Defunctioning stomas may reduce chances of a stoma-free outcome after anterior resection for rectal cancer
Funding information
Cancer Research Foundation in Northern Sweden, Agreement Concerning Research and Education of Doctors (ALFVLL-463921), Knut and Alice Wallenberg Foundation, and The Foundation for Medical Research in Skellefteå, Sweden.
Abstract
Aim
To investigate the conflicting consequences of faecal diversion on stoma outcomes and anastomotic leakage in anterior resection for rectal cancer, including interaction effects determined by the extent of mesorectal excision.
Method
Anterior resections between 2007 and 2016 were identified using the Swedish Colorectal Cancer Registry. National Patient Registry data were added to determine stoma outcome 2 years after surgery. Tumour distance from the anal verge constituted a proxy for extent of mesorectal excision [total mesorectal excision (TME): ≤10 cm; partial mesorectal excision (PME): 13–15 cm]. With confounder-adjusted probit regression, the total effect of defunctioning stoma on permanent stoma, and the interaction effect of extent of mesorectal excision, were estimated together with the indirect effect through anastomotic leakage. Baseline risks, risk differences (RDs) and relative risks (RRs) were reported.
Results
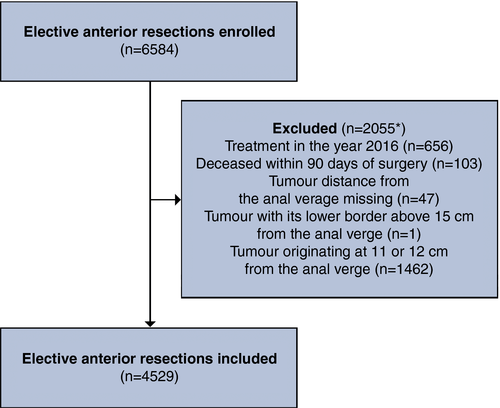
The main study cohort included 4529 patients. Defunctioning stomas influenced the absolute permanent stoma risk (TME: RD 0.11 [95% CI 0.09–0.13]; PME: RD 0.15 [95% CI 0.13–0.16]). The baseline risk was higher in TME, with a resulting greater RR in PME (2.23 [95% CI 1.43–3.02] vs 4.36 [95% CI 3.05–5.68]). The indirect reduction in permanent stoma rates, due to the alleviating effect of faecal diversion on anastomotic leakage, was small (TME: 0.89 [95% CI 0.81–0.96]; PME: 0.96 [95% CI 0.91–1.00]).
Conclusion
In anterior resection for rectal cancer, defunctioning stomas may reduce chances of a stoma-free outcome. Considering leakage reduction benefits, consequences of routine diversion in TME might be fairly balanced, while this seems questionable in PME.
What does this paper add to the literature?
Routine faecal diversion in anterior resection for rectal cancer remains controversial. Analysing 4529 anterior resections, this study found that defunctioning stomas caused a higher permanent stoma prevalence. This was especially pronounced for partial mesorectal excisions, with a lesser leakage reduction benefit, suggesting that routine diversion in these patients seems questionable.
INTRODUCTION
Although sphincter-preserving surgery is considered standard treatment for cancer of the mid- and upper rectum, the long-term permanent stoma prevalence after anterior resection amounts to 20%–25% [1, 2]. Anastomotic leakage is a major driver of this phenomenon [1, 2], while 20% of defunctioning stomas, originally fashioned to mitigate the consequences of a potential leak, are left in place or converted to end-colostomies [1, 3-5] and might add to the high stoma rate in themselves. Although their protective effects are at times clearly advantageous [6], the morbidity of defunctioning stomas [7, 8], and subsequent takedown surgery [2, 9], can be profound. Furthermore, research on leakage prediction has been inconclusive [10], and robust selection criteria for faecal diversion are generally lacking [11]. Additionally, while leakage rates were reduced in clinical trials, a corresponding effect with increased use of defunctioning stomas has been difficult to corroborate in population-based reports [12]. Routine use of defunctioning stomas seems to rather delay the leakage diagnosis [13], with less leaks acknowledged during the postoperative period and a decrease in reported leakage rates as a result.
While total mesorectal excision (TME) is always used for low or mid-rectal resections, partial mesorectal excision (PME) is, whenever oncologically feasible, an acceptable option for tumours in the upper rectum [14], allowing higher anastomoses with a lower risk of dehiscence [15, 16] and better functional results [17]. Randomised clinical trials supporting the use of defunctioning stomas have, nevertheless, been performed exclusively in patients undergoing TME surgery [18-22]. Thus, the role of defunctioning stomas in PME has not been appraised in a clinical trial; however, such stomas are in use for tumours in the upper rectum [15, 16, 23-26].
In light of the current evidence, a more selective use of defunctioning stomas seems warranted; however, population-based evidence is lacking as to whether such stomas lead to stoma permanence. In a nationwide cohort, we hypothesised a causal relationship [27] between a defunctioning stoma and a permanent stoma outcome, as well as an interaction effect depending on the extent of mesorectal excision.
METHODS
Study design
This was a nationwide, population-based, retrospective cohort study approved by the ethical review board at Umeå University. A total of 6584 patients treated with an elective anterior resection for rectal cancer in Sweden between 1 January 2007 and 31 December 2016 were identified using the Swedish Colorectal Cancer Registry (SCRCR). The last date of follow-up was 31 December 2017.
Registry-based stoma outcome
Stoma outcomes were ascertained by means of a previously validated registry-based method [24]. In brief, the method uses SCRCR data on primary faecal diversion, and subsequent procedure codes reported to the National Patient Registry, indicating stoma reversal, or further stoma construction. In the present report, stoma outcome was determined at the time point 2 years after anterior resection; this extended follow-up therefore takes into account stoma reversal delay, shown to sometimes exceed 12 months [2, 28]. In the main study cohort and analyses concerning stoma outcomes, operations on patients in 2016 were thus excluded. Also, to account for stoma non-reversals owing to fatal postoperative complications, patients deceased within 90 days of index surgery were also not considered in the main analysis.
Registry data
The SCRCR has been validated twice, displaying a near complete degree of coverage [29, 30]. It is continually cross-checked against the National Cancer Registry for missing registrations. Reporting to the registry includes patient demography, surgical details, postoperative course, final pathological assessment and a 5-year follow-up. Rectal cancer is defined by the registry as an adenocarcinoma, located at least partially within 15 cm from the anal verge, measured using rigid sigmoidoscopy.
In patients with high rectal cancer, PME is sufficient and advisable whenever a minimum 5 cm margin below the tumour is achievable [14, 15, 31, 32], while the majority of tumours originating above the peritoneal reflection, and at 13–15 cm from the anal verge in particular, are typically managed with such surgery [15, 16, 33-36]. Of note, the alternative term tumour-specific mesorectal excision is sometimes used, which is for all intents and purposes equivalent to PME. In the present study, registry data on tumour distance from the anal verge was used as a proxy to classify the extent of mesorectal excision. Any patient with a registered tumour at ≤10 cm was considered to have had undergone total mesorectal excision (TME), whereas patients with a tumour distance of ≥13 cm were deemed highly likely to have been treated with PME. Patients with tumours originating inbetween these levels were considered a stand-alone segment in which the extent of mesorectal excision could not reliably be determined, and were thus excluded (along with patients with missing data concerning tumour distance from the anal verge).
Anastomotic leakage was defined as dehiscence within 30 days of the index operation, and recorded in the registry. The leakage definition included rectovaginal or rectovesical fistula, or pelvic abscess, reported in the registry free text fields.
Statistical analyses
Although observational, the goal of the present report was to quantify causal effects. Thus, in the pursuit of making causal inferences based on unbiased total effect estimates, directed acyclic graphs (Figure S1) were drawn to display the causal structure of each statistical model [37-39], further explained in Appendix S1.
In the main analysis, a primary defunctioning stoma was considered the exposure for a permanent stoma outcome. Anastomotic leakage was acknowledged as a mediator (a variable influenced by the exposure and with effect on the outcome), while the American Society of Anesthesiologists’ (ASA) class, age, healthcare region, hospital volume, intraoperative bleeding, extent of mesorectal excision, neoadjuvant treatment, sex and clinical TNM (cTNM) stage were considered to be confounding variables. Subsequently, the protective effect of a primary defunctioning stoma on anastomotic leakage was assessed in a separate analysis, using the same confounder subset, except for cTNM and age.
To assess mediation and estimate total, direct and indirect effects, the multivariable probit regression method proposed by Lindmark et al. in 2018 [40] was used in the principal analyses. The method is described in detail in Appendix S1. Next, the protective effect of a defunctioning stoma on anastomotic leakage was estimated, also by means of multivariable probit regression; however, without exclusion of operations on patients in 2016 and those deceased within 90 days of index surgery. The latter analysis was performed with adjustment only for the specified confounder subset outlined above, and was confined to total effect measures, as there were no mediator variables of interest. Sensitivity analyses with outcomes determined at different time points and detailed statistical considerations, including dependence of observations within hospitals, handling of missing data with multiple imputation, and assessment of unmeasured confounding, are described in Appendix S1.
As a last step, the estimated effects of a defunctioning stoma on permanent stoma risks are illustrated in different type populations, with results from the regression models translated into number needed to harm (NNH); that is, the estimated number of defunctioning stomas required to result in a permanent stoma.
Results from the probit regression models are displayed with baseline risks (BRs), risk differences (RDs) and relative risks (RRs), and 95% confidence intervals (CIs). A p-value was considered statistically significant at a level below 0.05. All statistical analyses were performed using the computer software R version 3.5.2 (R Core Team).
RESULTS
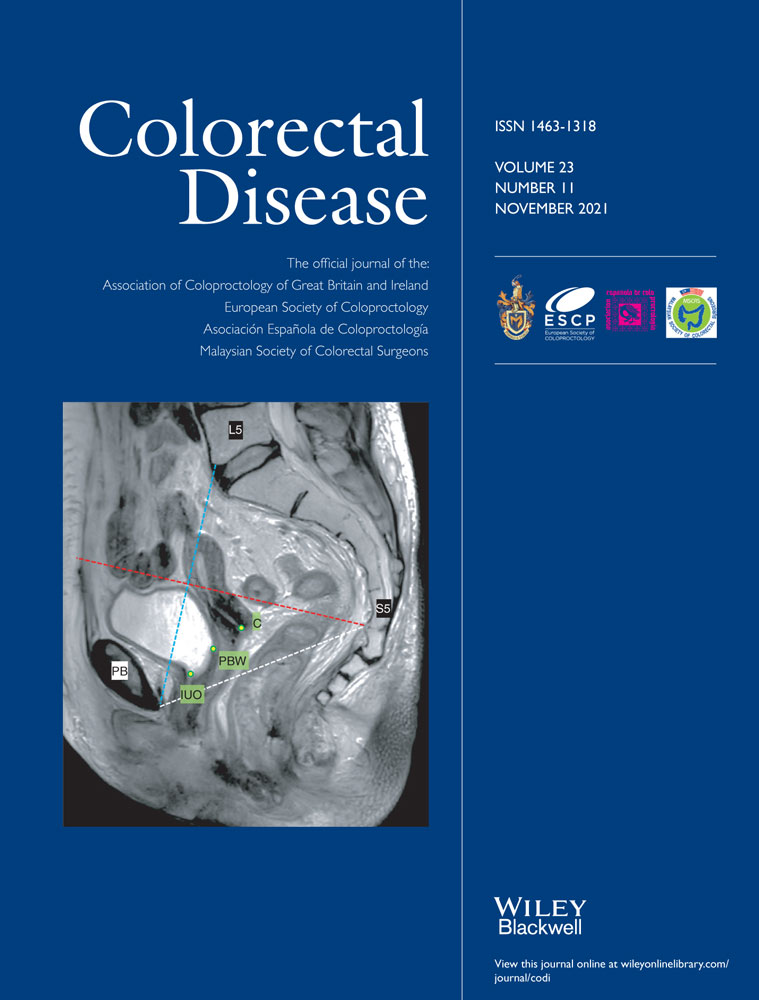
Between 1 January 2007 and 31 December 2016, 6584 patients underwent anterior resection for rectal cancer in Sweden. While some patients fulfilled more than one exclusion criterion, the exact number of ineligible patients amounted to 2055; of these, the majority (n = 1462) had tumours located at 11 or 12 cm above the anal verge (Figure 1). A total of 4529 patients were considered for further analysis, with 3007 (66.4%) registered with a tumour emerging within 10 cm of the anal verge, judged to have had TME surgery. The remaining 1522 (33.6%) patients had tumours registered at 13–15 cm and were classified as having undergone a PME.
Patient baseline demography, divided by the extent of mesorectal excision and stoma outcome, is shown in Table 1, with postoperative short- and long-term outcomes reported in Table 2. A defunctioning stoma was reported in 3573 patients (78.9%), while the overall anastomotic leakage incidence amounted to 419 events (9.3%); 21 leaks consisted of rectovaginal or rectovesical fistulae, or pelvic abscesses. In total, 768 (17.0%) of the 4529 patients had a permanent stoma at the end of follow-up. Figure S2 demonstrates all events with effect on stoma status registered between study inclusion and the end of follow-up.
| Categorical variables | No defunctioning stoma (n = 956) | Defunctioning stoma (n = 3573) | ||
|---|---|---|---|---|
| TME (n = 212) | PME (n = 744) | TME (n = 2795) | PME (n = 778) | |
| N (%) | N (%) | N (%) | N (%) | |
| Sex | ||||
| Male | 90 (42.5) | 407 (54.7) | 1653 (59.1) | 508 (65.3) |
| Female | 122 (57.6) | 337 (45.3) | 1142 (40.9) | 270 (34.7) |
| ASA class | ||||
| ASA I | 45 (21.2) | 178 (23.9) | 746 (26.7) | 161 (20.7) |
| ASA II | 123 (58.0) | 418 (56.2) | 1631 (58.4) | 445 (57.2) |
| ASA III–V | 29 (13.7) | 139 (18.7) | 375 (13.4) | 162 (20.8) |
| Missing† | 15 (7.1) | 9 (1.2) | 43 (1.5) | 10 (1.3) |
| Stage (clinical)‡ | ||||
| I | 63 (29.7) | 175 (23.5) | 521 (18.6) | 121 (15.6) |
| II | 29 (13.7) | 142 (19.1) | 576 (20.6) | 146 (18.8) |
| III | 53 (25.0) | 202 (27.2) | 1232 (44.1) | 323 (41.5) |
| IV | 16 (7.6) | 47 (6.3) | 138 (4.9) | 68 (8.7) |
| Missing† | 51 (24.1) | 178 (23.9) | 328 (11.7) | 120 (15.4) |
| Neoadjuvant therapy | ||||
| No neoadjuvant therapy | 108 (50.9) | 587 (78.9) | 627 (22.4) | 362 (46.5) |
| Radiotherapy | 89 (42.0) | 128 (17.2) | 1595 (57.1) | 259 (33.3) |
| Chemoradiotherapy | 15 (7.1) | 29 (3.9) | 573 (20.5) | 157 (20.2) |
| Healthcare region | ||||
| Stockholm-Gotland | 35 (16.5) | 118 (15.9) | 614 (22.0) | 202 (26.0) |
| Uppsala-Örebro | 33 (15.6) | 172 (23.1) | 686 (24.5) | 198 (25.5) |
| Southeast | 31 (14.6) | 123 (16.5) | 284 (10.2) | 60 (7.7) |
| Southern | 38 (17.9) | 115 (15.5) | 510 (18.3) | 147 (18.9) |
| Western | 59 (27.8) | 179 (24.1) | 468 (16.7) | 118 (15.2) |
| Northern | 16 (7.6) | 37 (5.0) | 233 (8.3) | 53 (6.8) |
| Type of surgery | ||||
| Open | 187 (88.2) | 558 (75.0) | 2434 (87.1) | 674 (86.6) |
| Laparoscopic | 18 (8.5) | 144 (19.4) | 263 (9.4) | 82 (10.5) |
| Conversion to open | 6 (2.8) | 36 (4.8) | 82 (2.9) | 17 (2.2) |
| Missing† | 1 (0.5) | 6 (0.8) | 16 (0.6) | 5 (0.6) |
| Stage (pathological)§ | ||||
| I | 66 (31.1) | 202 (27.2) | 881 (31.5) | 173 (22.2) |
| II | 52 (24.5) | 227 (30.5) | 728 (26.1) | 242 (31.1) |
| III | 68 (32.1) | 246 (33.1) | 975 (34.9) | 270 (34.7) |
| IV | 16 (7.6) | 48 (6.5) | 150 (5.4) | 75 (9.6) |
| Missing† | 10 (4.7) | 21 (2.8) | 60 (2.2) | 18 (2.3) |
| Continuous variables |
Median (IQR) Missing† N (%) |
Median (IQR) Missing† N (%) |
Median (IQR) Missing† N (%) |
Median (IQR) Missing† N (%) |
|---|---|---|---|---|
| Age (years) | 69 (61–77) | 70 (62–77) | 66 (59–73) | 67 (61–73) |
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Bleeding (ml) | 300 (150–600) | 200 (100–400) | 400 (200–700) | 350 (200–600) |
| 17 (8.0) | 22 (3.0) | 75 (2.7) | 25 (3.2) | |
| Operation time (min) | 177 (137–226) | 186 (142–240) | 251 (199–325) | 261 (208–338) |
| 10 (4.7) | 14 (1.9) | 63 (2.3) | 15 (1.9) | |
| Hospital volume (‖) | 14 (12–19) | 13 (18–24) | 18 (14–25) | 19 (13–25) |
| 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
- TME, total mesorectal excision; PME, partial mesorectal excision; ASA, American Society of Anesthesiologists; IQR, interquartile range.
- *The extent of mesorectal excision as derived using registry data on tumour distance from the anal verge as a proxy (≤10 cm: TME vs. 13–15 cm: PME). Another 515 patients had anterior resection for rectal cancer located at 11 or 12 cm during the specified study period and are excluded in the above table due to uncertainty in determining the extent of mesorectal resection. Additionally, 83 patients were deemed ineligible due to early mortality, and 5 were already excluded among the 515 patients outlined above; thus, amounting to 593 exclusions in total.
- †Missing = variable data missing in the Swedish Colorectal Cancer Registry.
- ‡Stage (clinical) = clinical TNM staging prior to surgery.
- §Stage (pathological) = final pathological assessment of TNM stage following surgery.
- ‖Anterior resections performed per year.
| Categorical variables | No defunctioning stoma (n = 956) | Defunctioning stoma (n = 3573) | ||
|---|---|---|---|---|
| TME (n = 212) | PME (n = 744) | TME (n = 2795) | PME (n = 778) | |
| N (%) | N (%) | N (%) | N (%) | |
| Anastomotic leak | ||||
| No | 183 (86.3) | 686 (92.2) | 2525 (90.3) | 716 (92.0) |
| Yes | 29 (13.7) | 58 (7.8) | 270 (9.7) | 62 (8.0) |
| Adjuvant chemotherapy | ||||
| No | 182 (85.9) | 585 (78.6) | 2127 (76.1) | 551 (70.8) |
| Yes | 30 (14.2) | 159 (21.4) | 668 (23.9) | 227 (29.2) |
| Cancer recurrence† | ||||
| No | 180 (84.9) | 673 (90.5) | 2457 (87.9) | 666 (85.6) |
| Yes | 31 (14.6) | 70 (9.4) | 333 (11.9) | 105 (13.5) |
| Missing | 1 (0.5) | 1 (0.1) | 5 (0.2) | 7 (0.9) |
| Permanent stoma‡ | ||||
| No | 195 (92.0) | 722 (97.0) | 2211 (79.1) | 633 (81.4) |
| Yes | 17 (8.0) | 22 (3.0) | 584 (20.9) | 145 (18.6) |
- Abbreviations: PME, partial mesorectal excision; TME, total mesorectal excision.
- *The extent of mesorectal excision as derived using registry data on tumour distance from the anal verge as a proxy (≤10 cm: TME vs. 13–15 cm: PME). Another 515 patients had anterior resection for rectal cancer located at 11 or 12 cm during the specified study period and are excluded in the above table due to uncertainty in determining the extent of mesorectal resection. Additionally, 83 patients were deemed ineligible due to early mortality, and 5 were already excluded among the 515 patients outlined above; thus, amounting to 593 exclusions in total.
- †Local and/or distant recurrence within 2 years of the index operation. A total of 14 patients were considered as erroneous registrations due to conflicting information about cancer recurrence and recoded as “missing” as a consequence.
- ‡Stoma outcome determined at 2 years after the index operation.
Defunctioning stoma and stoma outcome
Regardless of the extent of mesorectal excision, construction of a defunctioning stoma was significantly associated with an increased permanent stoma rate (Table 3). While confounder-adjusted total risk differences were similar between TME and PME (TME: RD 0.11 [95% CI: 0.09–0.13]; PME: RD 0.15 [95% CI: 0.13–0.16]), patients in the former group had a higher baseline risk of a permanent stoma outcome (TME: BR 0.09 [95% CI: 0.08–0.10]; PME: BR 0.04 [95% CI: 0.03–0.06]). This resulted in a higher relative risk for patients undergoing PME (TME: RR 2.23 [95% CI: 1.43–3.02]; PME: RR 4.36 [95% CI: 3.05–5.68]) (Figure 2). Mediation analyses indicated that the indirect effect through anastomotic leakage on stoma outcome was small, compared to the substantial direct effect of a defunctioning stoma (Table 3 and Figure 2). Sensitivity analyses with stoma outcomes determined at 3 years and at 1 year after surgery, respectively, showed similar results, albeit with a stronger effect of defunctioning stoma on permanent stoma outcome with a short follow-up time (Table S1 and Table S2). Results from imputed data sets did not differ from complete case analyses (data not shown). Moreover, any unmeasured confounding was estimated to have had limited impact on the results (Figure S3).
| Anterior resections | No defunctioning stoma | Defunctioning stoma |
|---|---|---|
| Baseline risk (95% CI) | Risk difference (95% CI) | |
| All | ||
| Direct effect | 0.08 (0.06–0.09) | 0.14 (0.13–0.15) |
| Indirect effect | 0.22 (0.20–0.23) | −0.02 (−0.03 to −0.01) |
| Total effect | 0.08 (0.06–0.09) | 0.12 (0.11–0.14) |
| TME | ||
| Direct effect | 0.09 (0.08–0.10) | 0.14 (0.12–0.15) |
| Indirect effect | 0.22 (0.21–0.24) | −0.03 (−0.04 to −0.02) |
| Total effect | 0.09 (0.08–0.10) | 0.11 (0.09–0.13) |
| PME | ||
| Direct effect | 0.04 (0.03–0.06) | 0.15 (0.14–0.17) |
| Indirect effect | 0.20 (0.19–0.21) | −0.01 (−0.02 to −0.00) |
| Total effect | 0.04 (0.03–0.06) | 0.15 (0.13–0.16) |
- Using probit regression models to quantify mediation, indirect effects correspond to the estimated effect attributable to anastomotic leakage. Analyses are based on a cohort of 4529 patients who had anterior resection for rectal cancer in Sweden between 1 January 2007 and 31 December 2015, with stoma outcome determined at 2 years after the index operation.*
- Abbreviations: PME, partial mesorectal excision; TME, total mesorectal excision.
- *The extent of mesorectal excision as derived using registry data on tumour distance from the anal verge as a proxy (≤10 cm: TME vs. 13–15 cm: PME). Another 515 patients had anterior resection for rectal cancer located at 11 or 12 cm during the specified study period and are excluded in the above table due to uncertainty in determining the extent of mesorectal resection. Additionally, 83 patients were deemed ineligible due to early mortality, and five were already excluded among the 515 patients outlined above; thus, amounting to 593 exclusions in total.
Defunctioning stoma and anastomotic leakage
For estimates concerning the protective effect of a defunctioning stoma on anastomotic leakage, only patients who could not be classified regarding mesorectal excision (i.e., had a tumour originating at 11 or 12 cm above the anal verge) were excluded, while operations on patients in 2016 and those deceased within 90 days of surgery remained. Consequently, 5122 patients were considered in these analyses.
Irrespective of the extent of mesorectal excision, a defunctioning stoma decreased the risk of symptomatic anastomotic leakage (TME: RR 0.51 [95% CI: 0.36–0.66]; PME: RR 0.73 [95% CI: 0.52–0.94]) (Table 4). TME had a higher baseline risk of symptomatic anastomotic leakage than PME (TME: BR 0.18 [95% CI: 0.16–0.20]; PME: BR 0.11 [95% CI 0.10–0.12]).
| Anterior resections | No defunctioning stoma | Defunctioning stoma | |
|---|---|---|---|
| Baseline risk (95% CI) | Risk difference (95% CI) | Relative risk (95% CI) | |
| All | 0.16 (0.15 to 0.17) | −0.07 (−0.09 to −0.06) | 0.56 (0.40 to 0.71) |
| TME | 0.18 (0.16 to 0.20) | −0.09 (−0.10 to −0.07) | 0.51 (0.36 to 0.66) |
| PME | 0.11 (0.10 to 0.12) | −0.03 (−0.04 to −0.02) | 0.73 (0.52 to 0.94) |
- Stratified by extent of mesorectal excision, calculations are based on 5122 patients who had anterior resection for rectal cancer in Sweden between 1 January 2007 and 31 December 2016.*
- TME, total mesorectal excision; PME, partial mesorectal excision.
- *The extent of mesorectal excision as derived using registry data on tumour distance from the anal verge as a proxy (≤10 cm: TME vs. 13–15 cm: PME). Patients with rectal cancer located at 11 or 12 cm are excluded in the above table due to uncertainty in determining the extent of mesorectal resection. All calculations were performed with adjustments for the confounding variables, ASA class, healthcare region, hospital volume, intraoperative bleeding, extent of mesorectal excision, neoadjuvant treatment and sex. Also, significant interactions between defunctioning stoma and extent of mesorectal excision, as well as defunctioning stoma and healthcare region were included in the statistical models.
Permanent stoma risk in different patient groups
Table 5 demonstrates the effect of faecal diversion on the permanent stoma risk, stratified for groups with specific baseline characteristics. Women treated with PME and chemoradiotherapy experienced the relatively highest risk for stoma permanence when diverted (NNH 4 [95% CI: 3–5]), in contrast to men operated with TME and no neoadjuvant treatment (NNH 14 [95% CI: 11–21]), where defunctioning stomas seemed to have had the least impact on long-term stoma outcomes.
| Type population | No defunctioning stoma | Defunctioning stoma | Relative risk (95% CI) | NNH (95% CI) |
|---|---|---|---|---|
| % Permanent stoma (95% CI) | % Permanent stoma (95% CI) | |||
| TME | ||||
| Female NCRT | 5.6 (4.3–7.0) | 15.2 (11.2–19.1) | 2.69 (1.60–3.78) | 10 (8–14) |
| Female RT | 9.9 (8.6–11.3) | 24.0 (20.1–27.9) | 2.41 (1.56–3.26) | 7 (5–9) |
| Female CRT | 9.7 (8.2–1.1) | 28.0 (24.1–32.0) | 2.91 (1.82–4.00) | 5 (4–6) |
| Male NCRT | 6.3 (4.9–7.7) | 13.10 (9.2–17.1) | 2.08 (1.24–2.92) | 14 (11–21) |
| Male RT | 11.0 (9.6–12.4) | 21.3 (17.4–25.2) | 1.94 (1.27–2.61) | 9 (7–12) |
| Male CRT | 10.5 (9.1–12.0) | 24.7 (20.8–28.7) | 2.35 (1.44–3.25) | 7 (5–8) |
| PME | ||||
| Female NCRT | 2.5 (1.2–3.9) | 14.4 (10.5–18.4) | 5.73 (3.78–7.68) | 8 (7–10) |
| Female RT | 4.9 (3.5–6.2) | 23.0 (19.0–26.9) | 4.71 (3.21–6.21) | 5 (4–6) |
| Female CRT | 4.7 (3.4–6.1) | 27.0 (23.0–30.9) | 5.71 (3.60–7.82) | 4 (3–5) |
| Male NCRT | 2.9 (1.5–4.2) | 12.5 (8.5–16.4) | 4.32 (2.97–5.67) | 10 (8–12) |
| Male RT | 5.5 (4.2–6.9) | 20.4 (16.5–24.3) | 3.69 (2.62–4.76) | 6 (5–7) |
| Male CRT | 5.2 (3.9–6.6) | 23.7 (19.7–27.7) | 4.53 (2.84–6.22) | 5 (4–6) |
- Analyses stratified by extent of mesorectal excision are based on a cohort of 4529 patients who had anterior resection for rectal cancer in Sweden between 1 January 2007 and 31 December 2015, with stoma outcome determined at 2 years after the index operation.*
- Abbreviations: CRT, chemoradiotherapy; NCRT, no neoadjuvant therapy; NNH, number needed to harm; PME, partial mesorectal excision; RT, radiotherapy; TME, total mesorectal excision.
- *The extent of mesorectal excision as derived using registry data on tumour distance from the anal verge as a proxy (≤10 cm: TME vs. 13–15 cm: PME). Patients with rectal cancer located at 11 or 12 cm are excluded in the above table due to uncertainty in determining the extent of mesorectal resection. All calculations were performed with adjustments for the confounding variables, ASA class, healthcare region, hospital volume, intraoperative bleeding, extent of mesorectal excision, neoadjuvant treatment, sex and clinical TNM stage. Also, significant interactions between defunctioning stoma and extent of mesorectal excision, as well as defunctioning stoma and healthcare region, were included in the statistical models.
DISCUSSION AND CONCLUSIONS
In this nationwide registry-based cohort of patients treated with anterior resection for rectal cancer in Sweden, construction of defunctioning stomas was estimated to have had a marked effect on the risk of ending up with a permanent stoma 2 years after surgery. The relative risk increase was especially pronounced in patients undergoing PME. The indirect effect on stoma outcome mediated through anastomotic leakage was negligible compared to the direct effect via defunctioning stoma construction, regardless of the extent of mesorectal excision.
The principal limitation of the present report is the use of registry data, including the main variables regarding estimated extent of mesorectal excision, construction of a defunctioning stoma, anastomotic leakage, as well as long-term stoma outcome. Although the validity of the SCRCR is regarded as high in general [30], underreporting of complications have been demonstrated [41] . Concerning the interaction with the extent of mesorectal excision, it is conceivable that most misclassifications would occur in the PME group, as tumours diagnosed in the lower and mid-rectum were in all likelihood managed with TME, while some patients with tumours located in the upper rectum might have had TME instead of PME. We would therefore expect misclassifications on mesorectal excision to attenuate effect estimates of defunctioning stoma construction on permanent stoma outcome, rather than the opposite. The same reasoning holds true for the registry-based stoma outcomes, as a previous validation study demonstrated that misclassifications only occurred in the permanent stoma group [24], which in turn would skew effect estimates towards the null hypothesis, in a corresponding manner. Additionally, while tumour distance from the anal verge was used to estimate the extent of mesorectal excision, the exact level of anastomosis was not possible to determine from registry data; this, together with other unobserved variables, potentially influencing the decision to divert the patient, or not, add to the study limitations. Any residual confounding due to the latter would, however, probably have had limited effect on the study results, according to the sensitivity analyses which assessed the robustness of the analyses to unmeasured confounding.
The study findings indicate that the extent of mesorectal excision did not influence the protective effect of a defunctioning stoma, despite the known higher leakage frequency for TME [15]. In other words, the advantageous effects were in absolute numbers comparable between patients undergoing TME and PME; however, the lower baseline risk of anastomotic leakage in patients treated with PME translated into a smaller risk reduction from faecal diversion, compared to TME patients. It is in this context important to point out that only leaks within 30 days of surgery are reported to the registry, and that the frequent use of defunctioning stomas in Sweden during the study decade [42] may have resulted in a decrease in registered leaks, as some actual leaks might have been diagnosed after the postoperative registration period; a delayed leakage diagnosis has previously been demonstrated in patients with a defunctioning stoma [13]. Together with substantial underreporting of anastomotic leaks to the registry [41], the indirect effect of leakage on stoma outcome is probably underestimated in the present report as some patients with a permanent stoma may actually have suffered from leakage, although this was not reported to the registry. This might have resulted in an artificially stronger effect on stoma permanence, attributed to the construction of a defunctioning stoma.
Although observational in its nature, several methodological considerations constitute strengths of the present report. While causality is difficult to prove in observational settings, we attempted to quantify unbiased effect estimates using prospectively collected data. With the help of directed acyclic graphs, the causal structure of the research question was defined and used to build statistical models with judicious confounder adjustment, and to clarify different pathways conveying the effect of interest. Additionally, sensitivity analyses were performed to assess plausible sources of error, but also to test assumptions as to when stoma reversals occur in practice. Moreover, the population-based study design limits the risk of selection bias, while the nationwide coverage of almost a decade's worth of surgical management reflects current practice in Sweden, resulting in high external validity and a large sample size.
While routine use of defunctioning stomas remains subject to controversy, attempts to quantify the actual long-term effects of temporary stoma construction on stoma outcomes have been sparse [25]. Although mitigating consequences of an anastomotic leak after low anterior resection with TME [18], the role of temporary faecal diversion in PME for high rectal cancer remains unclear. Together with the large variation in the use of defunctioning stomas [11], it is conceivable that some surgeons do not even consider patients undergoing PME for temporary faecal diversion at all. Nevertheless, figures from a previous chart-reviewed Swedish cohort demonstrate that 34% of patients undergoing PME received a defunctioning stoma [25], which is slightly less than our estimation of 51%. Again, misclassifications of patients treated with TME as PME are likely to have occurred and might contribute to the more frequent use of defunctioning stomas observed in the present report. Speculatively, the beneficial effects of defunctioning stomas derived from TME trials may to some extent have been extrapolated to also include PME surgery in Swedish practice. Thus, since the leakage incidence is lower for high rectal cancer and PME [15, 43], the more pronounced effect of defunctioning stoma on the permanent stoma rate was an expected finding.
Assuming that the present study findings are valid, the pathway from a primary defunctioning stoma to a permanent stoma outcome warrants further explanation. First, though anastomotic leakage itself is a major driver for stoma permanence [2], the protective effect of faecal diversion on the permanent stoma rate, by decreasing leakage occurrence, seemed to be small in the present report. This somewhat counterintuitive observation may to some extent be explained by earlier data indicating that defunctioning stomas merely delay, rather than reduce, leak rates [13]. Second, a more advanced tumour stage has also been shown to increase permanent stoma rates [2], while it seems reasonable to assume that cancer recurrence or disease progression, as well as unwillingness to undergo further surgery, may all be putative mechanisms when a primary defunctioning stoma remains in place. Thus, there are many events following anterior resection for rectal cancer predisposing to a non-reversal of a primary defunctioning stoma, whereas the present study findings suggest that there is a lesser need for creating a secondary stoma in those not defunctioned at the index operation.
In a recent study by Blok et al. results from an institutional shift from routine use of defunctioning stomas to highly selective diversion of low anastomosis after TME were reported [44]. Although the figures were derived from a single-centre experience, omission of routine diversion did not increase the rate of anastomotic leaks or the unintentional stoma rate. Moreover, all patients had tumours located within 7 cm from the anal verge, therefore with an expected higher risk of anastomotic leakage [15], as well as poor anorectal functioning [17], compared to tumours emerging in the upper rectum. Permanent stomas were also more frequent in the group undergoing routine diversion, also in line with our findings on stoma outcomes in TME patients. However, while the authors report some circumstances that entailed stoma construction in the highly selective stoma group, several previous studies on leakage prediction have been inconclusive [10]. Although robust selection criteria, crucial to implement a tailored defunctioning stoma strategy, remain elusive, our results suggest that most patients undergoing PME are probably better served without a defunctioning stoma. Some of these patients, including those with significant preoperative bowel dysfunction or a high likelihood of major anterior resection syndrome, might even fare better with a primary permanent stoma operation, for example, Hartmann's procedure, as there are known long-term consequences such as renal impairment due to loop ileostomies [45]. For illustrative purposes, we therefore also present permanent stoma risk figures for groups based on their baseline characteristics, although these estimates merit cautious interpretation.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
All authors fulfilled the ICMJE criteria for authorship.
ETHICAL APPROVAL
The research reported herein was approved by the ethical review board at Umeå University.
DATA AVAILABILITY STATEMENT
Upon reasonable request, data and methodology, including R software code, can be shared. This also applies to the registry-based data used in the present report, while access to such data might be subject to external review by the Swedish Colorectal Cancer Registry steering committee.