The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) burden of care study: Analysis of local treatments for lung metastases and systemic chemotherapy in 220 patients in the PulMiCC cohort
ClinicalTrials.gov Identifier: NCT01106261.
Funding information
Cancer Research UK, Grant/Award Number: C7678/A11393.
Abstract
Aim
The aim of this work was to examine the burden of further treatments in patients with colorectal cancer following a decision about lung metastasectomy.
Method
Five teams participating in the Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) study provided details on subsequent local treatments for lung metastases, including the use of chemotherapy. For patients in three groups (no metastasectomy, one metastasectomy or multiple local interventions), baseline factors and selection criteria for additional treatments were examined.
Results
The five teams recruited 220 patients between October 2010 and January 2017. No lung metastasectomy was performed in 51 patients, 114 patients had one metastasectomy and 55 patients had multiple local interventions. Selection for initial metastasectomy was associated with nonelevated carcinoembryonic antigen, fewer metastases and no prior liver metastasectomy. These patients also had better Eastern Cooperative Oncology Group scores and lung function at baseline. Four sites provided information on chemotherapy in 139 patients: 79 (57%) had one to five courses of chemotherapy, to a total of 179 courses. The patterns of survival after one or multiple metastasectomy interventions showed evidence of guarantee-time bias contributing to an impression of benefit over no metastasectomy. After repeated metastasectomy, a significantly higher risk of death was observed, with no apparent reduction in chemotherapy usage.
Conclusion
Repeated metastasectomy is associated with a higher risk of death without reducing the use of chemotherapy. Continued monitoring without surgery might reassure patients with indolent disease or allow response assessment during systemic treatment. Overall, the carefully collected information from the PulMICC study provides no indication of an important survival benefit from metastasectomy.
What does this paper add to the literature?
Lung metastasectomy has generally been reported in isolation with survival explicitly attributed to surgery and without reference to other treatments. Further metastasectomies and ablations are frequent, and chemotherapy is very often given with and without metastasectomy. This study adds an overview of the total management in a prospective cohort study.
INTRODUCTION
A 5-year survival rate of 40% following lung metastasectomy was reported in a meta-analysis of 2925 colorectal cancer (CRC) patients in 25 studies [1]. An editorial in the European Journal of Cardio-thoracic Surgery put this at 60% based on selected reports [2]. A consensus statement from the US Society of Thoracic Surgeons stated that without metastasectomy ‘survival is assumed to be zero’ [3]. The increased survival attributable to metastasectomy was thus widely believed to be up to 60%.
The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) study has reported an analysis of the full cohort of 512 patients. Of these, 263 nonrandomized patients had metastasectomy and 128 did not. Most if not all of the difference in the 5-year survival rates of 47% and 22%, respectively, appeared to be largely related to selection, with differences in the number of metastases, carcinoembryonic antigen (CEA), liver disease, age, lung function and performance status [4]. In the randomized controlled trial (RCT) nested within it, comparative survival, quality of life and health utility were also reported [5-7]. The RCT showed no difference in survival at any time point, but with 93 patients could not exclude the possibility of a small long-term survival benefit [5]. Taken together the PulMiCC RCT and the cohort preclude the assumption of zero survival [3] and show that any survival benefit from metastasectomy is much smaller than has been claimed. The recently published analysis of Surveillance, Epidemiology and End Results database also found no difference in survival associated with lung metastasectomy [8].
Since the publication of PulMiCC, commentators have shifted their emphasis towards local control of metastatic disease, including other ablative treatments, repeated interventions and chemotherapy [9, 10]. This includes the concept that lung metastasectomy spares patients the side effects of systemic treatments by providing a chemotherapy ‘holiday’ [11].
In this paper we report on second and subsequent local interventions for lung metastases and the use of systemic chemotherapy in subsets of patients treated at five of the PulMiCC study sites from which data were available for these treatments. This analysis suggests how the impression of benefit has arisen in uncontrolled analyses of survival. These data may make a useful contribution to the research initiative of the Association of Coloproctology of Great Britain and Ireland (ACPGBI) [12] and the IMPACT initiative (Improving Management of Patients with Advanced Colorectal Cancer) of the ACPGBI [13].
METHOD
Details of the methods of the PulMiCC study have been published [5, 6]. The UK National Research Ethics Service (NRES) granted ethical approval (10/H0720/5) and recruitment began at each site after approval from the local ethics committees. One-year follow-up, adverse events and the date and cause of death were collected on case report forms covered by Stage 1 enrolment written informed consent. In February 2019 the NRES gave ethical approval for an audit of clinical care.
Five trial sites, including the four largest recruiting centres, provided records of additional treatments. The selection of these sites and the collection of their data were carried out without prior knowledge of the treatments or outcomes, but solely on the availability of data (Table 1).
| No. of metastasectomies | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | ≥2 | |||||
| (a) Baseline characteristics | |||||||
| Sex | |||||||
| Totala | 51 | 114 | 55 | ||||
| Men | 36 | 71 | 34 | ||||
| Women | 15 | 43 | 21 | ||||
| % Men | 71% | 62% | 62% | ||||
| Prior liver metastasectomy | |||||||
| Total | 46 | 105 | 54 | ||||
| Yes | 23 | 32 | 18 | ||||
| No | 23 | 73 | 36 | ||||
| % Yes | 50% | 30% | 33% | ||||
| Solitary metastasis (%) | |||||||
| Total | 46 | 104 | 53 | ||||
| Solitary | 13 | 72 | 17 | ||||
| Multiple | 33 | 34 | 36 | ||||
| % Solitary | 28% | 68% | 32% | ||||
| ECOG PS | |||||||
| Total | 48 | 104 | 52 | ||||
| Zero | 27 | 62 | 42 | ||||
| One | 18 | 42 | 10 | ||||
| Two | 3 | 0 | 0 | ||||
| % Zero | 56% | 60% | 81% | ||||
| (b) Distributions of characteristic in quartiles | |||||||
| Age (years) | |||||||
| n a | 51 | 114 | 55 | ||||
| Min. | 45.4 | 35.3 | 30.8 | ||||
| 0.25 | 61.9 | 60.1 | 56.4 | ||||
| Med. | 66.9 | 65.4 | 64.7 | ||||
| 0.75 | 74.3 | 72.7 | 69.3 | ||||
| Max. | 85.6 | 85.6 | 81.7 | ||||
| CEA (ng/ml) | |||||||
| n a | 32 | 70 | 36 | ||||
| Min. | 1 | 0.3 | 1 | ||||
| 0.25 | 2 | 1.6 | 2 | ||||
| Med. | 4.3 | 2.7 | 2.6 | ||||
| 0.75 | 11.2 | 4.2 | 4.5 | ||||
| Max. | 57 | 151 | 23 | ||||
| Months since CRC resection | |||||||
| n a | 43 | 106 | 51 | ||||
| Min. | 1.6 | 1.4 | 5.6 | ||||
| 0.25 | 13.7 | 15.3 | 16 | ||||
| Med. | 25.3 | 26.1 | 25.3 | ||||
| 0.75 | 43.1 | 38.9 | 34.6 | ||||
| Max. | 82.6 | 89.4 | 91 | ||||
| No. of metastases | |||||||
| n a | 46 | 106 | 53 | ||||
| Min. | 1 | 1 | 1 | ||||
| 0.25 | 1 | 1 | 1 | ||||
| Med. | 2 | 1 | 2 | ||||
| 0.75 | 4 | 2 | 3 | ||||
| Max. | 9 | 9 | 8 | ||||
| FEV1 (litres) | |||||||
| n a | 42 | 109 | 50 | ||||
| Min. | 1.2 | 0.7 | 1.4 | ||||
| 0.25 | 1.9 | 2.2 | 2.2 | ||||
| Med. | 2.8 | 2.7 | 2.7 | ||||
| 0.75 | 3.3 | 3.4 | 3.4 | ||||
| Max. | 4.4 | 5.1 | 5.3 | ||||
| %FEV1 predictedb | |||||||
| n a | 42 | 108 | 49 | ||||
| Min. | 45 | 26 | 53 | ||||
| 0.25 | 73 | 86 | 78 | ||||
| Med. | 87 | 101 | 92 | ||||
| 0.75 | 103 | 111 | 111 | ||||
| Max. | 136 | 145 | 148 | ||||
- Abbreviations: CEA, carcinoembryonic antigen; CRC, colorectal cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; FEV, forced expiratory volume; Max., maximum; Med., median; Min., minimum.
- a The number for whom data were available. Some measurements such as CEA, ECOG and lung function tests were not always deemed necessary by clinical teams. Information for patients referred from other sites, for example the date of the primary colorectal cancer operation, was sometimes incomplete. Selection analyses were made on available data without interpolation.
- b Forced expiratory volume in the first second and its percentage of the predicted value for sex, age and height.
For comparative analyses the patients were divided into three groups: (1) no lung metastasectomy; (2) one lung metastasectomy; (3) second and subsequent lung metastasectomy and other local interventions.
The PulMiCC protocol allowed for other local interventions, including radiotherapy, stereotactic radiotherapy and image-guided thermal ablation.
Selection analyses
To examine the factors used in selection, this analysis excluded randomized patients. Univariate and multivariate analyses based on logistic regression were used to assess which baseline factors were associated with elective surgery. Similarly, logistic regression was used to examine what baseline factors predict a second metastasectomy, or other intervention, if a patient has had one metastasectomy.
Intervention modelling
To model the pattern of interventions over time for all patients in the PulMICC elective groups, a multistate model in continuous time was fitted. The model included five states: (1) entry; (2) metastasectomy only; (3) second intervention undertaken; (4) three or more interventions undertaken; (5) death. Patients were assumed to move progressively through the first four states with a possibility of death while in any of states 1 to 4. The model is represented in Figure 1.
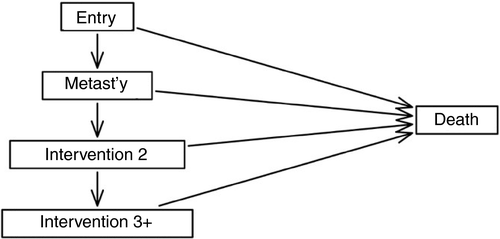
Separate (transition) rates, using a time scale in years, were estimated for moving through the first four states and for death rates from these states. For all rates a Markov assumption was made of constant rates over the follow-up period, except for the transition to the metastasectomy state that was allowed to change after 1 year (estimated to be a lower rate). The model was used to estimate the probability of being in each state at various follow-up times, both from the entry state and from the metastasectomy state. Maximum likelihood estimation of the model was implemented using the R package ‘msm’ [14, 15]
Survival analysis
For illustrative and descriptive purposes, Kaplan–Meier estimates of survival were prepared for the three groups. For a more detailed inspection of patients having just one metastasectomy operation and for those who had a second metastasectomy or other local treatments with various times of origin, a Cox relative regression model [16] was also fitted to compare the rate of death for patients with one metastasectomy with that of patients having had a second.
Exploration of guarantee-time bias
When classifying patient groups by events which occur some time after enrolment to the study—such as having one metastasectomy or having two or more interventions for metastases—the problem of guarantee-time bias (GTB) arises [17]. In comparing patients who had no metastasectomy with those who had one or more operations, the classifying events occur at various times after enrolment. In order to study the effect graphically, the life line displays as suggested by Maringe and colleagues have been adopted and adapted [18]. The three groups defined above were used to classify patients. The time components are colour coded in horizontal bars and the bars are stacked according to the length of the lines which end in either death or censoring.
RESULTS
Baseline data
The data collected during the PulMiCC trial for the five trial sites were used for this analysis of further treatments from 220 patients (see Table S1 in the Supporting Information). There was a high level of completion of baseline data with 1249/1329 (95%) present for the six items potentially available for each of 220 patients. Demographics, patient performance data and oncological characteristics are presented in Table 1 in the three specified groups. Numbers of alternative local interventions used to treat lung metastases are set out in Table 2.
| Surgery | Radiotherapy | SABR | RFA | Totals | |
|---|---|---|---|---|---|
| No metastasectomy | 51 | ||||
| First lung metastasectomy | 114 | 0 | 0 | 0 | 114 |
| Two local interventions | 36 | 4 | 2 | 2 | 44 |
| Three local interventions | 5 | 0 | 1 | 3 | 9 |
| Four local interventions | 0 | 0 | 0 | 2 | 2 |
| Total | 155 | 4 | 3 | 7 | 220 |
- Abbreviations: RFA, radio frequency ablation; SABR, stereotactic radiotherapy.
Selection analyses
The distributions and frequency of patient performance and oncological factors are in Table S2 and these baseline data were used in selection analyses. These results are provided in Tables S3 and S4.
Oncological factors that made metastasectomy significantly less likely were history of a prior liver resection, elevated CEA and more than two lung metastases. Patient factors making metastasectomy significantly less likely were impaired Eastern Cooperative Oncology Group (ECOG) performance status (PS) and poorer lung function. In multivariate analyses, if ECOG PS is not included then log(CEA), the number of metastases and a history of liver metastases remain influential but numbers for the analyses are reduced. If ECOG PS is included, the same variables remain significant but only the highest category for the number of metastases is significant.
There was a smaller influence of baseline factors on selection for a second metastasectomy for those patients who had an initial metastasectomy. Note that, in general, the influence of baseline factors, summarized in Table 2, on decision-making would have been reduced by the subsequent course of events and the current clinical and oncological status of the patient. A higher ECOG PS score (worse) is associated with a smaller likelihood of a second metastasectomy but there is a reversal of the effect of baseline metastasis counts on decision-making. Patients with more lung metastases at baseline were more likely to have second metastasectomy, which is contrary to the influence of metastasis count on the decision about a first metastasectomy, perhaps because it is quite a different process that is being examined.
Multistate model
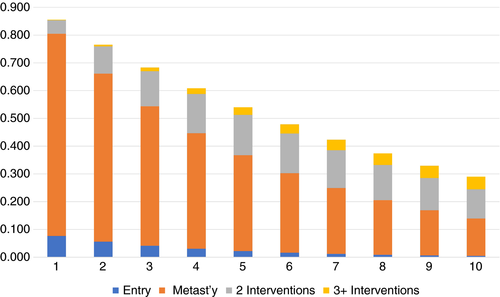
The observed data on state changes for the 220 patients which were used to fit the multistate model are in Table S5. The multistate model was used to provide the estimates of being in the various model states, including death, 1 to 10 years after cohort entry (Table S6, Figure 2). Similar estimates are provided in Table 3 for follow-up after an initial metastasectomy. The model represents patients who did not have a metastasectomy as remaining in the entry state or as having moved to the death state from the entry state. Tables S6 and Figure 2, as well as Table 3, show the expected increasing probability of having multiple interventions and of death as follow-up increases. For example, after 5 years, 38.6% of metastasectomy patients are expected to be alive and not to have had a second surgical intervention while 40.6% are expected to have died. Seventeen per cent are estimated to be alive and to have had only a second operation, while only 3.5% are estimated to have had more than two metastasectomies and still be alive.
| Year | One metastasectomy | Two interventions | Three or more interventions | Death | ||
|---|---|---|---|---|---|---|
| 1 | 0.826 | 0.081 | 0.003 | 0.089 | ||
| 2 | 0.683 | 0.132 | 0.010 | 0.175 | ||
| 3 | 0.565 | 0.159 | 0.018 | 0.258 | ||
| 4 | 0.467 | 0.172 | 0.027 | 0.334 | ||
| 5 | 0.386 | 0.174 | 0.035 | 0.406 | ||
| 6 | 0.319 | 0.168 | 0.042 | 0.471 | ||
| 7 | 0.263 | 0.159 | 0.047 | 0.531 | ||
| 8 | 0.218 | 0.147 | 0.051 | 0.585 | ||
| 9 | 0.180 | 0.134 | 0.053 | 0.633 | ||
| 10 | 0.149 | 0.120 | 0.054 | 0.677 | ||
Kaplan–Meier analyses
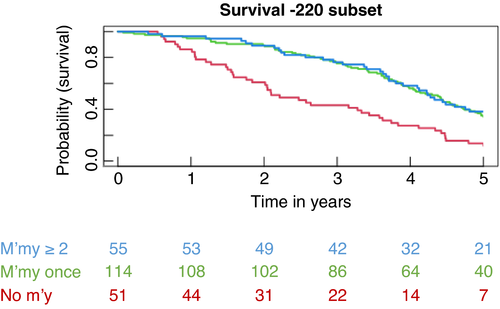
Estimated survival curves for the three groups, presented in Figure 3, show the similarity in the pattern of survival of patients in the two groups who had metastasectomy and the difference from those who did not. These curves serve to illustrate the data but they cannot be formally compared because the definition of the groups is based on events after the time of origin, which introduces GTB.
Estimated survival for patients who had just one metastasectomy and those who had two and more interventions are again depicted in Figure 4(A) and (B). The first is a comparison of patients who had one and those who had two or more metastasectomies, with both groups having a time of origin of the date of their first operation. In the second, the time of origin is the date of their defining metastasectomy, that is their first metastasectomy for those only having one and their second for the rest.
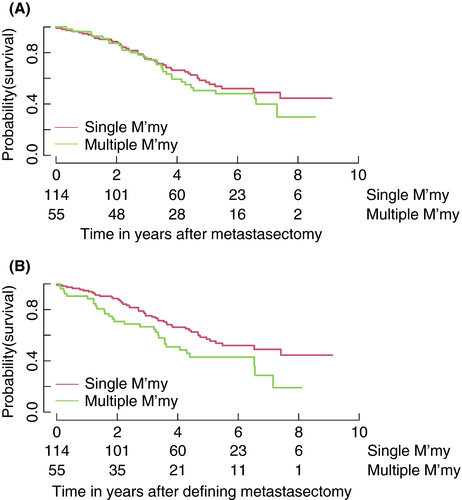
Again, these curves serve to illustrate the data but they cannot be formally compared because the definition of the groups is based on events after the time of origin. However, a formal, and appropriate, comparison of the risk of death after a second metastasectomy compared with that for patients at a comparable time after their first metastasectomy, but not having had a second, can be undertaken using a time-dependent indicator for the second metastasectomy in a Cox relative risk regression model. This leads to an estimated hazard ratio (HR) of 1.63 (95% CI 1.03–2.58; p = 0.04). Patients who had a second operation are at greater risk of death. In this observational comparison, all that can be said is that the second metastasectomy does not alter the risk of death to be equivalent to that of patients not selected to have the second intervention.
Guarantee-time bias in stacked life line diagrams
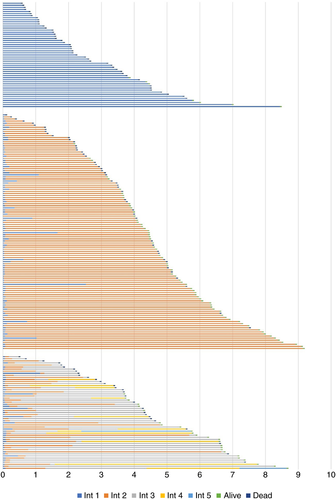
Figure 5 represents the lives of the 51, 114 and 55 patients in the three respective groups. Their baseline characteristics are in Table 1. For all patients the origin is at the time of entering the study and blue represents the metastasectomy-free lifetime. The median interval before metastasectomy for the 169 patients who had a metastasectomy was 22 days (range 0–607, interquartile range 20–35). For 14 patients the metastasectomy-free interval from enrolment was more than 6 months and for five of them it was more than a year. All these intervals add to the overall GTB. Orange represents the lifetime after a first metastasectomy and successive colours the time before a subsequent intervention, as shown in the legend. Patients alive at study close or censored are marked in green and deaths in dark blue.
Chemotherapy usage
Of the further subset of 139 patients at four sites for whom data were available, 79 (57%) had chemotherapy, with a total of 179 courses (Table 4). The 139 patients are grouped in the first column according to the number of interventions (zero to four) divided horizontally according to the number of courses of chemotherapy. Only 7/139 patients were recorded as having neither metastasectomy nor chemotherapy. These counts may be underestimates because unrecorded treatments may have been given elsewhere, but the recorded information, giving dates and named drugs, is probably reliable and unlikely to be an overestimate of chemotherapy use. The identifiable drugs used were bevacizumab, capecitabine, fluorouracil, irinotecan and oxaliplatin in various combinations. The Sankey diagram (Figure 6) illustrates the difficulty of unravelling any meaningful link between treatment and effect.
| No. of chemotherapy courses | No. of patients | 0 | 1 | 2 | 3 | 4 | 5 | TCC | CPP |
|---|---|---|---|---|---|---|---|---|---|
| No metastasectomy | 28 | 7 | 4 | 4 | 1 | 12 | 0 | 63 | 2.25 |
| Only one metastasectomy | 74 | 34 | 21 | 7 | 6 | 4 | 2 | 79 | 1.07 |
| Second local intervention | 32 | 17 | 6 | 5 | 0 | 4 | 0 | 32 | 1.00 |
| Third local intervention | 4 | 1 | 2 | 0 | 1 | 0 | 0 | 5 | 1.25 |
| Fourth local intervention | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 |
| Total | 139 | 60 | 33 | 16 | 8 | 20 | 2 | 179 |
- Abbreviations: CPP, courses per patient; TCC, total chemotherapy courses.
- Note: These are the data behind the Sankey flow chart (Figure 6). This does not include adjuvant therapy at the time of primary resection. These are treatments within the PulMiCC study so represent treatments for advanced colorectal cancer. A total of 139 patients had 179 courses. Patients who have not had a metastasectomy have about twice the number courses of chemotherapy per patient of those having one or more interventions. (p < 0.001, Poisson test). Further metastasectomy operations do not appear to make any further difference to chemotherapy usage (p = 0.74, Poisson test).
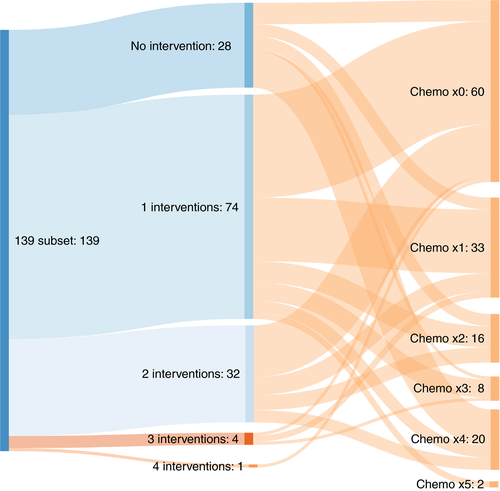
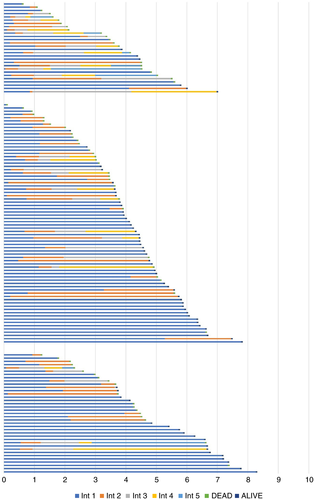
Figure 7 shows the 139 patients in the three groups, but here the colour changes indicate successive courses of chemotherapy. There are patients who had one or two metastasectomies who did not have chemotherapy and had relatively long survival. We cannot attribute any causal relationships but it does indicate that these patients were intensively treated with chemotherapy.
DISCUSSION
The fundamental limitation of this report is that no causal relationship can be deduced between survival time and additional local or systemic treatments. But it is clear that a second metastasectomy and/or use of chemotherapy is evidence of progressive disease. Among the 220 patients in this analysis 33.3% had a further metastasectomy. In our analysis, a Cox model demonstrated that a significantly greater likelihood of death remained after a second intervention.
The PulMICC data provide an explanation for an impression of benefit. GTB is an umbrella term for forms of bias which include immortal-time bias [17]. The effect was seen in the early days of heart transplantation when the worst affected patients died waiting for an available heart, thus inflating the apparent benefit [19]. This was illustrated in an analysis of liver resection data in the English National Health Service. The shape of the survival curve was in marked contrast to the cancer survival curves in the same publication [20]. The effect can be seen in Figure 3 and is explained in the stacked life lines (SLL) diagram in Figure 5. The top group containing patients who were selected to not have metastasectomy, and whose survival is measured from study entry, shows an initial steeper fall which then flattens. This is familiar in cancer registry data. The other curves show the effect of GTB because the interventions by which patients were classified into the two groups occurred some time after the date of registration from which their survival is measured [18]. This may result from the ‘test of time’ which is either explicitly or inherently part of the process of selecting patients for metastasectomy or ablation [21]. The SLL diagrams and the Kaplan–Meier survival plots (Figure 3) clearly illustrate GTB, a common source of bias in observational studies of lung metastasectomy.
Lung metastasectomy for CRC has been increasing and there are some recently published data on the rate of lung metastasectomy operations for CRC in the English National Health Service [22]. Between 2005 and 2013, 173,354 individuals had a colorectal resection, and of these 3434 (2%) had a lung resection within 3 years. If all lung resections were for CRC lung metastasis that would be the upper bound on the rate of lung metastasectomy. It remains highly selective in the UK but in an Editorial in the European Journal of Cardio-thoracic Surgery it is seen as ‘pillar of modern thoracic surgery’ and its effectiveness is ‘assumed’ by the US Society of Thoracic Surgeons (with recognition that there had been no trials before PulMiCC).
While PulMiCC was recruiting, a detailed systematic review was published of CRC survival gains with systemic treatments during the period from 1993 to 2015 [23]. The conclusion was ‘Gains from first-line therapies have been modest but consistent; however, gains from second-line therapies have been disappointing’. The authors also addressed the interaction between systemic treatments and metastasectomy, writing ‘Finally some may argue that while indeed more metastasectomies are being performed, they have been made possible by better therapies and that this benefit should be ascribed to the therapies’. This implies that any association between metastasectomy and longer survival may be reverse causation: that longer survival with more effective chemotherapy provides an opportunity to do more operations. Again, it is not possible to draw inferences about effectiveness but the cohort study provided an opportunity to examine practice. The PulMiCC data suggest that the use of chemotherapy has not been adequately protocolized and that the interplay between systemic treatments and local therapies is not clear. The use of chemotherapy has been studied in the Hospital Episode Statistics (HES) data and the National Bowel Cancer Audit, and the comparison suggests under-recording in the HES [24]. An outstanding example of an integrated system of assessing these patients has recently been reported from Finland [25]. However, without large and collaborative controlled trials we will not produce conclusive answers and we will fail to ensure that our patients have proven treatments and are spared the harm of treatments that are not beneficial. The IMPACT initiative is ideally placed to lead on such research in the treatment of metastatic cancer [13]
Lung metastases make little contribution to the lethal effects of CRC. Local control in the chest is largely irrelevant to survival or palliation. Survival is determined by extrathoracic disease and lung metastases are and remain asymptomatic in most patients. Lung metastases are blood borne and are usually a component of systemic disease, which is arguably better treated systemically. Once this is recognized, lung metastases may be viewed as a means of monitoring disease progression and its response to systemic treatments. The presence of easily measurable metastases may in fact allow chemotherapy to be delayed in patients with indolent disease. Metastases might also be selectively biopsied to inform the use of targeted drugs [26]
Taken together, all the evidence from the PulMiCC cohort study [4] and the nested RCT [5-7] undermines the belief that pulmonary metastasectomy significantly improves survival or symptoms. This study does not support the suggestion that, in practice, repeated metastasectomy spares patients systemic chemotherapy (Table 4). It would instead be entirely appropriate to use lung metastases, as the most readily imaged site of disseminated CRC, to monitor disease progress. Reassurance for patients with slow-growing disease might spare them chemotherapy at that stage and the response to treatment when needed could be more easily monitored.
ACKNOWLEDGEMENTS
Alison Hardwick, Aman Coonar, Amanda Stone, Belinda Lees, Benjamin Hyams, Bina Shah, Brian Davidson, Charlotte Jacobs, Christopher Rollinson, Cinzia Baldini, David Tsang, Elinor Pegg, Elizabeth Radford, Ian Morgan, Ingrid Potyka, Jacqueline Connell, Jakub Kablec, Jaqui Currie, Jo Allen, Joel Dunning, John Edwards, Julie Alderton, Jurjees Hasan, Kay Cash, Kerry Goodsell, Lucy Stelfox, Mark Cutting, Michael Shackcloth, Monica Narasingham, Rebecca Boyles, Robert Rintoul, Roxanne Todd, Sarah Feeney, Sarah Milgate, Simon Grumett, Trevor Thompson, Victoria Hughes, Victoria Lake. Many research staff and clinicians helped by returning data and responding to queries during the ten years of the study and its analysis. With apologies to those not mentioned we are grateful to those listed here.
CONFLICT OF INTEREST
None of the authors has a conflict of interest with respect to any of the contents of this submission.
AUTHOR CONTRIBUTIONS
Trial design, leadership and management: TT, VF, FM, LF, PL. Principal investigators: TB, MM, JK, YZ. Trial coordination: CBG, NRW. Manuscript drafting and editing: TT, VF, FM. All authors have seen draft versions and have approved the final version as submitted.
ETHICAL STATEMENT
Ethics approval was granted by the National Research Ethics Committee London – Hampstead 10/H0720/5. Approval for follow-up as an audit of practice 11 February 2019 HAMPSTEAD, NRES Committee, London (HEALTH RESEARCH AUTHORITY).
Open Research
DATA AVAILABILITY STATEMENT
Data are available on application to the corresponding author.