SteC and the intracellular Salmonella-induced F-actin meshwork
Abstract
Salmonella enterica serovars infect a broad range of mammalian hosts including humans, causing both gastrointestinal and systemic diseases. Following uptake into host cells, bacteria replicate within vacuoles (Salmonella-containing vacuoles; SCVs). Clusters of SCVs are frequently associated with a meshwork of F-actin. This meshwork is dependent on the Salmonella pathogenicity island 2 encoded type III secretion system and its effector SteC. SteC contains a region with weak similarity to conserved subdomains of eukaryotic kinases and has kinase activity that is required for the formation of the F-actin meshwork. Several substrates of SteC have been identified. In this mini-review, we attempt to integrate these findings and propose a more unified model to explain SCV-associated F-actin: SteC (i) phosphorylates the actin sequestering protein Hsp27, which increases the local G-actin concentration (ii) binds to and phosphorylates formin family FMNL proteins, which enables actin polymerisation and (iii) phosphorylates MEK, resulting in activation of the MEK/ERK/MLCK/Myosin II pathway, leading to F-actin bundling. We also consider the possible physiological functions of SCV-associated F-actin and similar structures produced by other intracellular bacterial pathogens.
Abbreviations
-
- FMNL
-
- formin-like proteins
-
- MLC
-
- myosin light chain
-
- MLCK
-
- myosin light chain kinase
-
- PMLC
-
- phosphorylated myosin light chain
-
- ROCK
-
- rho-activated kinase
-
- SCV
-
- Salmonella-containing vacuole
-
- T3SS
-
- type III secretion system
-
- T3SS2
-
- type III secretion system 2
1 INTRODUCTION
Actin is a major component of the eukaryotic cell cytoskeleton and has many crucial functions including structural support, cell migration and organelle transport. It exists in a highly dynamic flux between globular, monomeric G-actin and filamentous and branched, F-actin. The polymerisation of G-actin into F-actin is initiated by actin-nucleating proteins such as formins and the Arp2/3 complex, usually at membrane surfaces (Mullins, Bieling, & Fletcher, 2018). The actin cytoskeleton is targeted by many bacterial virulence proteins, which can cause both its polymerisation or depolymerisation. This can prevent (Yersinia) or promote (Salmonella, Shigella) internalisation of bacteria into host cells, subvert membrane trafficking (Chlamydia), induce bacterial motility in the cell cytoplasm (Shigella, Listeria) and provide structural support for vacuoles containing bacteria (Coxiella, Chlamydia) (Dowd, Mortuza, & Ireton, 2020; Jimenez, Chen, & Alto, 2016; Scidmore, 2011).
Serovars of Salmonella enterica cause many diseases of birds and mammals, including gastroenteritis and systemic typhoid fever in humans. These infections rely on numerous virulence (effector) proteins translocated into host cells by type III secretion system 1 (T3SS1) and type III secretion system 2 (T3SS2). T3SS1 effectors are translocated across the plasma membrane and induce bacterial uptake into non-phagocytic cells through membrane ruffling (LaRock, Chaudhary, & Miller, 2015). Membrane ruffling is the production of lamellipodia-like protrusions, caused by localised and transient actin polymerisation at the site of bacterial attachment (Hume, Singh, Davidson, & Koronakis, 2017). The process of actin remodelling is coordinated by the actions of several effectors, including SopE and SptP, which have GEF (stimulatory) and GAP (inhibitory) activity, respectively, on the small GTPases Cdc42 and Rac1 (Patel & Galán, 2006). Cdc42 and Rac1 control actin polymerisation through regulators of the Arp2/3 complex (Miki & Takenawa, 2003). These localised membrane ruffles engulf Salmonella, leading to their uptake into the host cell, where bacteria are enclosed in a vacuole derived from the plasma membrane. The Salmonella-containing vacuole (SCV) gradually matures into a compartment with some characteristics of late endosomes. Acidification and low nutrient levels of the SCV lumen trigger the expression of T3SS2 (Beuzón, Banks, Deiwick, Hensel, & Holden, 1999; Deiwick, Nikolaus, Erdogan, & Hensel, 1999). This translocates approximately 30 different effectors across the vacuole membrane into the host cell. T3SS2 effectors have a variety of different targets and functions, enabling nutrient acquisition, bacterial spread, induction of cell death and avoidance or suppression of innate and adaptive immune responses (Jennings, Thurston, & Holden, 2017).
The characteristics of the maturing SCV in host cells have been defined using high-resolution microscopy and flow cytometry (Alpuche-Aranda, Racoosin, Swanson, & Miller, 1994; Garcia-del Portillo & Finlay, 1995; Méresse, Steele-Mortimer, Finlay, & Gorvel, 1999; Rathman, Sjaastad, & Falkow, 1996). These and other studies showed that the mature SCV is an unusual late endosomal-like compartment, containing lysosomal membrane proteins but lacking hydrolytic enzymes that are typical of mature lysosomes. In 2001, a confocal fluorescence microscopy study revealed a meshwork of F-actin surrounding SCVs (Méresse et al., 2001). This meshwork first became apparent approximately 4 h after bacterial invasion and was the result of de novo actin polymerisation, rather than recruitment of pre-existing F-actin filaments. SCV-associated F-actin was dependent on a functional T3SS2 and was detected in human epithelial cells, mouse J774 macrophages and in mouse fibroblasts infected with S. Typhimurium or S. Typhi (Méresse et al., 2001).
The morphology of SCV-associated F-actin varies among different host cells and cell types. In some cells, the meshwork tends to surround (Figure 1, upper panel) or is interwoven between clusters of intracellular bacteria (Figure 1, lower panel), forming a nest-like structure; in other cells, it appears as a condensed focus within a mass of SCVs (Méresse et al., 2001; Miao et al., 2003). SCV-associated F-actin is rarely found around individual and isolated bacteria (Miao et al., 2003). The close association of F-actin with the SCV membrane and with tubular extensions from the SCV (Salmonella-induced filaments; Sifs) suggest that actin polymerisation is initiated at the membrane, as occurs normally in uninfected cells (Brumell, Goosney, & Finlay, 2002; Gov & Gopinathan, 2006; Méresse et al., 2001).
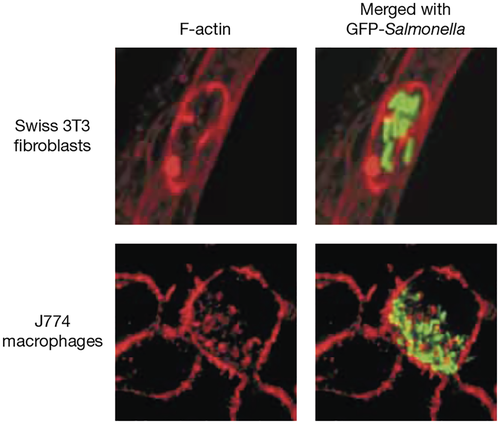
The purpose of this article is to review our understanding of the cause and mechanism of assembly of the intracellular Salmonella-associated F-actin meshwork, and to discuss its physiological significance in relation to bacterial virulence.
2 CHARACTERISATION OF SteC
Infection of mammalian cells with an S. Typhimurium ssaV mutant, which lacks an essential component of the T3SS2 apparatus, showed that the F-actin meshwork requires a functional T3SS2 and suggested that one or more of its effectors are required for its formation (Méresse et al., 2001). However, the use of strains carrying mutations in genes for effectors SseE, SseF, SseG, SseI and SspH2 showed that these proteins are dispensable (Miao et al., 2003). In 2008, our group discovered that the T3SS2 effector SteC is required and sufficient to induce F-actin polymerisation in mammalian cells (Poh et al., 2008). The steC gene (STM1698) was originally identified by a genome-wide reporter screen for translocated effectors and its product was shown to be translocated by the T3SS2 but not T3SS1 (Geddes, Worley, Niemann, & Heffron, 2005). Independently, STM1698 was found to be part of the ssrAB regulon, which controls the expression of T3SS2 and many associated effector genes (Rytkönen et al., 2007). STM1698 was also found in a signature-tagged mutagenesis screen to be important for colonisation of chick intestine by S. Typhimurium (Morgan et al., 2004).
Translocated SteC is localised to SCV membranes and Sifs, consistent with F-actin localisation, which suggests that under physiological conditions, polymerisation is initiated at the SCV membrane (Engelenburg & Palmer, 2010; Méresse et al., 2001; Poh et al., 2008). Initial analysis of the 457 amino acid sequence of SteC revealed weak similarity to subdomains I, II and III of eukaryotic kinases (Figure 2a) and most closely to human serine/threonine-protein kinase RAF-1 (Poh et al., 2008; Wellbrock, Karasarides, & Marais, 2004). However, SteC lacks the conserved central core of the catalytic domain present in other subdomains of eukaryotic kinases, including highly conserved residues in the catalytic loop as well as other motifs, which are important for phosphotransferase activity and substrate recognition (Hanks & Hunter, 1995; Hanks, Quinn, & Hunter, 1988). Nevertheless, biochemical analysis of the purified protein established that SteC has kinase activity, and substitution of a critical ATP-anchoring lysine in subdomain II showed that formation of the SCV-associated F-actin meshwork is dependent on kinase activity (Poh et al., 2008). Structural characterisation of SteC is now required to understand its mechanisms of substrate binding, catalysis and phosphate transfer.
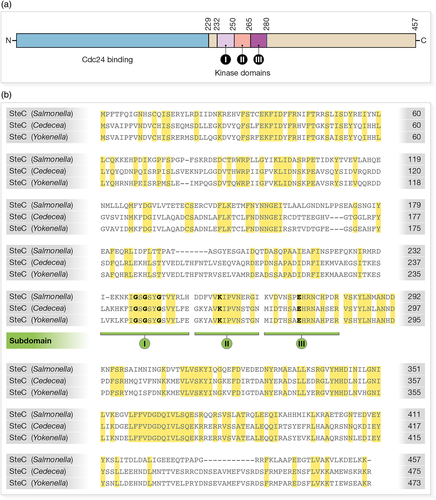
Other bacterial pathogens translocate effector kinases into host cells (Galyov, Håkansson, Forsberg, & Wolf-Watz, 1993; Kim et al., 2005; Pruneda et al., 2014; Teper et al., 2018), but SteC appears to be the only Salmonella T3SS effector with kinase activity. Bioinformatic searches have revealed steC homologues in the poorly-studied organisms Yokenella regensburgei and Cedecea sp. P7760 (Figure 2b). Both Yokenella and Cedecea are members of the Enterobacteriaceae, are opportunistic human pathogens and encode a T3SS (Chan & Tan, 2017; Hu et al., 2017; Kirzinger, Butz, & Stavrinides, 2015). Y. regensburgei has some biochemical similarity to S. enterica and causes a disease similar to enteric fever (Jain et al., 2013; Stock, Sherwood, & Wiedemann, 2004). There is approximately 41% amino acid sequence identity between SteC of Salmonella and the Yokenella and Cedecea proteins, which together share 82% identity (Figure 2b). Amino acids in kinase subdomains I, II and III that are known to be crucial for the activity of other kinases are present in the Yokenella and Cedecea proteins, suggesting that these proteins are active kinases (Figure 2b), although their targets might be different to those of SteC.
3 MECHANISM OF SCV-ASSOCIATED F-ACTIN FORMATION BY SteC
An early study by our group into pathways leading to SCV-associated actin polymerisation did not provide evidence for the involvement of the Arp2/3 complex or either of its upstream effectors, Cdc42 or Rac1 (Unsworth, Way, McNiven, Machesky, & Holden, 2004). We also ruled out a contribution of the mammalian diaphanous-related formin mDia1, which has been implicated in stress fibre formation (Satoh & Tominaga, 2001; Unsworth et al., 2004).
Expression of SteC after transfection of fibroblasts results in the formation of thick cables of F-actin between large foci of highly condensed F-actin (Poh et al., 2008). These structures are very similar in appearance to those produced upon expression of an active version of the Rho effector ROCK, a serine–threonine kinase that induces the formation of stress fibres by activation of myosin II (Katoh, Kano, & Noda, 2011; Poh et al., 2008). ROCK and myosin light chain kinase (MLCK) induce F-actin bundling by phosphorylating myosin light chain (MLC), which can then bind and bundle F-actin (Bresnick, 1999; Gallagher, Herring, Griffin, & Stull, 1991). Since SteC induces stellate F-actin bundles similar to those induced by active ROCK, we examined the involvement of the ROCK/myosin pathway.
In mouse fibroblasts expressing SteC, phosphorylated MLC (pMLC) co-localised with SCV-associated F-actin. We found that SteC-associated F-actin requires the Myosin IIB but not Myosin IIA isoform (Odendall et al., 2012). Knockdown of MLCK reduced SCV-associated F-actin, indicating that MLCK is likely to be regulated by SteC. However, ROCK is unlikely to be involved as both treatment with a ROCK inhibitor, and the combined knockdown of two ROCK isoforms had no effect on SCV-associated actin formation (Odendall et al., 2012; Poh et al., 2008).
The MAP kinase ERK directly phosphorylates MLCK, increasing its ability to phosphorylate MLC and activate Myosin II (Klemke et al., 1997). ERK is regulated through phosphorylation by MEK, which is in turn regulated through phosphorylation by Raf (Roberts & Der, 2007; Shaul & Seger, 2007). As SteC displays weak similarity to C-Raf, the roles of MEK and ERK in SCV-associated actin were investigated. A combination of siRNA-mediated knockdown and knockout cells, and pharmacological inhibitors were used to establish that MEK and ERK are both involved in SCV F-actin assembly (Odendall et al., 2012).
In vitro kinase assays followed by mass spectrometry revealed that SteC phosphorylates MEK1 directly on residue S200. This phosphorylation was predicted by molecular dynamics analysis to displace an inhibitory helix, inducing autophosphorylation of S218/222 of MEK, which is known to cause its activation. Phosphorylation by SteC on S200 therefore represents a non-canonical mechanism of MEK activation. MEK isoforms contributed to SteC-dependent phosphorylation of ERK (Odendall et al., 2012). However, in these experiments, inhibition of MEK or MLCK did not completely abrogate SCV-associated F-actin structures, which suggested that SteC activates one pathway involving MEK, ERK, MLCK and Myosin II, and that a second, independent pathway must exist. Furthermore, although the MEK/ERK/MLCK/Myosin II pathway has an important role in the bundling of F-actin fibres into a meshwork, it does not explain how SteC initiates actin polymerisation.
A subsequent phosphoproteomic study identified HSP27 as a host protein that is phosphorylated by SteC (Imami et al., 2013). HSP27 is an actin monomer-sequestrating protein and its stimulus-driven phosphorylation on residues S15, S78 and S82 induces dissociation, which frees up G-actin monomers for nucleation, thereby promoting F-actin polymerisation (Doshi, Hightower, & Lee, 2009; During et al., 2007; Mounier & Arrigo, 2002). In vitro evidence showed that SteC phosphorylates at least six residues of HSP27 including two of the naturally phosphorylated residues, S15 and S82. Knockdown of HSP27 in cells overexpressing SteC resulted in a significant decrease in F-actin bundles (Imami et al., 2013). Therefore, Hsp27 could provide a source of G-actin for SteC but this is unlikely to initiate polymerisation (Mounier & Arrigo, 2002). Imami et al. (2013) were unable to reproduce the reported effect of the MEK inhibitor PD98059 in reducing SteC-induced F-actin (Odendall et al., 2012). However, Imami et al. (2013) measured F-actin in a HeLa cell line stably overexpressing SteC, whereas Odendall et al. (2012) measured SCV-associated F-actin in infected cells, where translocated SteC is likely to be much less abundant.
Recently, a study investigating S. Typhimurium effector-host protein–protein interactions identified an interaction between SteC and formin family FMNL proteins (Walch et al., 2020). Salmonella-infected FMNL2/3 knockout fibroblasts showed reduced actin bundling, suggesting that SteC initiates F-actin formation through FMNLs (Walch et al., 2020). FMNL proteins initiate actin polymerisation and bundle F-actin (Courtemanche, 2018). Their activation has in some cases been shown to involve the small GTPase Cdc42 (Kühn et al., 2015). FMNLs contain an FH2 domain, which is sufficient to catalyse actin nucleation in vitro (Otomo et al., 2005). However, rather than binding monomeric actin, it is thought that the FH2 domain binds and stabilises dimeric and/or trimeric actin (which normally form and disassociate spontaneously in the cell), thereby initiating polymerisation (Courtemanche, 2018). As mentioned above, the monomeric G-actin pool is tightly controlled in vivo by its sequestration with actin binding proteins such as profilin and Hsp27. Phosphorylation of Hsp27 by SteC could therefore result in a localised increased concentration of free G-actin that could undergo spontaneous dimerization and trimerisation and provide substrates for formin-mediated nucleation.
Walch et al. (2020) showed that SteC phosphorylates FMNL on several residues. A phosphomimetic mutant of one of these increased its binding affinity to Cdc42 (Kühn et al., 2015). Walch et al. (2020) also found that Cdc42 co-purified with SteC and FMNL1. Since FMNL1/2 are substrates of Cdc42 (Kühn et al., 2015), Walch et al. (2020) proposed that phosphorylation of FMNL proteins by SteC could promote interactions with Cdc42 and lead to actin polymerisation via this GTPase. This finding is interesting in light of a previous study that identified Cdc42 as a target of SteC in S. cerevisiae (Fernandez-Piñar et al., 2012). The N-terminal region of SteC, which is not involved in kinase activity, was shown to regulate Cdc42 through binding Cdc24 (Figure 2a), the sole GEF for yeast Cdc42. This interaction did not inhibit its catalytic activity but prevented nuclear localisation of Cdc24, resulting in a downregulation of the signalling pathways under its control (Fernandez-Piñar et al., 2012). In addition, overexpression of SteC in yeast inhibited Cdc42-dependent pathways and resulted in a growth inhibition, which was rescued by overexpressing Cdc42 (Alemán et al., 2009; Fernandez-Piñar et al., 2012). A GST-SteC fusion also bound to the human homologue of Cdc24, Vav1. However, GST-SteC did not alter the exchange activity of Vav1 on Rac1, one of its GTPase substrates (Fernandez-Piñar et al., 2012). Since binding of SteC to Vav1 was not investigated in infected mammalian cells, the possibility remains that binding to Cdc24 and Vav1 in vitro or in yeast are artefacts. Furthermore, the canonical Cdc42-dependent pathway for actin polymerisation involves WASP/WAVE and the Arp2/3 complex (Miki & Takenawa, 2003; Rohatgi et al., 1999;); together with Cdc42, these proteins had been previously excluded from involvement in the SCV-associated F-actin meshwork (Unsworth et al., 2004). However, in the study by Unsworth et al. (2004), the direct involvement of Cdc42 was tested using a dominant negative form of the protein, which is also prone to artefacts. Therefore, further work is warranted to test more rigorously the requirement for Vav1 and Cdc42 in the effect of SteC on SCV-associated F-actin. In addition, the functional importance of SteC-induced phosphorylation within the formin FH2 domain needs to be investigated.
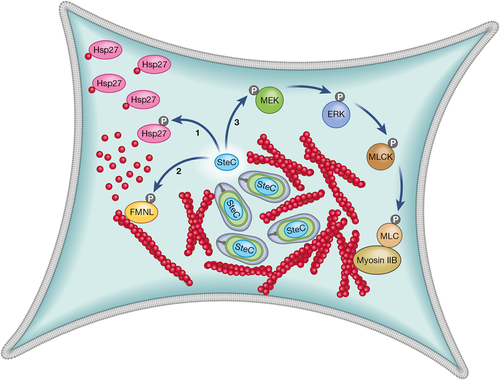
Together, the work described above on Hsp27, FMNL and Myosin II can be incorporated into a tentative but more unified model for the mechanism of action of SteC. We propose that SteC has coordinated action on these proteins. First, SteC phosphorylates Hsp27 in its G-actin bound state; this releases Hsp27, thereby increasing a local concentration of G-actin. SteC independently binds to and phosphorylates FMNL proteins, somehow enabling formin-mediated nucleation of the localised free G-actin and resulting in the production of F-actin filaments. Then, through phosphorylation of MEK and activation of the MEK/ERK/MLCK/Myosin II pathway, SteC coordinates the bundling of these F-actin filaments into larger cables and a meshwork (Figure 3). One problem with this model is that it does not explain the F-actin structures that were detected in 50% of cells lacking Myosin IIB (Odendall et al., 2012). However, in that study, we scored F-actin structures in a binary manner and it is possible that some of those scored as “positive” represented cells with clusters of filaments rather than the more pronounced bundles that are produced by the action of myosins (Odendall et al., 2012). In light of the recent work by Walch et al. (2020), it would be interesting to repeat these experiments, analysing in more detail the type of actin structures that occur in cells lacking Myosin IIB.
4 PHYSIOLOGICAL FUNCTION OF SteC
Prior to the discovery of SteC, actin depolymerising drugs were used to assess the influence of SCV-associated F-actin on the vacuole membrane and intracellular bacterial replication. Results suggested that it helps maintain the integrity of the SCV and is important in bacterial growth in macrophages (Méresse et al., 2001), but it is also possible that the reduced number of wild-type bacteria recovered from macrophages exposed to latrunculin B or cytochalasin D may have been due to increased host cell death after liberation of bacteria into the cytosol, which would stimulate innate immune signalling and pyroptosis (Thurston et al., 2016). Furthermore, a subsequent examination of vacuole membrane integrity using an steC mutant strain ruled out an effect of the protein on vacuole stability (Poh et al., 2008). Myosin II has an important function in host vesicular traffic and fusion events (Bond, Brandstaetter, Sellers, Kendrick-Jones, & Buss, 2011). Therefore, the recruitment of Myosin II and F-actin to the SCV could redirect host vesicular transport. However, if this were the case then one would expect to detect defects in vacuole membrane and possibly intracellular growth of an steC mutant, and so far there is no solid evidence for this (see below). Myosin-containing stress fibres in non-muscle cells are also important for cell migration (Shutova & Svitkina, 2018). Another T3SS2 effector, SseI, is known to interfere with the directional migration of dendritic cells (Brink et al., 2018; Carden et al., 2017). Although an steC mutant was included in a screen for Salmonella effectors that affect dendritic cell migration, it did not pass the threshold that differentiated strains having an effect on this process (McLaughlin et al., 2014). Therefore, it appears unlikely that SteC affects host cell migration – at least in dendritic cells.
Several studies have examined the contribution of steC to intracellular bacterial growth and virulence in vivo. steC (STM1698) was identified as having a possible role in the caecal colonisation of chicks by S. Typhimurium (Morgan et al., 2004). However, the mutant was attenuated in only 50% of infected birds and the possibility of polar effects of the transposon were not excluded. A survey of different T3SS2 effector mutants indicated that although an S. Typhimurium steC mutant had a growth defect in mouse (RAW264.7) macrophages, it was not attenuated at systemic sites following oral inoculation of mice (Buckner, Croxen, Arena, & Finlay, 2011). In our earlier work, we found no evidence for a growth defect in either human epithelial cells or RAW264.7 macrophages (Poh et al., 2008). Furthermore, our analysis of bacterial replication in mouse bone marrow-derived macrophages (using a sensitive method based on fluorescence dilution) also failed to reveal a growth defect of the steC mutant (Figueira, Watson, Holden, & Helaine, 2013). In other work, neither our group (Poh et al., 2008) nor Geddes et al. (2005) detected a virulence defect of an S. Typhimurium steC mutant compared to the wild-type strain in competitive infections after intraperitoneal inoculation of mice. By contrast, in another study from our group, we found that following oral inoculation of mice the numbers of steC mutant bacteria were greater than those of the wild-type strain, and both absence of SteC or expression of a kinase-dead point mutant conferred a growth advantage in epithelial cells and macrophages at late stages of infection (Odendall et al., 2012). Therefore, it is possible that SteC functions to restrain intracellular growth, as has been found for other Salmonella effectors (Baek, Wang, Roland, & Curtiss, 2008; Rosenberger & Finlay, 2002). However, the conflicting results on the contribution of SteC to intracellular growth and virulence – both within and between different research groups – means that more work will have to be done to clarify its contribution to bacterial survival and growth in hosts and host cells.
The steC gene appears to be highly conserved and functional among most serovars of S. enterica that cause gastrointestinal disease, whereas serovars targeting extraintestinal regions frequently contain premature stop codons, frameshift errors or other mutations (Jennings et al., 2017). In this regard, it is interesting that two other bacteria containing steC homologues (Yokenella and Cedecea) are both intestinal colonisers. Together with the virulence studies mentioned above, this supports the idea that SteC might have a specific function in intestinal infection.
The physiological function of SteC and SCV-associated F-actin remains unclear, and it is formally possible that the meshwork is an epiphenomenon – a by-product of another, as yet unidentified but physiologically relevant effect. In the absence of a clear and consistent intracellular growth phenotype, and since phosphorylation of ERK would be predicted to lead to significant changes in gene expression, it would be worthwhile to investigate the effects of SteC on host cell transcriptional responses. It is noteworthy that the recent proteomic study showing that SteC interacts with FMNLs also revealed other interacting proteins that are involved in mRNA splicing (Walch et al., 2020). Follow up validation work on these is needed to establish if SteC has an additional function in the regulation of transcript splicing. However, if the model outlined here (Figure 3) is broadly correct, then the concerted effect of SteC on three different host targets, each contributing to the formation of the F-actin meshwork, makes an “epiphenomenon” explanation very unlikely. The production of SteC is regulated both at the transcriptional level by the SsrA/B two component system (Rytkönen et al., 2007) and also post-transcriptionally by the small RNA PinT (Santos, Bischler, Westermann, & Vögel, 2020), suggesting that the amount of SteC translocated into host cells is very important to its function.
F-actin also assembles around vacuoles containing other bacterial pathogens. Recently it was found that actin polymerises to form a dynamic “cocoon”-like structure around the nascent Shigella vacuole (Kühn et al., 2020). The cocoon forms after bacterial uptake and its disassembly precedes the normal process of vacuole rupture, after which bacteria undergo characteristic F-actin “comet tail” motility in the host cell cytosol (Choe & Welch, 2016). As with Salmonella, the development of the cocoon involves a T3SS, and its assembly seems to be driven through the well-characterised Cdc42/N-WASP/Arp2/3 signalling pathway. Interference with cocoon formation impairs the normal rupturing process, suggesting that it helps to control maturation of the vacuole and its rupture (Kühn et al., 2020). The Chlamydia trachomatis vacuole (inclusion body) is also surrounded by cytoskeletal proteins, including F-actin. Its assembly is driven by RhoA and, unlike the Shigella cocoon, it functions to maintain inclusion integrity (Kumar & Valdivia, 2008). Coxiella burnetii also forms a large vacuole that is associated with F-actin. The use of actin depolymerising agents suggested that the structure might be involved in trafficking of vesicles to and from the vacuole and for its maturation, but further work on the mechanism of assembly is needed to confirm this (Aguilera et al., 2009).
In conclusion, work over the last several years has established that several intracellular bacterial pathogens induce vacuole-associated actin rearrangements. However, a detailed understanding of the physiological consequences of these phenomena is lacking. The discovery that FMNL is a target for Salmonella SteC by Walch et al. (2020) represents a significant advance in terms of understanding its mechanism of action in producing SCV-associated F-actin, and further work based on this and the other targets of SteC will help us understand its significance and overall impact on Salmonella virulence.
ACKNOWLEDGEMENT
We thank members of our laboratory, Dr Charlotte Odendall and two Reviewers for their thoughtful and helpful comments.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study