Intraoperative indocyanine green fluorescence angiography in colorectal surgery to prevent anastomotic leakage: A single-blind phase III multicentre randomized controlled trial (FLUOCOL-01/FRENCH 21/GRECCAR 19 intergroup trial)
See Appendix A for the members of FLUOCOL study group.
Trial registration: NCT05168839.
Abstract
Aim
Anastomotic leak (AL) is a major problem in colorectal surgery, and its prevention is crucial for patient safety. The scientific literature shows that optimal anastomotic perfusion is essential for anastomotic healing. However, in cases of left colon or rectal cancer requiring high vessel ligation for oncological reasons, anastomotic blood supply relies mainly on the pericolic arterial arcades. Consequently, assessing anastomotic perfusion using intraoperative fluorescence angiography with indocyanine green might be relevant to reduce the risk of AL. Although evidence of its positive impact on the risk of AL is growing in the literature, most studies are descriptive prospective cohorts or retrospective comparative series with controversial findings. Furthermore, no other studies specifically address left-sided colon or high rectal tumours. FLUOCOL-1 is a large multicentre randomized controlled trial (RCT) that aims to demonstrate that assessing anastomotic perfusion using intraoperative fluorescence angiography with indocyanine green will reduce ALs in left-sided or high anterior resections with intraperitoneal anastomosis
Method
FLUOCOL-1 is a French multicentre, single-blind, randomized, two-arm, phase III superiority clinical trial. Patients will be randomized in a 1:1 ratio to either the intervention group (FLUO+) or the control group (FLUO−). A total of 1010 patients will be randomized. The primary endpoint is the occurrence of an AL within 90 days postsurgery. AL is defined as any anastomotic dehiscence with leakage into the pelvic cavity diagnosed by imaging or surgical exploration, or any isolated pelvic organ-space infection with no evidence of fistula, as defined by the International Study Group of Rectal Cancer.
Discussion
Prevention of AL is one of the most important questions to be addressed in colorectal surgery. The FLUOCOL-1 multicentre RCT described herein aims to demonstrate that assessment of anastomotic perfusion using intraoperative fluorescence angiography with indocyanine green will reduce ALs in certain resections with intraperitoneal anastomosis.
INTRODUCTION
Colorectal cancer (CRC) is the fourth leading cause of cancer death worldwide [1]. In France, CRC is the third leading cause of cancer death in men and the second leading cause in women [2].
Anastomotic leak (AL) is a major problem in colorectal surgery affecting at least 7% of patients operated on for left-sided colonic cancer. It is the most feared complication after colorectal anastomosis. It is associated with mortality, prolonged hospitalization, impaired health-related quality of life and increased health-care costs [3, 4]. Preventing AL is crucial for ensuring patient safety in colorectal surgery.
Risk factors for AL after colorectal anastomosis are extensively documented in the literature [5-7]. Among these factors, optimal anastomotic perfusion is crucial for successful anastomotic healing [8, 9]. However, in cases of left colon or rectal cancer requiring high vessel ligation for oncological reasons, anastomotic blood supply primarily depends on the pericolic arterial arcades. Consequently, assessing anastomotic perfusion using intraoperative fluorescence angiography (IOFA) with indocyanine green (ICG) might be relevant to reduce AL [10]. However, although evidence of its positive impact on the risk of AL is increasing in the literature, the available studies exhibit heterogeneity [11-20]. While the EssentiAL trial focused on rectal cancer patients and demonstrated a significant 4.2% reduction in the AL rate in the ICG group [21], the AVOID trial found no benefit across a broader colorectal resection population including all types of colorectal resections [22]. Our team published a retrospective case-matched study suggesting a positive impact in a single-surgeon prospective cohort where AL rates in the group without IOFA and the group with IOFA were comparable with those reported in the literature [23]. Clinically relevant AL was significantly reduced in the IOFA group with no necrosis of the descending colon compared with the group without IOFA (16.7% vs. 2.4%, p = 0.026). It can be hypothesized that IOFA helps prevent clinically relevant AL caused by insufficient perfusion of the descending colon, which could lead to necrosis requiring emergency reintervention with Hartmann's procedure. The use of IOFA led to a change in the surgical strategy in 10.9% of cases in our cohort. This observation is within the range of existing studies (5%–27.1%). This primarily led to adjustments in the level of transection of the descending colon. Consequently, IOFA might be considered as one of the currently available tools for routinely preventing AL. Additionally, intravenous ICG was found to be safe with no related adverse effects observed in our cohort and with a very low risk of allergy reported in the literature (1/300 000) [17]. Finally, upon blind review our study showed a strong interobserver agreement between the intraoperative assessment of colic perfusion by the operating surgeon and the postoperative assessment by a second surgeon.
The reported rates of AL range from 5% to 10% specifically in left and sigmoid resections in Western countries [24, 25]. A meta-analysis conducted by Rausa et al. [26] showed promising results with IOFA use which might lead to a reduction in the AL rate with a relative risk of 0.44 (range 0.14–0.87) suggesting that IOFA could potentially halve the AL rate. Four more recent meta-analyses confirmed the capacity of IOFA in reducing the AL rate. They emphasized the need for high-quality randomized controlled trials with a placebo control [27-30].
The main objective of the FLUOCOL-1 study is to demonstrate that the assessment of anastomotic perfusion using IOFA with ICG will reduce AL in left-sided or high anterior resection with intraperitoneal anastomosis.
METHODS AND ANALYSIS
Study setting
We designed FLUOCOL-1 as a French multicentre, single blind, randomized, two-arm, phase III superiority clinical trial.
Following baseline clinical assessment, patients will be randomly allocated to one of two groups. In the experimental group (FLUO+), cancer resection and anastomosis will be performed following the assessment of descending colon perfusion using intravenous ICG injection. In the control group (FLUO−), cancer resection and anastomosis will be performed without IOFA.
Participating centres
A total of 1010 patients will be enrolled, with 505 allocated to the control arm and 505 to the intervention arm, across 35 French clinical and university hospital centres. The recruitment period began in September 2022.
Study objectives and outcomes
The primary objective is to demonstrate that ICG angiography reduces the rate of AL after resection of left colic or upper rectal cancer with intraperitoneal anastomosis.
The primary outcome is the occurrence of any anastomotic fistula within 90 days following surgery. We defined anastomotic fistula as any anastomotic dehiscence with communication into the pelvic cavity diagnosed by imaging or surgical exploration, or any isolated pelvic organ space infection without evidence of fistula, as defined by the International Rectal Cancer Study Group [31].
- To determine the association of ICG angiography and the change in the planned anastomosis during surgery. (A change in planned anastomosis during surgery is defined as any decision change on perfusion assessment such as modifying the initially planned level of transection of the descending colon or refashioning anastomosis including the decision to undertake a permanent stoma rather than an anastomosis.)
- To evaluate the rate of defunctioning and unplanned end stomas.
- To evaluate the rates of operative and postoperative complications (as per the Clavien–Dindo classification) at 30 and 90 days postsurgery.
- To assess the mortality rates at 30 and 90 days postsurgery.
- To evaluate the length of postoperative hospitalization.
- To assess the rate of reintervention at 30 and 90 days postsurgery.
- To assess patients’ quality of life before surgery and at 90 days postsurgery (QLQ-C30 and QLQ-CR29 questionnaires).
Study population
Recruitment
The surgeon will provide an information leaflet, present the study and invite eligible patients to participate. After obtaining the patient's signed informed consent, the physician will proceed with randomization.
Patient selection
The FLUOCOL-1 study aims to include 1010 male or female patients, of legal age, scheduled for CRC treatment through left colic or upper rectal resection at participating digestive surgery departments.
- adult patients (age >18 years);
- scheduled for elective left colectomy or high rectal resection for cancer with intraperitoneal anastomosis;
- signed informed consent;
- affiliated to the French social security system (including CMU).
- emergency surgery;
- rectal cancer requiring total mesorectal excision and expected anastomosis below the peritoneal reflection;
- colon cancer requiring total or subtotal colectomy defined as a right colectomy extended to the splenic flexure or more;
- colon cancer requiring transverse colectomy;
- recurrent CRC;
- locally advanced CRC requiring multivisceral excision;
- history of colectomy;
- associated concomitant major resection of another organ (liver, etc.);
- previous pelvic radiotherapy for pathology unrelated to diagnosis with colon cancer, for example treatment for prostate cancer;
- inflammatory bowel disease;
- history of known allergy to indocyanine;
- pregnant patients;
- refusal to participate or inability to provide informed consent;
- patient under legal protection (individuals under court-appointed guardianship).
Study schedule and duration
Patients will be recruited over a period of 36 months. The study schedule comprises three visits which coincide with routine visits as depicted in Figure 1, which presents the recruitment and data collection process.
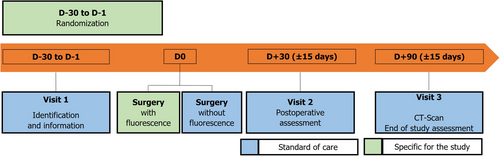
Randomization
Eligible patients will be randomized via an eCRF (Cleanweb™). Randomization will occur between the inclusion visit (V1) and the day prior to surgery (D0). The randomizing investigator will gain access to the internet site through a personal password.
Interventions details
Surgery will adhere to the standard practices of each participating centre and align with the current French recommendations for CRC surgery.
Each investigating surgeon must have performed a minimum of 10 IOFA procedures prior to starting inclusion. In addition, a surgical film describing the injection technique and fluorescence detection will be presented to all the participating surgeons at the time of the study set-up visits.
Open laparotomy, laparoscopy and robotic-assisted surgery are viable options as they are associated with comparable rates of anastomotic fistula. All patients will receive routine intraoperative intravenous antibiotic prophylaxis in accordance with the recommendations of the French Society of Anaesthesiologists [32]. Other aspects of patient care during the perioperative period, including induction and maintenance of general anaesthesia and postoperative recovery procedures, will be conducted based on the specific expertise and current practices of each centre.
In the experimental arm, ICG (Infracyanine®) will be injected by the anaesthesiologist intravenously at least once, with a bolus of 0.1 mg/kg. The detection of ICG in the proximal colic segment will be performed either open or intracorporeally using the dedicated infrared camera available in each centre at the time of the study set-up. An additional injection will be allowed at the surgeon's discretion if deemed necessary (change of anastomosis site). The time from injection to ICG detection and any adverse events will be meticulously recorded.
Apart from the randomization, patients will receive the same treatment as usual.
Data collection
With the exception of the use of ICG, patient management will follow standard procedures. We will collect the data describing each patient's evolution and management using an electronic data collection system (Cleanweb™) (Table 1).
| V1, preoperative visit (D–30 to D–1) | Surgery (D0) | V2, postoperative visit (D30 ± 15 days) | V3, postoperative visit (D90 ± 15 days) | |
|---|---|---|---|---|
| Eligibility screen | X | |||
| Patient information | X | |||
| Informed consent signature | X | |||
| Randomization | X | |||
| Demographic data | X | |||
| Surgery | X | |||
| Clinical data | X | X | X | |
| Quality of life questionnaires (QLQ-C30 and QLQ-CR29) | X | X | ||
| CT scan | X | |||
| Anastomotic fistula and other operative complications | X | X | X | |
| Adverse events | X | X |
Monitoring, including data management and quality assurance, will be conducted by the study sponsor. The sponsor will assume responsibility for monitoring the safety of all study participants.
Statistical analysis
Statistical analyses of the data will be performed using R version 3.6.1 and SAS version 9.4 software.
Continuous variables will be described using the median with its interquartile range, while categorical variables will be described using frequency and percentage in each study arm.
The primary endpoint, i.e. the rate of occurrence of anastomotic fistula within 90 days of colonic cancer resection, will be compared using a Z-test with pooled variance. The significance level will be set at 5%.
Quality of life (QoL) data will be described at the inclusion visit and at visit 3 (D90) with the mean and standard deviation, accounting for randomization arm. The completion rate for each questionnaire at each visit will be described. The time to deterioration of the QoL score will be analysed longitudinally, defined as the time from study inclusion to the first deterioration of at least five points from baseline, estimated using the Kaplan–Meier method.
The analysis of the primary objective will adhere to the modified intention-to-treat (mITT) principle, including all randomized patients regardless of compliance with eligibility criteria and the use of ICG angiography, provided planned resection with anastomosis has been performed and a 90-day postoperative status regarding the occurrence of an anastomotic fistula is available.
Confirmatory sensitivity analyses will be conducted, first in the strict ITT population (patients not evaluable and/or lost to follow-up within 90 days postoperatively will be considered failures if an anastomotic fistula is present) and second in the per protocol population, defined by patients who received the allocated procedure by randomization, underwent planned resection with anastomosis and have a 90-day postoperative status regarding the occurrence of an anastomotic fistula. An additional sensitivity analysis will be conducted to test whether an open fluorescence system used for laparotomy procedures influences the results.
All statistical tests will be two-sided with an alpha risk of 5%.
Sample size
- surgical centre
- body mass index (<30 kg/m2/≥30 kg/m2)
- American Society of Anesthesiologists score (1; 2/3; 4; 5)
- preoperative treatment (none/chemotherapy and/or radiotherapy)
- tumour location (left colon; sigmoid/upper rectum).
The minimization randomization algorithm considers previously randomized patients to allocate a new treatment while minimizing differences between stratification criteria. The randomization result provided by the system will be allocated in 80% of the cases, otherwise the other treatment will be allocated. In 20% of the cases the system will not use the randomization algorithm and will allocate treatment randomly to reduce the clinician's ability to predict the assigned treatment.
To minimize study bias, patients will be blinded to the assigned arm.
In this study, we hypothesize that the use of ICG angiography during surgery in patients with left colon or upper rectal cancer will reduce the rate of development of anastomotic fistula within 90 days after surgery from 7% to 3% [24, 26].
Using a Z-test with pooled variance, an intermediate efficacy and futility analysis at 50% of the information fraction, a 1:1 ratio for randomization, a two-sided type 1 error of 5% and 80% power, 958 evaluable patients for the rate of development of anastomotic fistula within 90 days of surgery must be randomized (479 in each arm).
Considering a 5% rate of patients lost to follow-up and/or with an unperformed anastomosis and/or not evaluable for the primary endpoint, a total of 1010 patients (958 × 100/95) must be randomized in the study.
The enrolment calculation was performed in East 6.4 (Statistical software for the design, simulation and monitoring clinical trials. Cytel Inc., Cambridge, MA).
Interim analysis
An interim analysis using the alpha risk expenditure function with the Lan–DeMets method (O'Brien–Fleming limits) is planned at 50% of the available information fraction (479 evaluable randomized patients).
Decision rules for both interim and final analyses of the primary objective are depicted in Figure 2.
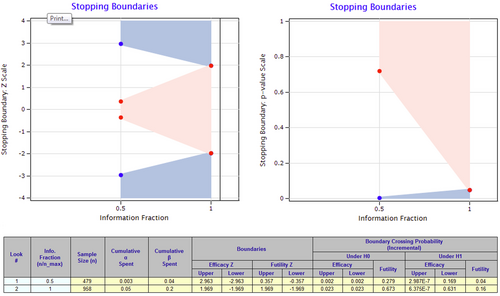
The trial can be prematurely terminated by either the coordinating investigator or the sponsor based on medical and/or administrative reasons or the results of the interim analysis. This decision will be made after mutual consultation, with reasons for discontinuation appropriately documented and notification provided to the relevant competent authorities.
Description of permanent or temporary withdrawal rules
- Decision of the participating individual (withdrawal of consent). Each participant has the right to leave the trial at any time and for any reason without affecting their relationship with the principal investigator.
- If technical incidents lead to the cessation of the ongoing experiment, a new appointment will be proposed.
- If participants in the research are unable to perform the tasks during the experimental phase, the experiment will be stopped and the results will be recorded.
- Tumour progression.
- Patient death.
- Loss to follow-up.
At any time the investigator as well as the sponsor reserve the right to prematurely terminate the trial for medical and/or administrative reasons. This will only occur after mutual consultation. Reasons for termination must be appropriately documented, and the trial termination must be reported to the relevant competent authorities.
Strategies for achieving adequate participant enrolment to reach the target sample size
The participating surgeons are all members of Groupe de Recherche en CHirurgie du Rectum (French Research Group of Rectal Cancer Surgery) and Fédération de Recherche en chirurgie (French Federation for Surgical Rsearch). They are therefore accustomed to working together on randomized studies.
No competing studies are expected during the enrolment period of the FLUOCOL-1 study.
Attrition bias will be minimized due to the short follow-up period (90 days), and the alignment of study visits with the surgeon's regular schedule.
Unbinding procedure
As the surgeon is informed of the randomization arm, an unblinding procedure is not necessary.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Ethics and dissemination
This trial will be conducted according to good clinical research practice and the latest Declaration of Helsinki [33].
FLUOCOL-1 has been submitted and approved by the Comité de Protection des Personnes in April 2022 and by the Agence nationale de sécurité du médicament et des produits de santé (ANSM) in June 2022.
Any important protocol modification will be communicated to all relevant parties by the sponsor.
A signed informed consent will be obtained from all participating patients. Patient anonymity will be safeguarded through the use of subject identification codes, and all study information will be securely stored in restricted areas. Participants’ study information will not be disclosed outside of the study without the written permission of participants.
Participants will have the freedom to withdraw their consent at any time for any reason and will still be invited to participate in follow-up assessments.
All results will be disseminated in international, peer-reviewed journals and presentation at international congresses. Authorship will be determined according to the recommendations from the International Committee of Medical Journal Editors [34].
In the case the results are significantly positive, IOFA will be easy to implement.
DISCUSSION
The prevention of AL stands as one of the paramount inquiries in colorectal surgery. While the literature increasingly suggests the substantial potential of fluorescence angiography with ICG in mitigating the rate of AL, the absence of high-quality randomized controlled trials with a placebo control remains a critical gap. We anticipate that FLUOCOL-1, as a single-blind, randomized study involving a substantial cohort of patients (1010) across numerous hospital centres will address this gap.
We deliberately chose not to include lower rectal cancer in this trial. While we acknowledge that lower rectal resections are associated with the highest rates of AL, several studies had already focused on this population at the time the FLUOCOL trial was designed. The novelty of this trial lies in its exclusive inclusion of patients with intraperitoneal tumours undergoing anastomosis above the pouch of Douglas. In such cases, a diverting stoma is not considered standard of care, given the lower risk of leakage. However, in the absence of a stoma, any leak that does occur is more likely to result in significant clinical consequences.
Recently, the ICG-COLORAL trial did not demonstrate a significant clinical benefit of the use of ICG in colorectal surgery overall. However, in the subgroup of left-sided resections (n = 472), the AL rate was lower in the ICG group compared with the control group (5.2% vs. 9.5%; OR 0.55, 95% CI 0.29–1.05), suggesting a potential benefit of ICG fluorescence imaging in this specific surgical context. Although the difference did not reach statistical significance, the observed trend supports further investigation in well-defined subgroups such as patients undergoing left-sided colorectal resection [35]. The FLUOCOL trial is therefore the first randomized study specifically addressing left-sided colon and high rectal cancer resections with intraperitoneal anastomosis. Furthermore, the study is designed with substantial statistical power, ensuring robust results that will provide valuable insights into the outcomes of these procedures. Should the superiority of ICG fluorescence be demonstrated, the findings will carry significant clinical implications for current colorectal surgical practices, yielding substantial benefits for patients.
AUTHOR CONTRIBUTIONS
Jean-Baptiste Pretalli: Writing – original draft; writing – review and editing; funding acquisition. Dewi Vernerey: Methodology; writing – review and editing; formal analysis. Philippe Evrard: Writing – review and editing. Astrid Pozet: Writing – review and editing; project administration. Anne-Laure Clairet: Writing – review and editing. Stéphane Benoist: Writing – review and editing; conceptualization. Mehdi Karoui: Writing – review and editing; conceptualization. Eddy Cotte: Writing – review and editing. Bruno Heyd: Writing – review and editing. Zaher Lakkis: Conceptualization; writing – original draft; funding acquisition; writing – review and editing.
ACKNOWLEDGEMENTS
The authors would like to thank Serb Laboratories for supplying the doses of ICG (Infracyanine®).
FUNDING INFORMATION
Programme Hospitalier de Recherche Clinique—Cancer (PHRC-K-20-044) and French Ministry of Health. ICG doses were provided free of charge by SERB Laboratory.
CONFLICT OF INTEREST STATEMENT
None.
ETHICS STATEMENT
FLUOCOL-1 has been submitted and approved by the Comité de Protection des Personnes in April 2022 and by the Agence nationale de sécurité du médicament et des produits de santé (ANSM) in June 2022.
DISCLOSURE
The sponsor is the owner of the data and bears responsibility for data management and analysis (Dr Vernerey, Methodology and Quality of Life Unit in Oncology).
The trial can be prematurely terminated by either the coordinating investigator or the sponsor based on medical and/or administrative reasons or the results of the interim analysis. This decision will be made after mutual consultation, with reasons for discontinuation appropriately documented and notification provided to the relevant competent authorities. The sponsor is responsible for the conduct of the trial, including covering research-related costs, promptly reporting serious adverse events and safety monitoring.
The content of this protocol is described according to the relevant items of the SPIRIT checklist (Standard Protocol Items: Recommendations for Interventional Trials) [36].
Serb supplies the ICG (Infracyanine®) used in the study free of charge. Serb is not involved in any other part of the work, including the design or analysis of the results.
APPENDIX A
FLUOCOL study group: Jean-Marc Regimbeau; Antoine Dabrowski; Pierre Goubault; Nathan Moreno-Lopez; Bertrand Trilling; Guillaume Piessen; Mehrdad Jafari; Benoit Gignoux; Diane Mege; Laura Beyer-Berjot; Régis Fara; Cécile De Chaisemartin; Mahaut Leconte; Jérémie Lefevre; Léon Maggiori; Jérôme Loriau; Nelson Trelles; Cyril Perrenot; Jean-Jacques Tuech; Antoine Sina; Benoit Romain; Mehdi Ouaissi; Léonor Benhaim; Olivier Oulie; David Orry; Gilles Manceau; Etienne Buscail; Véronique-Desfourneaux Denis; Quentin Denost; Cécilia Ceribelli; Caroline Rossi.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




