The prognostic value of extramural venous invasion in preoperative MRI of rectal cancer patients
Abstract
Aim
This study aimed to examine the prognostic value of extramural venous invasion observed in preoperative MRI on survival and recurrences.
Method
In total, 778 rectal cancer patients were evaluated in multidisciplinary meetings in Helsinki University Hospital during the years 2016–2018. 635 patients met the inclusion criteria of stage I–III disease and were intended for curative treatment at the time of diagnosis. 128 had extramural venous invasion in preoperative MRI.
Results
The median follow-up time was 2.5 years. In a univariate analysis extramural venous invasion was associated with poorer disease-specific survival (hazard ratio [HR] 2.174, 95% CI 1.118–4.224, P = 0.022), whereas circumferential margin ≤1 mm, tumour stage ≥T3c or nodal positivity were not. Disease recurrence occurred in 17.3% of the patients: 13.4% had metastatic recurrence only, 1.7% mere local recurrence and 2.2% both metastatic and local recurrence. In multivariate analysis, extramural venous invasion (HR 1.734, 95% CI 1.127–2.667, P = 0.012) and nodal positivity (HR 1.627, 95% CI 1.071–2.472, P = 0.023) were risk factors for poorer disease-free survival (DFS). Circumferential margin ≤1 mm was a risk factor for local recurrence in multivariate analysis (HR 5.675, 95% CI 1.274–25.286, P = 0.023).
Conclusion
In MRI, circumferential margin ≤1 mm is a risk factor for local recurrence, but the risk is quite well controlled with chemoradiotherapy and extended surgery. Extramural venous invasion instead is a significant risk factor for poorer DFS and new tools to reduce the systemic recurrence risk are needed.
What does this paper add to the literature?
The article offers more information on the importance of extramural venous invasion and its relationship to rectal cancer recurrence. The value of the study is enhanced by the quite large study population (635 consecutive patients) which were all evaluated in rectal cancer multidisciplinary meetings and treated in a single centre.
INTRODUCTION
Colorectal cancer is the second most common cancer in Europe and in Finland. Rectal cancer constitutes about one-third of the cases. In 2018, the incidence of colorectal cancer in Finland was 51/100 000 for women and 72/100 000 for men. Based on the Finnish Cancer Registry database, the 5-year overall survival (OS) rate in 2016–2018 was 66% [1]. In rectal cancer, survival and recurrence rates vary considerably depending on the tumour stage at the time of diagnosis and tumour characteristics. The incidence of local recurrence varies from 4.4% to 26% [2, 3]. The risk of local recurrence started to decrease with improvements in surgical techniques such as total mesorectal excision (TME) [4] and extended abdominoperineal resection [5]. The other major contributors have been the use of neoadjuvant (chemo)radiotherapy [2, 3, 6, 7] and the centralization of management to tertiary centres [8, 9].
Extramural venous invasion (EMVI) detected in histological specimens is defined as the involvement of the veins beyond the muscularis propria [10]. It is an independent risk factor for local recurrence, metachronous nodal and distant metastases and also for overall mortality [10, 11]. Involved surgical margin and mucinous histology are additional risk factors for a local recurrence [12]. After adoption of the TME technique, the effect of mesenteric nodal involvement on local recurrence risk is less clear [13-15].
The aim of preoperative neoadjuvant treatment including chemoradiotherapy (CRT) is to reduce the risk of local and distant recurrences [3]. The decisions regarding neoadjuvant treatment are taken in multidisciplinary meetings, and MRI has proven to be the best modality to estimate the possibly threatened circumferential margin (CRM) and EMVI [16, 17]. For nodal involvement, MRI is the best method available but yet not particularly accurate, as it is highly sensitive but not specific [16, 17]. For locally advanced rectal cancers, preoperative CRT has been shown to be beneficial in ensuring cancer-free surgical margins and thus decreasing the risk of local recurrence [7]. So far, CRM, T class and nodal status in MRI have been the main parameters guiding decision making on neoadjuvant therapy. Even though the treatment planning has improved local control, metachronous distant metastases remain a problem impacting survival, and preoperatively identifying EMVI has not changed the situation [18, 19]. Therefore, we aimed to study the effect of EMVI seen in preoperative MRI (mrEMVI) on survival and local recurrence risk, and to study the prognostic impact of the nodal status in MRI.
PATIENTS AND METHODS
Patients
Patients diagnosed with rectal cancer who were evaluated in multidisciplinary rectal cancer meetings in Helsinki University Hospital between January 2016 and December 2018 were included. Helsinki University Hospital serves as a secondary referral centre for 1.1 million and a tertiary referral centre for 1.6 million inhabitants.
The data comprised 778 patients. The eligibility criteria were rectal adenocarcinoma or mucinous adenocarcinoma with distal margin at or below 15 cm from the anal verge on clinical examination or MRI, and the intent for curative treatment at the time of diagnosis.
Exclusion criteria were metastatic disease at the time of diagnosis, no MRI available before surgery, and final histology other than predominantly adenocarcinoma or mucinous adenocarcinoma. Finally, 635 eligible patients were included (Table 1).
| n = 635 | % | ||
|---|---|---|---|
| Age (years) | Median (range) | 70.1 | 24.1–98.2 |
| Gender | Female | 273 | 43.0 |
| Male | 362 | 57.0 | |
| BMI (kg/m2) | Mean (±SD) | 25.7 | (±4.7) |
| ASA | 1 | 43 | 6.8 |
| 2 | 243 | 38.3 | |
| 3 | 277 | 42.6 | |
| 4 | 62 | 9.8 | |
| Missing | 62 | 1.6 | |
| Distance from the anal verge | 6 cm or less | 286 | 45 |
| Over 6 cm | 349 | 55 | |
| Histology | Adenocarcinoma | 600 | 94.5 |
| Mucinous adenocarcinoma or signet cell features | 35 | 5.5 | |
| MRI T stage | mrTx | 9 | 1.4 |
| mrT1 | 6 | 0.9 | |
| mriT2 | 114 | 18.0 | |
| mriT3 | 233 | 36.7 | |
| mrT3a | 28 | 4.4 | |
| mrT3b | 149 | 23.5 | |
| mrT3c | 53 | 8.3 | |
| mrT3d | 3 | 0.5 | |
| mrT4a or T3mrf+ | 180 | 28.3 | |
| mrT4b | 93 | 14.6 | |
| MRI N stage | mrN0 | 285 | 44.9 |
| mrN1 | 218 | 34.3 | |
| mrN2 | 132 | 20.8 | |
| MRI EMVI | EMVI+ | 128 | 20.2 |
| EMVI− | 503 | 79.2 | |
| Missing | 21 | ||
| MRI CRM | mrCRM ≤1 mm | 279 | 43.9 |
| mrCRM >1 mm | 335 | 52.8 | |
| Missing | 21 | ||
| Pathological T stage | pTx | 30 | 4.7 |
| pT1 | 50 | 7.9 | |
| pT2 | 185 | 29.1 | |
| pT3 | 306 | 48.2 | |
| pT4 | 64 | 10.1 | |
| Pathological N stage | pN0 | 405 | 63.8 |
| pN1 | 153 | 24.1 | |
| pN2 | 77 | 12.1 | |
| Pathological lymphovascular invasion | Positive | 80 | 12.6 |
| Negative | 552 | 86.9 | |
| Missing | 3 | ||
| Neoadjuvant treatment | Long-course (chemo)radiotherapy 50 Gy | 197 | 31.0 |
| Short-course radiotherapy 5 × 5 Gy | 182 | 28.7 | |
| No preoperative radiotherapy | 256 | 40.3 | |
| Procedure | Anterior resection | 461 | 72.6 |
| Abdominoperineal resection/ELAPE | 153 | 24.1 | |
| Hartmann | 19 | 3.0 | |
| Other | 2 | 0.3 | |
| Postoperative adjuvant therapy | No | 300 | 47.2 |
| Yes | 309 | 48.7 | |
| No, even though planned | 24 | 3.8 | |
| Missing | 2 |
- Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CRM, circumferential resection margin; ELAPE, extralevator abdominoperineal excision; EMVI, extramural venous invasion.
Our primary aim was to examine death due to all causes OS, death from rectal cancer disease-specific survival (DSS), disease recurrence disease-free survival (DFS) and local recurrence. The primary test factor was mrEMVI status in the preoperative MRI. As secondary points of interest we examined the above-mentioned outcomes according to mrT stage, mrN stage and mrCRM. We also did a subgroup analysis of the post-CRT patients with the above-mentioned outcomes.
The data on the patients were obtained from electronic medical records. Information on neoadjuvant treatment, radiotherapy (RT), patient demographics, surgical technique, postoperative adjuvant therapy and histopathological records was acquired. Long-course 45–54 Gy CRT (capecitabine as a radiosensitizer) and long wait (7–11 weeks) before surgery were preferred for tumours with threatened margin or high volume. Alternatively, long-course RT or short-course 5 × 5 Gy RT with long wait before surgery was chosen for fragile patients not suitable for long-course CRT. The data on patients not attending follow-up in the Helsinki University Hospital were obtained from the electronic patient records of local healthcare centres (primary healthcare providers). Causes of death were identified from the Finnish Population Register Centre. The study was approved by the local institutional review board.
Imaging assessment
MRI was performed using, in most cases, the Siemens scanner 1.5 T or 3 T using high resolution multichannel phased-array pelvic surface coils. Bowel preparation for the rectum was used.
The imaging interpretation was done by five specialized gastrointestinal radiologists regularly attending the rectal cancer multidisciplinary meetings. The structured report of the preoperative MRI included mrT stage (T1, T2, T3a–d, T4a–b), primary tumour extramural extension, mrCRM, distance from the anal verge, nodal status (mrN0, mrN1, mrN2) and mrEMVI [20]. The tumours with threatened margins (mrCRM ≤ 1 mm) above the peritoneal reflection were reported T4a and the tumours below the peritoneal reflection T3mrf+.
The nodes were reported positive if their size was 5 mm or greater, had heterogeneity or had irregular margins. Patients were classified as mrN0 in the absence of lymph node metastasis, as mrN1 in the case of one to three regional positive lymph nodes and as mrN2 for four or more positive nodes.
mrEMVI+ was reported when the tumour penetrated through the bowel wall and invaded at least one extramural vascular structure [21, 22].
The radiological assessment of distant metastases (M0, M1a–c) was based on the structured report of whole body CT that was performed at the time of the diagnosis.
Only the patients undergoing long-course 45–54 Gy (C)RT with long wait or short-course RT 5 × 5 Gy with long wait before surgery had a response MRI. Response MRI was performed approximately 6–7 weeks after the end of (C)RT.
Histopathological assessment
Histopathological information was gathered from pathological reports. Structured pathological reports were based on the Union for International Cancer Control TNM 7th edition. When the 8th edition was issued in 2018, mismatch repair immunohistochemical staining and budding were added to the structured report. The pathological report included information on histological type (World Health Organization 2010) [23], size of the tumour, tumour grade, depth of invasion (T class), the integrity of the mesorectal fascia and possible tumour perforations, and the resection margins including CRM. In the pathology report the number of metastatic and normal lymph nodes (N class) and the possible invasion of blood vessels, lymphatic vessels or nerves were listed. Response to RT was estimated at 1–5 (Dvorak classification) [24].
Postoperative follow-up
The follow-up visits were scheduled at 6 weeks and 6, 12, 18 and 24 months after the operation and included laboratory tests (carcinoembryonic antigen [CEA] and haemoglobin) and endoscopy of the colorectal/anal anastomosis; further, haemoglobin and CEA were taken yearly after 2 years until 5 years. In addition to routine protocol, for patients with increased risk of recurrence (node positive, high grade histology, lymphovascular invasion, mucinous histology) whole body CT was performed at 1 and 2 years after operation. For patients with coloanal anastomosis, flexible sigmoidoscopy was performed also at 3, 4 and 5 years from the operation. For patients with long-course CRT pelvic MRI, whole body CT, haemoglobin and CEA were arranged at 4 and 6 years. The follow-up of elderly patients (>80 years) was decided individually. No patients were lost to follow-up.
Statistical analysis
Patient characteristics and surgical and pathology results were described using frequency and percentage for categorical variables and median and range for continuous variables. Data were compared using the Pearson chi-squared test for categorical variables. DSS and DFS were calculated with Kaplan–Meier analysis, and the survivals were compared with the log-rank test. The risk for disease recurrence or disease-specific death was analysed using Cox regression analysis. Potential risk factors with a P < 0.1 in univariate analysis were incorporated into multivariate logistic regression. All tests were two-sided, and P < 0.05 was considered statistically significant. A biostatistician was consulted on the methodology. The statistical analysis was conducted using SPSS 25 software.
RESULTS
Median age of the patients was 70.1 years (range 24.1–98.2 years) and 362 (57.0%) were men. Median body mass index (BMI) was 25.0 kg/m2 (16.3–45.7 kg/m2) and 520 (82%) of the patients were American Society of Anesthesiologists Physical Status Classification class II–III. In 286 patients (45%) the tumour was located at 6 cm or less from the anal verge. The tumours were predominantly adenocarcinomas (94.5%), and 5.5% had mucinous histology. T-stage distribution in the MRI is presented in Table 1: 306 patients (48.2%) had mrT1-T3b tumour and 329 (51.8%) mrT3c-T4b tumour. Nodal status was mrN0 in 285 (44.9%), mrN1 in 218 (34.3%) and mrN2 in 132 (20.8%) patients. Altogether, 197 (31%) of the 635 patients were planned to receive long-course CRT as neoadjuvant treatment. Due to the side effects of the capecitabine or poor general condition, eight received only RT (50.4 Gy). mrEMVI was found in 128 (20.2%) patients.
mrEMVI-positive patients
Of the 128 mrEMVI-positive patients, 76 (59.4%) underwent CRT before surgery, of whom two received only long-course RT, six patients received short-course RT (5 × 5 Gy) and had surgery after a long waiting interval, 23 received short-course RT (5 × 5 Gy) and had surgery within the following 5 days, and the remaining 23 patients received no neoadjuvant treatment. A report of the response MRI with EMVI information was available in 75 of the mrEMVI+ patients, and in the following response MRI 30 patients out of 75 (40%) mrEMVI+ patients turned mrEMVI negative.
Disease-specific survival and disease-free survival
The median follow-up time was 2.5 years (0–4.7), during which time 71 (11.2%) deaths occurred, 39 (6.1%) being cancer related. During the follow-up time, OS was 88.8% and DSS rate was 93.7%. There were 110 (17.3%) recurrences. Altogether 25 (3.9%) local recurrences were found; of these 11 (1.7%) were local recurrences only, 14 (2.2%) were a combination of local and metastatic recurrences. 85 (13.4%) were metastatic recurrences.
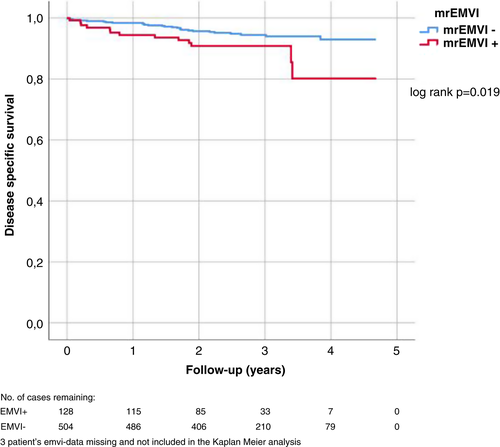
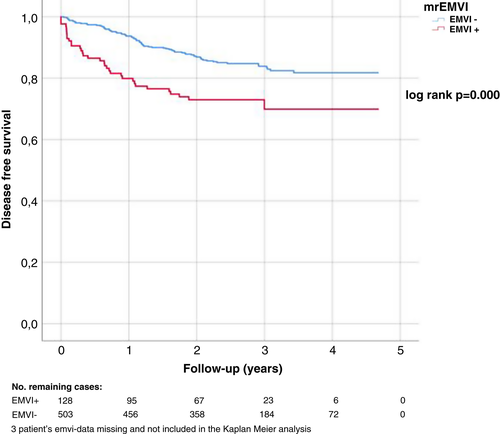
In univariate analyses, mrEMVI+ disease was associated with poorer DSS (P = 0.019) (Table 2, Figure 1), whereas mrCRM ≤1 mm, mrT class ≥T3c or mrN+ were not. We also calculated the cumulative DSS at 3 years, to check that the smaller number of patients in follow-up after 3 years did not affect the result, and found that the difference between the groups remained (P = 0.027). In multivariate analysis, none of these was associated with DSS. mrEMVI+ disease and mrN+ were associated with poorer DFS in both univariate and multivariate analyses (Table 2, Figure 2) and mrEMVI+ patients developed metastases more often and earlier than mrEMVI− patients (Figure 2). Mean time until recurrence was 0.67 years in EMVI+ patients versus 1.26 years in EMVI– patients.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Disease-specific survival | ||||||
| mrEMVI+ | 2.174 | 1.118–4.224 | 0.022 | 1.700 | 0.787–3.675 | 0.177 |
| mrCRM ≤ 1 mm | 1.788 | 0.903–3.541 | 0.095 | 1.596 | 0.784–3.248 | 0.197 |
| mrT3c–T4 | 1.628 | 0.858–3.089 | 0.136 | |||
| mrN+ | 1.347 | 0.709–2.556 | 0.363 | |||
| Disease-free survival | ||||||
| mrEMVI+ | 2.129 | 1.421–3.192 | 0.000 | 1.734 | 1.127–2.667 | 0.012 |
| mrCRM ≤ 1 mm | 1.387 | 0.933–2.062 | 0.106 | |||
| mrT3c–T4 | 1.609 | 1.098–2.358 | 0.015 | 1.259 | 0.838–1.892 | 0.267 |
| mrN+ | 1.912 | 1.281–2.854 | 0.002 | 1.627 | 1.071–2.472 | 0.023 |
| Local recurrence | ||||||
| mrEMVI+ | 1.764 | 0.735–4.231 | 0.204 | |||
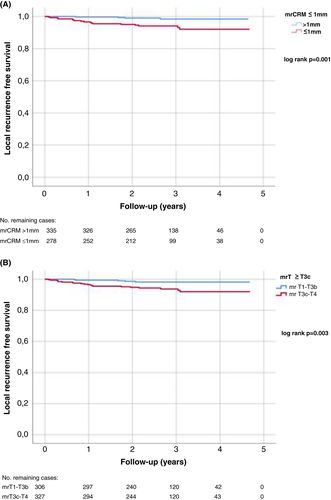
| mrCRM ≤ 1 mm | 5.481 | 1.844–16.293 | 0.002 | 5.675 | 1.274–25.286 | 0.023 |
| mrT3c–T4 | 3.963 | 1.487–10.559 | 0.006 | 0.954 | 0.241–3.783 | 0.947 |
| mrN+ | 1.469 | 0.649–3.326 | 0.356 | |||
| Low tumour (≤6 cm) | 1.844 | 0.828–4.105 | 0.134 | |||
- Abbreviations: CRM, circumferential resection margin; EMVI, extramural venous invasion; HR, hazard ratio.
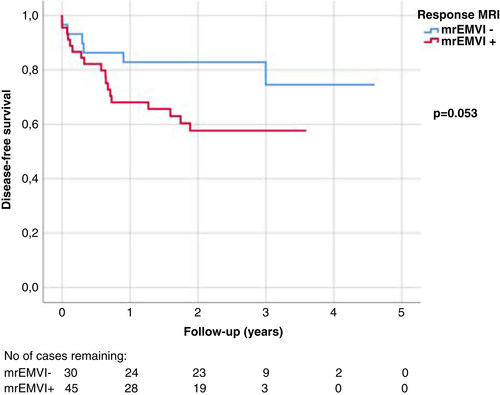
We also did a subgroup analysis of the 75 patients who were mrEMVI+ at the beginning and had a response MRI available. In the group of 30 patients who turned mrEMVI− in response MRI, DFS seemed to be better than for those 45 patients who remained mrEMVI+ (Figure 3), although the difference did not reach statistical significance (P = 0.053). There was no significant difference in OS (P = 0.116) nor in DSS (P = 0.224).
Risk factors for local recurrence in MRI
There were 25 (3.9%) local recurrences, of which 11 (1.7%) were solitary local recurrences without metastatic disease. In univariate analyses, mrCRM ≤ 1 mm and mrT class ≥T3c were significant risk factors for local recurrence (Table 2, Figure 4A,B), but mrEMVI+ and mrN+ were not, nor was the location of the tumour ≤6 cm from the anal verge (Table 2). In Cox regression multivariate analysis, CRM remained an independent risk factor for local recurrence (Table 2).
DISCUSSION AND CONCLUSIONS
In this study clarifying the prognostic significance of preoperative MRI in 635 consecutive rectal cancer patients treated with curative intent, mrEMVI+ was a risk factor associated with poorer DFS, but not for local recurrence. In univariate analysis, mrEMVI+ was also a risk factor for DSS. Statistical significance was not reached in multivariate analysis, most probably depending on the relatively short follow-up time. During the follow-up time, 3.9% of the patients had local recurrence and 15.6% developed metachronous distant metastases.
Centralization of rectal cancer treatment, and at the same time the adoption of multidisciplinary teams in planning the therapy of each individual rectal cancer patient, along with a high quality TME technique [25, 26], has resulted in a decreasing number of local recurrences [8, 9]. Perhaps the most important single tool in this preoperative evaluation has been rectal MRI and especially dedicated gastrointestinal radiologists interpreting it. MRI has been proven to be quite precise in estimating CRM [17, 20, 21] and therefore in guidance to choosing long-course CRT in order to down-size the tumour. Before the introduction and adoption of the TME technique mesenteric nodal positivity seemed to be a risk factor for local recurrence [3, 27], most probably because of leaving part of the mesentery behind during surgery. Since then, nodal positivity seen in rectal MRI has been an indicator for the recommendation of neoadjuvant RT in many rectal cancer centres. However, the evaluation of nodal positivity in rectal MRI is not that accurate [28], notably not in so-called good T3 tumours, leading to overtreatment with RT. In these tumours, about 4% local recurrence rates have been achieved with meticulous TME alone [4]. It is also worth considering that the benefit of adjuvant therapy in stage III rectal cancer is far less clear than in colon cancer, and the results are somewhat contradictory [29-31]. In the present study, nodal positivity in MRI was not a risk factor for decreased DSS or local recurrence. However, mrN+ was a risk factor for poorer DFS.
Lately, increasing attention has been paid to the possible prognostic significance of EMVI in rectal MRI [32], although it has not been used as an independent factor to guide the decision making in the guidelines for the time being. In our study, mrEMVI+ was a highly significant factor predicting poorer DFS and most of the recurrences appeared as distant metastases; just 1.7% of the patients had local recurrence only. The effect on DSS was seen in univariate analysis but not in multivariate analysis. This may be due to the relatively short follow-up time. Previously, in patients treated with neoadjuvant CRT, post-CRT mrEMVI positivity has been reported to be the only significant MRI-related factor for DFS [33], which was also noticed in our study. The recurrences also appeared earlier in mrEMVI+ patients in our series, in line with earlier reports [34]. This suggests that there is a high risk of micrometastases already when mrEMVI+ is noticed. Accordingly, mrEMVI+ patients also develop synchronous metastases more frequently [32]. Obviously, this raises the question whether it is possible to decrease the risk of distant recurrence in mrEMVI+ patients with more comprehensive systemic neoadjuvant chemotherapy such as the total neoadjuvant chemotherapy approach [35]. No controlled studies using mrEMVI+ as an indicator for systemic neoadjuvant chemotherapy have been published but they are being conducted (ClinicalTrials.gov NCT04842006). The results of an observational open-label and non-comparative study in which patients were treated with neoadjuvant chemotherapy before surgery were not encouraging; 3-year DFS for mrEMVI+ patients was 44% versus 96% for mrEMVI− patients [36], which is an encouragement to improve the systemic control.
Only mrCRM ≤1 mm was a risk factor for local recurrence in our study, which follows the results of previous reports [37-39]. However, considering that over a third of the patients had mrCRM ≤1 mm in our study population, selective long-course CRT combined with extended surgery resulted in an acceptable 3.9% rate of local recurrence.
Retrospective study design and relatively short follow-up time concerning DFS and DSS are the limitations of our study. It is possible that, with longer follow-up time, in addition to mrEMVI also some other factors in MRI might have an influence on survival. For example, tumour deposits, which seem to be an interesting prognostic factor [40], were not comprehensively mentioned in the radiology reports in the time period of our study. On the other hand, the strength of the study lies in the number of patients analysed (n = 635) and especially the number of mrEMVI+ patients (n = 128). Moreover, all the patients were evaluated and treated in one tertiary high-volume centre.
In conclusion, mrCRM ≤1 mm is a risk factor for local recurrence in rectal cancer, but the risk is quite well controlled with neoadjuvant (chemo)radiotherapy and extended surgery. In contrast, mrEMVI+ is a significant risk factor associated with poorer DFS after curative rectal cancer surgery and new tools to mitigate the systemic recurrence risk are needed.
FUNDING INFORMATION
The study received no external funding.
ETHICAL APPROVAL
The study was approved by the hospitals ethical board.
AUTHOR CONTRIBUTION
All authors contributed to the writing, analyzing or design of the study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




