Acute PresentatiOn of coLorectaL cancer - an internatiOnal snapshot (APOLLO): Protocol for a prospective, multicentre cohort study
Correspondence
Chris Varghese, Department of Surgery, University of Auckland, Auckland, New Zealand.
Email: [email protected]
EuroSurg Collaborative, [email protected], @EuroSurg
Abstract
Aim
The primary aim of the study is to describe the variation in the operative and nonoperative management of emergency presentations of colon and rectal cancer in an international cohort. Secondary aims will be to develop a risk prediction model for mortality and primary anastomosis and validate risk criteria of large bowel obstruction (LBO) in patients with previously known colorectal cancer undergoing neoadjuvant chemotherapy or awaiting elective surgery.
Method
This prospective, multicentre audit will be conducted via the student- and trainee-led EuroSurg Collaborative network internationally over 2023 with 90-day follow-up. Data will be collected on consecutive adult patients presenting to the hospital in an unplanned and urgent manner with colorectal cancer (CRC) due to malignant LBO, perforation, CRC-related haemorrhage, or other related reasons. Primary outcome is 90-day mortality. Secondary outcomes include rates of stomas, primary anastomosis, stenting, preoperative imaging, and complications or readmissions.
Conclusion
This protocol describes the methodology for the first international audit on the management of acutely presenting CRC. This study will utilise a large collaborative network with robust data validation and assurance strategies. APOLLO will provide a comprehensive understanding of current practice, develop risk prediction tools in this setting, and validate existing trial results.
INTRODUCTION
Colorectal cancer (CRC) presents as an emergency in as many as a third of patients [1, 2], 80% of the time with obstruction and, less commonly, perforation and haemorrhage [3]. Emergency surgery for CRC is associated with mortality in 15%–34%, morbidity in 32%–64% [4], higher ostomy rates, and poorer health related quality of life [4]. Patients presenting emergently with CRC also tend towards more deranged physiology and advanced tumour biology [3, 5].
Existing guidelines on the optimal management of such cases are based on predominantly low grade evidence [6], and current series remain small. Global practices in the management of emergency CRC remain unquantified. A significant number of patients presenting with malignant large bowel obstruction (LBO) also have disseminated disease [7]. For these patients, self-expandable metal stents may offer a lower risk of permanent stoma compared to traditional surgical options, but also may result in perforations [8, 9]. The use of stents worldwide remains unclear.
There is therefore an impetus for a global snapshot audit of emergency presentations of CRC to capture prospective outcome data and describe the variation in management worldwide. Acute PresentatiOn of coLorectaL cancer: an internatiOnal snapshot (APOLLO) is an international, multicentre, prospective observational study which will address this need and aims to describe the operative and nonoperative management of emergency presentations of colon and rectal cancer in an international cohort.
METHODS
This study protocol is reported according to relevant items of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist.
Study objectives
The primary aim of the APOLLO study is to describe the operative and nonoperative management of emergency CRC presentations in an international cohort. Secondary aims will be to describe 30- and 90-day management outcomes, identify the risk factors for intraoperative, 30-, and 90-day mortality and ostomy rates in patients deemed for active management (i.e., not for palliative management), and develop a mortality and ostomy risk prediction model for patients undergoing active management for CRC. This study will also aim to validate risk criteria of LBO in patients with previously known CRC undergoing neoadjuvant chemotherapy or awaiting elective surgery.
Study design
An international, multicentre, prospective observational study of patients presenting acutely with CRC will be delivered by the EuroSurg Collaborative using the trainee- and student-led collaborative model. EuroSurg is an international collaborative group supported by national collaborative groups including Student Audit and Research in Surgery (STARSurg, UK), the Portuguese Surgical Research Collaborative (PTSurg, Portugal), Student-Initiated German Medical Audit (SIGMA, Germany), the Italian Surgical Research Group (ItSURG, Italy), and Trials and Audit in Surgery by Medical students in Australia and New Zealand (TASMAN, Australia and New Zealand).
“Mini-teams” of collaborators will participate at each hospital. Team members will include medical students, junior doctors, trainees, registrars, and supervising consultants (Figure 1).
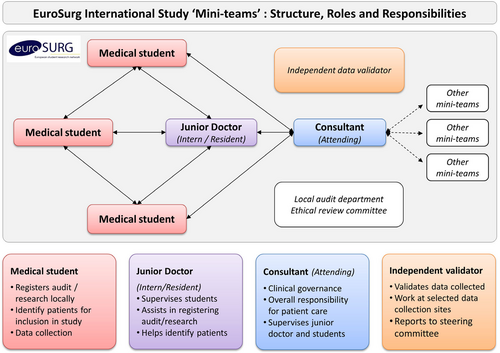
Study setting
The study is open to any secondary or tertiary hospital across the world with a general or colorectal surgery department performing major CRC surgery. All participating centres will be required to register the study according to local regulations prior to the commencement of data collection from each respective site. As this is an audit of practice, limited to using data obtained as part of usual care, no change to normal patient management is required.
At each hospital, one mini-team per period will collect data on eligible patients admitted over any consecutive 6-week period between January and June 2023. Patients will be followed up at 30- and 90-days after admission. Future dates and sites able to participate may be added at the discretion of the steering committee.
Eligibility criteria
- Adult patients (18 years and above)
-
Patients admitted to the hospital acutely with primary colon AND/OR rectal adenocarcinoma and referred to general/colorectal surgical departments
- Including operative patients for both curative and palliative reasons
- Including those who are referred to general surgery for assessment without surgery
Patients should be included regardless of cancer metastatic extent. Patients with known CRC presenting with progression of disease, that is, new acute obstruction, and not for side effects of treatment (chemotherapy/radiotherapy), are also included.
Patients should be excluded if they present acutely for the side effects of chemotherapy or radiotherapy. Nonadmitted patients, patients without adenocarcinoma, or patients with noncolorectal primary cancers that have metastasised to the colon or rectum (e.g., melanoma or lymphoma) should be excluded. Patients with primary neuroendocrine tumours, gastrointestinal lymphomas, gastrointestinal stromal tumours, and primary colorectal squamous cell carcinoma should also be excluded.
Outcomes
The primary outcome is 90-day mortality from the day of presentation. Secondary outcomes include (a) mortality at other timepoints (operative, in-hospital, 30-day), (b) rates of primary anastomosis, (c) rates of stoma formation and reversal, (d) rate and grade of surgical complications measured by Clavien Dindo Classification [10], (e) rate of colonic stenting and their associated complications, (f) representation, (g) rates of radiological assessment on admission, and (h) the proportion of patients presenting acutely with LBO with known CRC who had high risk criteria for obstruction (as defined by the FOXTroT obstruction criteria) at index assessment.
Audit standards
- All patients with suspected LBO should have a contrast-enhanced computed tomography (CT) scan [11],
- A 90-day mortality of <20% after emergency CRC surgery should be achieved (consistent with NBOCA recommendations)
Other variables
Additional variables for risk-adjustment of analyses will be collected (Table S1). These include demographics, comorbidities, frailty score [23], reason for presentation, cancer type and location, cancer TNM and mutation status, CRC screening status, relevant investigations, management strategies, surgical details, and surgeon/proceduralist skill levels.
Data collection and management
Data will be collected and stored online via the Research Electronic Data Capture (REDCap) web application [12, 13], hosted and managed by the Birmingham Surgical Trials Consortium (BiSTC) REDCap system hosted at the University of Birmingham, UK.
REDCap allows collaborators to enter and store data in a system that is encrypted and compliant with HIPAA-Security Guidelines in the United States. The security of the study database system is governed by the policies of the University of Birmingham. Data management and data security within the BiSTC REDCap will abide by the requirements of the General Data Protection Regulations and any subsequent amendments. Collaborators will be given secure REDCap project server login details, allowing secure data storage on the REDCap system. No identifiable patient data will be uploaded or stored on the REDCap database without prior local permissions.
Patients will be followed up for 90-days after presentation. No change in routine follow-up will take place.
Study recruitment and power calculation
Patients will be included if their hospital admission starts (defined as “date of hospital admission”) within the time during the data collection periods as specified above.
Teams should collect data on consecutive eligible patients at their hospital during the data collection period. Strategies to identify consecutive patients could include daily review of theatre lists, handover meetings/sheets, and ward lists, new inpatient referrals to surgical teams, CRC multidisciplinary team meetings, and daily review of emergency department admissions.
Based on previous EuroSurg studies, APOLLO is anticipated to include 150 centres in the UK and 150 centres in Europe and other countries [14]. We anticipate that on average, around five patients will present per 6-week data collection period at each participating site giving a minimum sample size of 1500.
Statistical analysis
The primary descriptive analysis will be investigating emergency CRC presentation, their respective management, and short-term outcomes. Frequency of different presenting symptoms such as obstruction, perforation and bleeding will be described. Comparisons will be made between patients with different management approaches such as resection, primary anastomosis, end-stoma formation, stents, and palliation.
Planned secondary analyses include the development of a mortality risk prediction tool in emergency CRC surgery with curative intent as per the TRIPOD statement [15]. If the recruitment numbers allow, this aims to let surgeons preoperatively predict short term morbidity and mortality, in turn determining which patients may benefit from alternative bridging stents, and which may benefit from a primary anastomosis.
For model development, a mixed effects multivariable regression model will be built with candidate variables selected based on clinical plausibility. Relative model fits will be assessed using Akaike information criterion to obtain the most parsimonious model. Area under the receiver operating characteristic curve will be used to assess discrimination. Validation of the model will be performed as the methods of Bonsdorff et al. [16]. Statistical analysis will be completed using R (R Foundation Statistical Program).
Study deliverance and quality assurance
This protocol has been written with guidance from an international expert cross-speciality advisory group and with the contribution of patient representatives from ACPGBI. A data dictionary will be developed to help collaborators in collecting data and patient inclusion. E-learning materials will be available on the EuroSurg website (eurosurg.org).
Following data collection, only data sets with >95% data completeness will be accepted for pooled analysis. To emphasise the importance of data completeness to collaborators, centres with >5% missing data points will be excluded from the study and collaborators from those centres withdrawn from the published list of citable collaborators. Data validation will be performed to ensure all eligible cases were recruited.
Data governance and ethics
Ethical approval according to the requirements of each participating country will be completed. The study will be submitted for consideration as a clinical audit at eligible centres. Ethical approval documentation will be required from collaborators at each centre and country prior to the commencement of data collection. Data including ethical approval documentation will be stored in the REDCap secure online database provided by BiSTC.
Any changes to protocols will be mediated by the steering committee and communicated to all relevant ethical boards through local hospital teams.
DISCUSSION
Colorectal cancer incidence and death counts have doubled globally in the previous three decades, with significant regional and national disparities [17]. In this protocol, we present an international prospective observational student- and trainee-led study conducted across the EuroSurg collaborative network. Patients who have emergency surgery for CRC have poorer outcomes, but little international and prospective data exists on this topic to inform guidelines and decision making [5, 6]. This study aims to provide a contemporary and international snapshot of current practice.
There are various options for patients presenting acutely with CRC, such as primary resection with anastomosis, colonic stenting as a bridge to elective surgery, primary resection with an end colostomy, or defunctioning stomas. Furthermore, with the rising use of neoadjuvant chemotherapy for colonic cancer [18], management pathways have become increasingly complex.
As such, significant controversy exists in the management of acute malignant CRC [6]. Difficulties in generating randomised studies in an acute setting, institutional practices, and availability of specialist CRC surgeons or specialist endoscopists, all contribute to varying practices [6, 9]. Outputs from this study, such as accurate operative and anastomosis risk predictions, may help guide clinical decision making in acutely presenting CRC. Important arising data may also inform areas of priority for future prospective and randomised studies.
Some limitations and challenges have been identified for this study. Due to the study's observational design, a pragmatic decision was made to rationalise the volume of data points, maximising feasibility across a network of medical students and trainees. This foregoes the collection of detailed pathological variables as well as presenting laboratory results. The observational nature of this study also limits the causal relationship that can be inferred between interventions and patient outcomes. Finally, given the low volume of acute CRC procedures, the committee opted for longer data collection periods compared to previous studies to maximise patient capture [19, 20]. Additionally, given the low incidence of acute presentations of CRC [3], adequate power for risk prediction models will be contingent on resultant sample sizes accrued.
APOLLO will be delivered through the well-established collaborative research model which has been validated across several international cohort studies [21, 22]. This model facilitates the inclusion of large numbers of patients in “snapshot” research studies across short study periods, particularly advantageous in low-incidence presentations such as acutely presenting CRC. APOLLO will extend this model across multiple countries, promoting multinational collaboration and supporting national research collaboratives. Concurrently, it will serve as an opportunity to upskill medical students and young trainees to engage in surgical research early in their careers and learn more about CRC pathways. Collaborators will have the opportunity to appraise their current practice with acute CRC patients to be compared at global scale, which can help to develop focused guidelines based on the findings from APOLLO study.
The accuracy and completeness of data collected will be ensured using the following strategies: a local senior consultant will supervise students and trainees; online tutorials will provide training in assessment of primary and secondary outcome measures, eligibility criteria and data collection (http://eurosurg.org/e-learning/); and, finally, independent collaborators at each participating centre will perform data validation.
The study is open to new registrations until 31 April 2023. International collaboration is essential to define current practices in colorectal surgery and improve patient outcomes.
AUTHOR CONTRIBUTIONS
All collaborators from the Steering Committee participated in drafting the manuscript and all individuals agreed for its submission. The Study Advisory Group supervised the protocol design and final manuscript. The corresponding author attests that all listed collaborators meet required criteria and that no others have been omitted.
ACKNOWLEDGEMENTS
We are grateful for support from the ACPGBI Patient Liaison Group, ESCP Trials, and the ESCP Annual Conference 2022 in Dublin. We would like to thank the University of Birmingham and BiSTC for managing the study data and REDCap hosting capabilities. Open access publishing facilitated by University of Auckland, New Zealand as part of the Wiley - University of Auckland, New Zealand agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
No funding was received for the completion of this study.
CONFLICT OF INTEREST
None to declare.
ETHICAL APPROVAL
Ethical approval processes will be sought according to the requirements of each participating country. Evidence of ethical approval will be required from national or local collaborators prior to the commencement of data collection. In the UK, an ethical review by the South-east Scotland Research Ethics Service has confirmed that COMPASS does not require formal ethical approval and it can be submitted for consideration as a clinical audit at eligible centres. Data will be stored in the REDCap secure online database provided by BiSTC.
Open Research
DATA AVAILABILITY STATEMENT
Data will be collected and stored online through the Research Electronic Data Capture (REDCap) managed by the Birmingham Surgical Trials Consortium (BiSTC), hosted at the University of Birmingham, UK. The security of the study database system is governed by the policies of the University of Birmingham. Data collected during the APOLLO study can be used for future analyses at the Study Management Group's discretion and agreement.




