TTE) and r = 0·63 versus 0·82 (TEE), with a somewhat closer relation to intra-operative score for TAV than for BAV when analysed separately, see Table 2.
The same still frames as for the visual analysis were used for the quantitative measurement of the GSMn (Table 1). GSMn scores differed for TTE and TEE images. TTE still frames showed better correlation (r = 0·68) with the intra-operative AVC assessment than TEE (r = 0·52), see Table 2. It was noted that the correlation coefficients for GSMn and intra-operative scores were close to the values obtained for IOS and visual AVC scores using the same echocardiographic still frames as for GSMn analysis. The mean values of GSMn for different subgroups of diagnoses are illustrated in Figs 1 and 2.
Valve phenotype determination
To diagnose the anatomical type of AV lesion, we used real-time images of TTE and TEE. The results were compared with the surgical determination of the valve phenotype (Table 3). Using real-time TEE images, we diagnosed the presence of BAV with high sensitivity 92% and specificity 94%, and a high kappa value of 0·86, indicating excellent agreement with the intra-operative surgical assessment. The results obtained using TTE images (sensitivity 77%, specificity 82%, and kappa value of 0·57) showed moderate agreement with the surgeon's diagnosis. The specificity and agreement of diagnosing BAV were not considerably different with a low AVC score (≤3) compared with more calcified valves (AVC score >3) for TTE or TEE (Table 3). Interobserver agreement for diagnosing BAV showed again the superiority of TEE, with excellent agreement between the two independent observers (κ = 0·90).
| Compared with intra-operative assessment | True positive | True negative | False positive | False negative | Agreement (κ value)a | Sensitivity,% | Specificity,% |
|---|---|---|---|---|---|---|---|
| Mild or no AVC (AVC score ≤3) | |||||||
| TTE | 18 | 30 | 5 | 7 | 0·58 | 72 | 86 |
| TEE | 22 | 34 | 1 | 3 | 0·86 | 88 | 97 |
| Moderate to severe AVC (AVC score >3) | |||||||
| TTE | 57 | 28 | 8 | 16 | 0·53 | 78 | 78 |
| TEE | 68 | 33 | 3 | 5 | 0·84 | 93 | 92 |
| All valves | |||||||
| TTE | 75 | 58 | 13 | 23 | 0·57 | 77 | 82 |
| TEE | 90 | 67 | 4 | 8 | 0·86 | 92 | 94 |
- AVC, aortic valve calcification score; κ, kappa value.
- a Total interobserver agreement (κ value) = 0.38 for transthoracic (TTE) and 0.90 for transoesophageal (TEE) echocardiography.
Discussion
We found a high correlation between semi-quantitative echocardiographic AVC assessment of real-time cine loops and intra-operative AVC evaluation, which was better than assessment of still frames, not only for TTE as previously reported (Yousry et al., 2012) but also for TEE. TEE was superior to TTE in the evaluation of aortic valve phenotype, but not in the estimation of AVC where similar results were obtained.
Clinical implications of aortic valve calcification have been demonstrated in patients with aortic stenosis (Morgan-Hughes et al., 2003; Bonow et al., 2006). In asymptomatic AS, advanced AVC is a strong predictor of poor prognosis, with increased risk of death and aortic valve replacement (Rosenhek et al., 2000). Moreover, numerous studies have shown an association between AVC and adverse clinical events (Rosenhek et al., 2000, 2004; Pohle et al., 2001; Branch et al., 2002; Nemcsik et al., 2007; Palmiero et al., 2007; Ishii et al., 2009; Corciu et al., 2010; Pradelli et al., 2013). Recently, AVC assessment by TEE has been shown useful in transcatheter aortic valve implantation procedures for predicting postprocedural aortic regurgitation (Colli et al., 2011; Koos et al., 2011).
In spite of the clinical importance of AVC, quantitative methods for its evaluation are limited, especially for echocardiography. For other diagnostic modalities such as CT, quantitative methods for AVC assessment have been developed and are in use (Agatston et al., 1990; Acarturk et al., 2003). For echocardiography, different scoring systems have been described for the visual assessment of real-time echocardiographic images, ranging from a simple ‘yes/no sclerosis/calcification’ score to a 4-grade system, or even more complicated scoring (Rosenhek et al., 2000, 2004; Rossi et al., 2013). In a previous study, we proposed a 5-grade scoring system to standardize the evaluation of AVC and validated it for TTE against surgical assessment (Yousry et al., 2012). In the current study, we evaluated the same scoring system for visual analysis of TEE real-time loops and still frames.
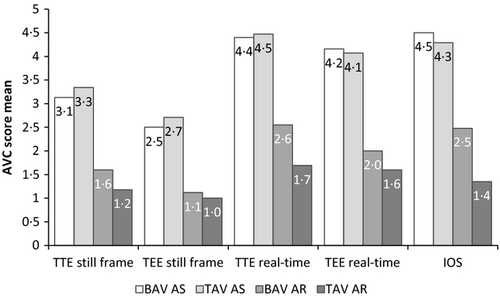
The AVC scores for visual evaluation of real-time images obtained by TTE and TEE (Fig. 3) showed high correlation with the intra-operative score and to each other. The best results for TEE were obtained when real-time loops were used, which is in line with our previous TTE study (Yousry et al., 2012). Using still frames from TEE or TTE for AVC scoring revealed only a moderate correlation with IOS. This is not surprising as the aortic valve is a three-dimensional structure, and some thickening and calcification of the valve may be appreciated only in systole or diastole. In our study, using a 5-grade scoring system resulted in an excellent interobserver agreement on the degree of AVC, with a high ICC of 0·94 for TEE images and 0·93 for TTE images.
In an effort to find a quantitative and reproducible score for AVC in echocardiographic images, we adapted dedicated GSM software using the mean pixel density within the region of interest as the digital numerical value for AVC. The software calculations were based on 256 degrees of brightness of each pixel in the selected valve area, ranging from total white (255) to total black (0), and where a higher average value indicated more AVC. The GSM software was only used on still frames and therefore subject to the same limitations as visual assessment of still images. Consequently, the correlations between GSMn and IOS were close to those obtained by visual assessment of still frames of both TTE and TEE images. The findings further strengthen the importance of using real-time images when assessing AVC by TEE and TTE, as previously shown (Yousry et al., 2012). These results also suggest that the lower correlation values obtained with GSM software were mainly the result of using echocardiographic still frames for the analysis. Further, although GSM in still frames is a more objective estimate of AVC, visual assessment seems to be as accurate.
Aortic valve phenotype
The prevalence of BAV in the general population is around 1–2% (Siu & Silversides, 2010). However, BAV is more prone to develop aortic stenosis than TAV, and in patients requiring aortic valve replacement congenitally malformed aortic valves (unicuspid or bicuspid) are even more common than TAV (Roberts & Ko, 2005; Roberts et al., 2005). Below about 70 years of age BAV was more prevalent and above this age TAV was a more common finding in three different aortic valve replacement cohorts (Roberts et al., 2005). This is well in line with our material (average age 64 years) with 58% BAV. We found TEE was more sensitive and specific in diagnosing BAV than TTE using intra-operative evaluation as the gold standard, which is in line with previous reports (Espinal et al., 2000), where TEE diagnosed up to 47% more cases of BAV than TTE (Alegret et al., 2005). The high sensitivity and specificity of TEE might be attributed to a closer approach to the aortic valve and higher image resolution than with TTE (Espinal et al., 2000). The clinical significance of BAV is high (Ward, 2000). Although it may not be desirable to perform TEE in all patients with aortic valve disease to increase the likelihood of identifying the correct number of leaflets, TEE has particular value in patients with a dilated aorta, and use of suboptimal TTE images to diagnose BAV may affect clinical decision-making. Our results regarding determination of aortic valve phenotype showed that TEE was more accurate than TTE in diagnosing BAV, with a sensitivity of 92% versus 77% and a specificity of 94% versus 82%. Interestingly, Roberts et al. also noted that diagnosing TAV during surgery was more accurate than diagnosing BAV when using pathological examination as the gold standard (Roberts et al., 2009). Two reasons were proposed to explain those results; BAV is usually more calcified than TAV, and the presence of a calcified raphe is usually misleading and considered as a border between cusps.
Strengths and limitations
Our study was performed at a single institution, all TTE and TEE examinations were carried out by experienced echocardiographers, and offline AVC and valve type analyses were performed by two independent investigators for the evaluation of interindividual variability.
Furthermore, surgery was performed by two senior cardiac surgeons, which facilitated a uniform intra-operative valve assessment. Using intra-operative scores as a gold standard has been questioned (Roberts et al., 2009). We did not evaluate interindividual variability of the surgeon′s scoring and interpretation of valve phenotype for practical reasons, as the senior surgeon had better view of the valve in situ and more extensive contact with the valve tissue than the assisting surgeon. It would also be difficult to perform unbiased individual interpretations during surgery. However, intra-operative scoring has been successfully used as the reference in other studies aimed at evaluating accuracy in diagnosing AVC and BAV (Roberts et al., 2009; Alkadhi et al., 2010; Joo et al., 2012). One may argue that surgeons do not focus on the details of the structure of valves explanted during surgery and that the valve details are usually not included in the routine report, or the report is not written in direct relation to surgery. However, none of these arguments were applicable in our study because the experienced surgeons participated in the establishment of the IOS and thus were well aware of the distinctions between grades. Furthermore, the results were recorded in direct connection with the surgical procedure.
Conclusion
In systematic semi-quantitative evaluation of aortic valve calcification (AVC score), real-time images obtained by TTE and TEE are superior compared with still frames. Using a predefined semi-quantitative scoring system resulted in high interobserver agreement. As TEE has superior accuracy in determining the aortic valve phenotype, it should be applied if uncertainty by TTE in cases of aortic dilation where indications for aortic surgery is different in BAV and TAV.
Acknowledgments
The performance of transthoracic echocardiography by Mahmood Farasati MSc and Kamel Ramak MSc, Department of Clinical Physiology, Karolinska University Hospital, Stockholm, Sweden, is highly appreciated. Dr Yousry is a recipient of the Atherothrombosis Research grant from the European Society of Cardiology. The study was supported by the Swedish Research Council, the Swedish Heart Lung Foundation, the European Commission, the Stockholm County Council and a donation by Fredrik Lundberg.
Conflict of interest
The authors have no conflict of interest.




