The Coxiella burnetii effector protein CaeB modulates endoplasmatic reticulum (ER) stress signalling and is required for efficient replication in Galleria mellonella
Funding information: Bundesministerium für Bildung und Forschung, Grant/Award Number: 01KI1726A; Deutsche Forschungsgemeinschaft, Grant/Award Number: LU1357/5-1
Abstract
The obligate intracellular pathogen Coxiella burnetii is the causative agent of the zoonosis Q fever. C. burnetii infection can have severe outcomes due to the development of chronic infection. To establish and maintain an infection, C. burnetii depends on a functional type IVB secretion system (T4BSS) and, thus, on the translocation of effector proteins into the host cell. Here, we showed that the C. burnetii T4BSS effector protein CaeB targets the conserved endoplasmatic reticulum (ER) stress sensor IRE1 during ER stress in mammalian and plant cells. CaeB-induced upregulation of IRE1 RNase activity was essential for CaeB-mediated inhibition of ER stress-induced cell death. Our data reveal a novel role for CaeB in ER stress signalling modulation and demonstrate that CaeB is involved in pathogenicity in vivo. Furthermore, we provide evidence that C. burnetii infection leads to modulation of the ER stress sensors IRE1 and PERK, but not ATF6 during ER stress. While the upregulation of the RNase activity of IRE1 during ER stress depends on CaeB, modulation of PERK is CaeB independent, suggesting that C. burnetii encodes several factors influencing ER stress during infection.
1 INTRODUCTION
The obligate intracellular bacterium Coxiella burnetii is the causative agent of the zoonosis Q fever (Maurin & Raoult, 1999). Infection in humans generally occurs by inhalation of contaminated aerosols. Alveolar macrophages are the first line of defense against C. burnetii and take up the bacterium into a unique phagosome termed the Coxiella-containing vacuole (CCV). The CCV matures into a phagolysosomal-like compartment (Kohler & Roy, 2015). Most bacteria are killed within such a compartment. However, C. burnetii survives this harsh environment. Essential for this is a functional Dot/Icm type IVB secretion system (T4BSS), which is used to translocate effector proteins into the host cell cytoplasm (Beare et al., 2011; Carey, Newton, Lührmann, & Roy, 2011). Approximately 150 T4BSS effector proteins have been identified (Larson et al., 2016), but the mode of action for only a small proportion is known. These effectors have numerous functions: including subversion of vesicle trafficking (Larson, Beare, Howe, & Heinzen, 2013; Latomanski, Newton, Khoo, & Newton, 2016), CCV biogenesis (Kohler et al., 2016), modulation of host gene expression (Weber et al., 2016) or inhibition of host cell apoptosis (Klingenbeck, Eckart, Berens, & Lührmann, 2013; Lührmann, Nogueira, Carey, & Roy, 2010).
Apoptosis or programmed cell death is a tightly regulated and genetically controlled process that occurs in all eukaryotes during development as well as in response to stress or pathogen infection (Lawen, 2003). Apoptosis in mammalian cells can be subdivided into two main pathways: intrinsic and extrinsic. The extrinsic pathway depends on the activation of membrane-bound death receptors via binding of pro-apoptotic ligands, while the intrinsic pathway is activated by intracellular signals such as radiation, hypoxia, free radicals, bacterial and viral infection or the absence of growth factors or nutrients (Elmore, 2007). Activation of the intrinsic apoptosis pathway leads to mitochondrial outer membrane permeabilisation (MOMP), the release of cytochrome c and subsequent cell death induction. In contrast to animals, plants are sessile organisms missing a somatic immune system. Thus, they rely on the innate immunity to defend against pathogens (Dangl & Jones, 2001). In plants, cell death referred to as the hypersensitive response (HR), is part of the innate immunity (Pontier, Balague, & Roby, 1998). Induction of the HR is a mechanism to prevent spread of pathogens by rapid cell death of infected tissue. HR activation is mediated by recognition of microbial effectors by intracellular receptors, initiating a complex signalling cascade that involves changes in protein phosphorylation, production of reactive oxygen species and modification of ion fluxes (Pontier et al., 1998). Despite huge differences between plants and animals, there are distinct features of cell death regulation, which are conserved between the kingdoms. For instance, the pro-apoptotic mammalian Bax protein induces cell death in yeast and plants even though it does not exist in these organisms (Lacomme & Santa Cruz, 1999; Zha et al., 1996).
In the recent years it has become evident that many pathogens target endoplasmatic reticulum (ER) stress signalling pathways to support their pathogenesis (Celli & Tsolis, 2015). ER stress occurs when unfolded or misfolded proteins accumulate in the ER lumen leading to induction of a process called the unfolded protein response (UPR) (Hetz, 2012). ER stress is induced by different abiotic or biotic stress factors such as heat or pathogen infection, and by chemical compounds like tunicamycin (TM). TM inhibits the N-linked glycosylation of proteins and therefore triggers the accumulation of unfolded proteins in the ER (Takatsuki, Arima, & Tamura, 1971). The aim of the UPR is to re-establish ER homeostasis (Walter & Ron, 2011). In animals, the UPR depends on the signalling of the three ER stress sensors: protein kinase RNA (PKR)-like ER kinase (PERK), activating transcription factor 6 (ATF6) and inositol-requiring enzyme 1 (IRE1) (Walter & Ron, 2011). Among those, IRE1 is a key regulator of cell fate (Sano & Reed, 2013) and the most conserved arm of the ER stress sensors, as it also exists in yeast and plants (Chen & Brandizzi, 2013). IRE1 possesses dual enzymatic activities by containing both a serine/threonine kinase and endoribonuclease (RNase) domain. Activation of IRE1 induces a conformational change which activates the RNase domain, promoting the unconventional splicing of X-box-binding protein 1 (XBP1)-specific mRNA to produce XBP1s mRNA which is then translated into an active transcription factor in mammalian cells (Yoshida, Matsui, Yamamoto, Okada, & Mori, 2001). XBP1s binds the promoter regions of several genes involved in restoration of cellular homeostasis (Lee, Iwakoshi, & Glimcher, 2003). Similarly, in planta, the RNase domain of IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce an active transcription factor (Nagashima et al., 2011). Under prolonged ER stress conditions, when protein-folding homeostasis cannot be restored, UPR signalling shifts to promote apoptosis in mammalian cells (Hetz & Papa, 2018). However, the molecular switch from the adaptive phase to apoptosis induction is not completely understood. Several studies support the idea that PERK and IRE1 signalling largely contribute to apoptosis induction (Hetz & Papa, 2018). Sustained activation of IRE1 may lead to recruitment of tumour necrosis factor receptor associated factor 2 (TRAF2), which subsequently leads to activation of apoptosis signal-regulating kinase 1 (ASK1). This activates Jun-N-terminal kinase (JNK) and p38 MAPK that stimulates pro-apoptotic pathways (Ron & Hubbard, 2008). The execution of ER stress-induced cell death largely depends on the intrinsic apoptotic pathway (Urra, Dufey, Lisbona, Rojas-Rivera, & Hetz, 2013). As C. burnetii is able to inhibit intrinsic host cell apoptosis (Lührmann & Roy, 2007) it seems likely that C. burnetii is able to also prevent ER stress-induced apoptosis using its repertoire of T4BSS effector proteins. Indeed, a very recent publication suggests that this is the case (Brann, Fullerton, & Voth, 2020). However, the T4BSS effector protein(s) involved in the inhibition of the ER stress-induced apoptosis was not identified.
The aim of this study was therefore to investigate whether C. burnetii interferes with ER stress signalling and if the T4BSS effector protein CaeB plays a role therein.
CaeB (CBU1532) was identified as a T4BSS effector protein of C. burnetii by Carey et al. in 2011 and was shown to inhibit intrinsic apoptosis by preventing mitochondrial outer membrane permeabilisation (Klingenbeck et al., 2013). Initially ectopically expressed CaeB was described to co-localize with mitochondrial marker proteins and to induce aggregation of mitochondria (Carey et al., 2011). In later reports, ectopic expression of CaeB displayed a clear co-localisation with the ER (Rodriguez-Escudero et al., 2016). Thus, the exact localisation of CaeB in host cells remains to be clarified. Here, we analysed the molecular mechanism of CaeB in more depth and in the context of an infection. In addition, we analysed the ER stress induction during infection with C. burnetii wild type and ΔcaeB mutant. Our results indicate that C. burnetii modulates ER stress signalling and reduces ER stress-induced cell death. The effector protein CaeB is involved in these activities. Moreover, analysis of an in vivo infection model revealed that CaeB is required for full pathogenicity.
2 RESULTS
2.1 CaeB localizes at the ER
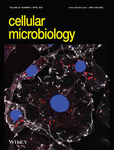
To determine the subcellular localisation and molecular activity of CaeB, we chose the plant Nicotiana benthamiana as a model system. We hypothesised that CaeB is also functional in planta, since CaeB acts downstream of Bax (Klingenbeck et al., 2013) and Bax induces cell death in planta (Lacomme & Santa Cruz, 1999). In addition, the plant model system allowed us to concentrate on evolutionary conserved pathways. To investigate the subcellular localisation of CaeB in planta, N. benthamiana leaves were infiltrated with agrobacteria containing plasmids to express GFP-tagged CaeB or an established ER marker fused to mCherry (Nelson, Cai, & Nebenfuhr, 2007). Two days post infiltration leaf discs were analysed by confocal laser scanning microscopy. Co-expression of both proteins resulted in a clear co-localisation at the ER (Figure 1a) similar to the results by Rodriguez-Escudero et al. (2016). As ER-mitochondria contact sites are involved in cell death modulation (van Vliet, Verfaillie, & Agostinis, 2014) and co-localisation of CaeB with mitochondria has been shown (Carey et al., 2011), we infiltrated N. benthamiana leaves with agrobacteria containing plasmids to express GFP-tagged CaeB and the established mitochondria marker IVD (Isovaleryl-coenzyme A dehydrogenase) fused to mCherry. Confocal microscopy analysis showed neither co-localisation nor association of GFP-CaeB with IVD-mCherry (Figure 1a). Similarly, in HeLa cells, we could detect partial co-localisation of GFP-CaeB with the ER marker calnexin, but not with mitotracker (Figure S1). The Pearson's correlation coefficient was 0.6 for GFP-CaeB and calnexin, but 0.0 for GFP-CaeB and mitotracker (Figure S1). These data suggest that GFP-CaeB partially localizes to the ER.
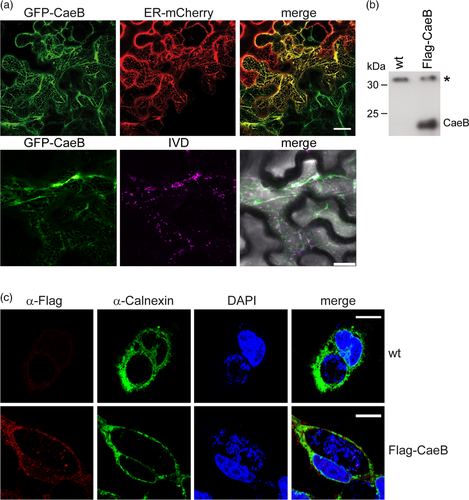
Next, we analysed the subcellular localisation of CaeB when translocated by C. burnetii. Therefore, we generated a C. burnetii strain expressing 3xFlag-tagged CaeB (Figure 1b) and determined its localisation in infected mouse embryonic fibroblasts (MEFs) by confocal microscopy. A signal for 3xFlag-CaeB was hardly detectable in the infected cells. However, if we detected a signal it showed ER localisation (Figure 1c), demonstrated by co-localisation with the ER marker calnexin. Thus, translocated CaeB also localizes to the ER during infection.
2.2 CaeB suppresses cell death in N. benthamiana induced by Bax or Pto + AvrPto
To determined whether CaeB also suppresses cell death in planta, we analysed the effect of CaeB expression in N. benthamiana challenged with different cell death inducing proteins. First, we investigated if CaeB interferes with Bax-induced cell death in plants, as in mammalian cells CaeB inhibits staurosporine-induced apoptosis downstream of Bax-activation (Klingenbeck et al., 2013). N. benthamiana leaves were transiently transformed using agrobacteria to mediate the expression of Bax in the presence or absence of GFP-tagged CaeB. Since the presence of Bax results in a rapid cell death response, Bax expression was kept under control of a dexamethasone (Dex) inducible promotor, which was activated 1 day post infiltration by spraying leaves with Dex. As expected, leaves expressing Bax showed a strong and fast induction of cell death, which was visible by the formation of necrotic lesions (Figure 2a). In contrast, co-expression of GFP-CaeB resulted in a clear suppression of Bax-induced cell death as there were no cell death symptoms visible after 2 days post Bax induction (Figure 2a). To quantify the degree of cell death over time, leaf discs were sampled and carefully transferred to water. Subsequently, the loss of membrane integrity of leaf discs was measured as changes in conductivity of the surrounding water, which is indicative of ion leakage. This confirmed rapid induction of Bax-induced cell death and its suppression by CaeB as indicated by the significantly lower increase in conductivity (Figure 2b). Thus, CaeB suppresses Bax-induced cell death in mammalian cells (Klingenbeck et al., 2013) and in planta (Figure 2a,b).
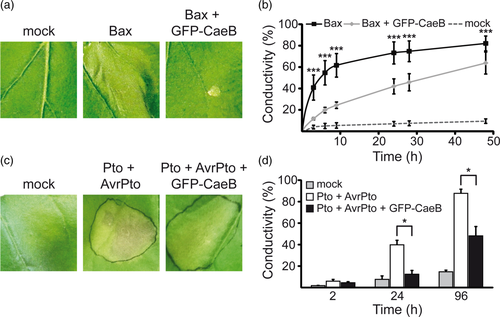
Next, cell death was induced by co-expression of the Pseudomonas syringae type III secretion system (T3SS) effector protein AvrPto and the tomato serine/threonine kinase Pto which recognises AvrPto; leading to the induction of R-gene mediated cell death in N. benthamiana (Frederick, Thilmony, Sessa, & Martin, 1998). However, how the AvrPto-Pto-interaction leads to R-protein activation, and how this causes cell death remains still elusive (Balint-Kurti, 2019; Martin, 1999). It is known that the kinase activation domain of Pto is important for AvrPto recognition (Frederick et al., 1998). In addition, kinase activity of Pto is required for the induction of the HR response (Sessa, D'Ascenzo, & Martin, 2000), for example, by Pto-mediated phosphorylation of Pti1 (Zhou, Loh, Bressan, & Martin, 1995).
In fact, co-expression of both proteins caused a clear induction of cell death, which was inhibited by the presence of GFP-CaeB (Figure 2c). Visual changes were confirmed via ion leakage measurement of leaf discs expressing Pto and AvrPto with or without GFP-CaeB (Figure 2d). These results demonstrate that expression of CaeB suppresses cell death in N. benthamiana induced by different stimuli, indicating that CaeB might target an evolutionarily conserved pathway to inhibit cell death. Although, it is currently unknown, which cell death pathways are induced by Bax or AvrPto-Pto expression in N. benthamiana.
2.3 CaeB inhibits ER stress-induced cell death in N. benthamiana
Since CaeB co-localizes with the ER and has global cell death suppression activity we analysed if CaeB interfered with ER stress-induced cell death in N. benthamiana. Leaves expressing the ER-mCherry marker alone or together with GFP-CaeB were treated with tunicamycin (TM). After 24 hr, structural changes in the ER network were inspected with a confocal laser scanning microscope. TM treatment led to the disruption of the typical ER network structure in ER-mCherry expressing leaves, whereas only minor changes in the net-like ER structure were detectable in cells expressing GFP-CaeB (Figure 3a). To clarify that this is not only a general consequence of the CaeB-mediated inhibition of cell death, we analysed the ER structure in TM-treated N. benthamiana leaves expressing the Pseudomonas syringae T3SS effector protein HopG1. Like CaeB, HopG1 was shown to prevent Bax-induced cell death in N. benthamiana (Block et al., 2010; Jamir et al., 2004). However, HopG1 did not prevent disruption of the ER network after TM treatment (Figure S2). Thus, CaeB prevents ER stress and, thus, directly protects the cell from TM-induced structural disturbances in planta.
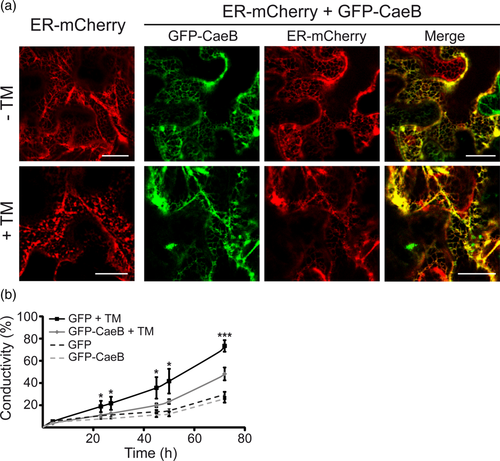
We further investigated the role of CaeB in ER stress signalling in planta by introducing prolonged ER stress. N. benthamiana leaf discs expressing either GFP or GFP-CaeB were floated on an aqueous solution containing either TM or DMSO as a control and cell death development via ion leakage measurement was monitored over a period of 72 hr. Under control conditions leaf discs expressing GFP or GFP-CaeB showed only a slight increase in conductivity over time. In contrast, GFP expressing leaf discs treated with TM displayed a stronger increase in conductivity (Figure 3b) compared to GFP-CaeB expressing leaf discs, indicating that CaeB reduces ER stress-induced cell death in planta (Figure 3b).
2.4 CaeB suppresses cell death in mammalian cells
Intrigued by the results obtained in N. benthamiana, we analysed the ability of CaeB to suppress ER stress-induced cell death in mammalian cells. Therefore, HEK293T cells stably expressing GFP or GFP-CaeB (Figure S3) were treated with TM and the cleavage of poly (ADP-ribose) polymerase (PARP) was analysed as a well-known readout for apoptosis induction (Oliver et al., 1998). TM treatment led to PARP cleavage in GFP expressing cells, but not in GFP-CaeB cells (Figure 4a,b), indicating that CaeB prevents ER stress-induced apoptosis. In addition, the expression of GFP-CaeB prevents PARP cleavage after staurosporine (Klingenbeck et al., 2013) or etoposide treatment (Figure 4c). Both agents activate apoptosis via the mitochondrial apoptotic pathway, suggesting that CaeB might influence not only ER, but also mitochondrial activity.
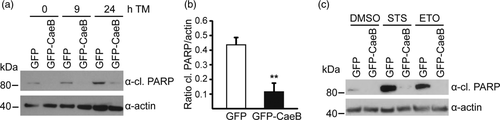
2.5 CaeB modulates IRE1 signalling during ER stress conditions in HEK293T cells
In mammalian cells, ER stress triggers the activation of the unfolded protein response (UPR), which is controlled by three major sensors: IRE1, PERK and ATF6 (Walter & Ron, 2011). Therefore, we analysed the effect of CaeB on PERK, ATF6 and IRE1 signalling. Stable GFP or GFP-CaeB HEK293T cells were treated with TM for different periods of time and western blot analysis using antibodies against ER stress marker proteins were used to determine the role of CaeB in ER stress responses. First, we analysed whether CaeB alters PERK signalling. Activation of PERK leads to oligomerisation and trans-autophosphorylation (Hetz & Papa, 2018). Active PERK phosphorylates eIF2a on serine 51, which results in global repression of translation initiation (Walter & Ron, 2011). However, some transcripts (e.g., ATF4) are translated more efficiently during PERK-dependent translation repression. ATF4 is normally inefficiently translated, whereas translation is very efficient in response to PERK activation (Hetz & Papa, 2018). Thus, we analysed the level of ATF4 in cells expressing either GFP or GFP-CaeB after ER stress induction. As shown in Figure 5a, TM treatment led to a similar accumulation of ATF4 in the presence of both GFP and GFP-CaeB, indicating that CaeB does not influence ATF4 protein levels.
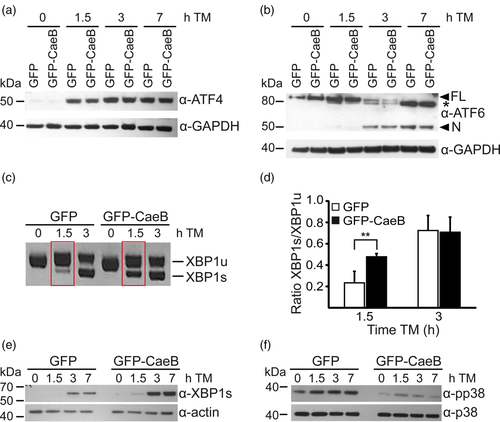
Next, we determined whether CaeB alters ATF6 signalling during ER stress. The transmembrane glycoprotein ATF6 is exported to the Golgi upon ER stress and is cleaved by two Golgi-resident proteases, releasing its cytosolic domain (Haze, Yoshida, Yanagi, Yura, & Mori, 1999). Under ER stress, the cleaved cytosolic domain of ATF6 translocates to the nucleus to induce UPR gene expression (Yamamoto et al., 2007). As shown in Figure 5b TM treatment led to similar cleavage of ATF6 in cells expressing GFP or GFP-CaeB, indicating that CaeB does not influence ATF6 activation.
We also analysed the influence of CaeB on IRE1 signalling during ER stress. TM treatment of GFP and GFP-CaeB cells led to splicing of XBP1. However, splicing of XBP1 mRNA was significantly enhanced in GFP-CaeB expressing cells at earlier time points after TM induction compared to GFP control cells (Figure 5c,d). In addition, the amount of XBP1s protein was increased in GFP-CaeB cells following TM treatment as compared to GFP expressing cells (Figure 5e). Thus, GFP-CaeB cells showed an earlier and higher rate of XBP1 mRNA splicing compared to GFP expressing cells after ER stress induction in mammalian cells. As activation of IRE1 may also lead to phosphorylation of downstream targets like p38 we analysed activation of p38 after TM treatment. The expression of GFP-CaeB resulted in a reduced p38 phosphorylation after TM treatment compared to controls (Figure 5f). Taken together, these data demonstrate that CaeB exclusively modulates the IRE1 signalling branch during ER stress while leaving the signalling of the UPR arms PERK and ATF6 unaltered.
2.6 CaeB modulates IRE1 signalling during ER stress conditions in N. benthamiana
UPR signalling is mediated by different ER stress sensors of which IRE1 was shown to be the most conserved between species (Chen & Brandizzi, 2013). Because we showed that CaeB expression modulates IRE1 signalling in mammalian cells and that CaeB is able to suppress ER stress-induced cell death in planta, we asked whether CaeB also modulates the activity of IRE1 in planta. IRE1 is responsible for the unconventional splicing of bZIP60 mRNA in N. benthamiana (Nagashima et al., 2011). Thus, the splicing of bZIP60 mRNA, the functional homologue of the mammalian XBP1, was analysed after TM treatment of N. benthamiana leaves expressing either GFP or GFP-CaeB. GFP-CaeB expressing leaves showed a significantly higher amount of spliced bZIP60 mRNA at early time points after ER stress induction (Figure 6a), indicating that CaeB alters the RNase activity of IRE1.
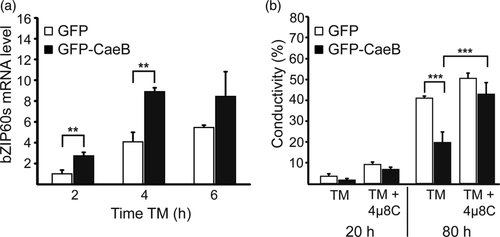
Next, we asked whether the CaeB-mediated increase in the RNase activity of IRE1 is required for the inhibition of ER stress-induced cell death. To specifically inhibit RNase activity of IRE1 without interfering with dimerization and phosphorylation of IRE1 we used 4μ8C, a small molecule inhibitor (Cross et al., 2012), which was successfully used before in Arabidopsis thaliana seedling (Zhang, Zhang, & Wang, 2016). As 4μ8C has never been used in N. benthamiana we initially adjusted experimental conditions in which 4μ8C prevents bZIP60 mRNA splicing without influencing cell viability. As shown in Figure S4 concentration above 2 μM and below 5 μM fulfils this requirement. Therefore, N. benthamiana leaf discs expressing either GFP or GFP-CaeB were floated on an aqueous solution containing TM or DMSO as a control with or without 2.5 μM 4μ8C and cell death development was analysed after 20 and 80 hr of treatment via conductivity measurement. As shown in Figure 6b, GFP-CaeB expressing leaf discs showed a significantly lower increase in conductivity after TM treatment as compared to GFP expressing leaf discs, confirming the results shown in Figure 3b. Simultaneous treatment of TM and 4μ8C strongly increased the conductivity in GFP-CaeB producing leaves, indicating that the RNase activity of IRE1 is important for CaeB-mediated inhibition of ER stress-induced cell death.
2.7 C. burnetii infection alters IRE1 signalling during ER stress
Our results indicate that CaeB modulates ER stress signalling by altering the signalling of the conserved ER stress sensor IRE1. Therefore, we wanted to address the question if IRE1 signalling is also modulated during C. burnetii infection. Therefore, HeLa229 cells were infected with C. burnetii and 3 days post-infection ER stress was induced by TM treatment. Importantly, C. burnetii infection alone did not induce activation of p38 (Figure 7a) or splicing of XBP1 mRNA (Figure 7b). However, treatment with TM resulted in a reduced activation of p38 and increased splicing of XBP1 mRNA in C. burnetii infected cells compared to uninfected cells (Figure 7a,b). This led us to conclude that C. burnetii infection modulates IRE1 activity similar to CaeB in TM treated cells.

2.8 C. burnetii-mediated modulation of ER stress signalling partially depends on CaeB
To investigate the role of CaeB in C. burnetii-mediated modulation of IRE1 signalling during ER stress, a CaeB deletion mutant (ΔcaeB) was generated using a newly developed nutritional selection system, which is based on lysine auxotrophy (Beare, Jeffrey, Long, Martens, & Heinzen, 2018). The ΔcaeB mutant is capable of growing in axenic culture, infecting and forming replicative CCVs in HeLa cells (Figure S5a–c). We then determined whether the infection with wild type or ΔcaeB C. burnetii leads to an altered signalling of ER stress sensors in presence or absence of ER stress induction. As shown in Figure 8, infection of HeLa cells with wild type or ΔcaeB bacteria did not alter the signalling of PERK, IRE1 or ATF6 in control conditions. In contrast, induction of ER stress by TM application resulted in activation of all three ER stress sensors in infected and uninfected cells. However, infection resulted in reduced ATF4 production under ER stress, suggesting decreased activation of PERK signalling during C. burnetii infection (Figure 8a and Figure S5). Processing of ATF6 was not changed by infection after TM treatment (Figure 8b). Phosphorylation of p38 was decreased (Figure 8c and Figure S5e) and XBP1 splicing increased in cells infected with C. burnetii (Figure 8d,e), demonstrating a shift of IRE1 activity towards the pro-survival signalling arm. Infection with C. burnetii ΔcaeB also resulted in reduced ATF4 induction, suggesting that other C. burnetii factors are involved in modulation of PERK signalling. Importantly, increased splicing of XBP1 was not detectable in cells infected with the CaeB deletion mutant, demonstrating that CaeB is required for upregulation of XBP1 splicing during ER stress. Furthermore, C. burnetii ΔcaeB only partially reduced activation of p38 (Figure 8c and Figure S5e), suggesting that in addition to CaeB other bacterial proteins influence p38 activation.
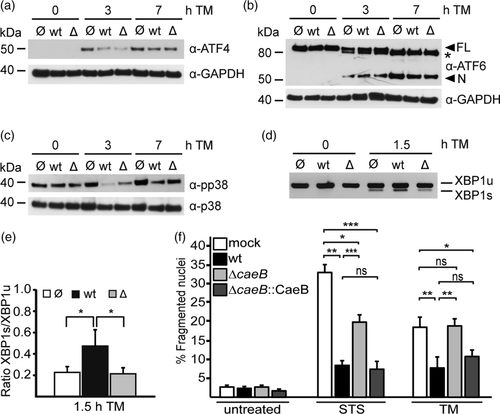
Together these data suggest that CaeB is essential for C. burnetii-mediated upregulation of XBP1 splicing during ER stress. Furthermore, the data reveal that CaeB in cooperation with other factor(s) is involved in the inhibition of p38 phosphorylation. In addition, the results suggest that C. burnetii modulates PERK, but not ATF6 activation independent of CaeB during ER stress.
2.9 The ΔcaeB mutant has impaired anti-apoptotic activity
As the ΔcaeB mutant is impaired in the upregulation of IRE1 during ER stress conditions, we wondered whether the ΔcaeB mutant is influenced in its ability to prevent apoptosis induction. Thus, we infected HeLa cells with either wild type, a CaeB deletion mutant or a CaeB deletion mutant complemented with HA-tagged CaeB for 3 days. The cells were treated with TM to induce ER stress-mediated cell death or with staurosporine to induce intrinsic apoptosis. Wild type C. burnetii inhibited TM- as well as staurosporine-induced cell death (Figure 8f). In contrast, the ΔcaeB mutant only partially diminished apoptosis induced by staurosporine and completely failed to prevent TM-induced cell death. Importantly, the complemented strain restored the anti-apoptotic activity of C. burnetii (Figure 8f). This data demonstrate that CaeB participates in the anti-apoptotic activity of C. burnetii.
2.10 The ΔcaeB mutant is attenuated in the Galleria mellonella in vivo model
To unravel whether CaeB is important for C. burnetii pathogenesis, we investigated the role of CaeB using the in vivo G. mellonella model, which was used before to study C. burnetii pathogenesis (Kohler et al., 2016; Martinez et al., 2016; Norville et al., 2014). Larvae were injected in the upper right proleg with either wild type C. burnetii or the ΔcaeB mutant and survival rate was monitored over 10 days. Larvae infected with the ΔcaeB mutant survived significantly longer as larvae infected with the wild type bacteria (Figure 9a). This attenuation in virulence was not mediated by a reduced infection rate (Figure 9b). However, we observed reduced replication and reduced size of the CCV in larvae infected with the ΔcaeB mutant (Figure 9c–e), suggesting virulence of C. burnetii lacking CaeB is attenuated in vivo. Whether this attenuation is mediated by reduced IRE1 activation is unclear and needs further study.
3 DISCUSSION
Pathogens interfere with a wide range of different signalling pathways to overcome host cell-mediated defense mechanisms such as cell death (Friedrich, Pechstein, Berens, & Lührmann, 2017). Induction of cell death is a common and conserved strategy in human and plant cells to efficiently repel microbial invaders. In many cases, microbes hijack the host cell apoptosis machinery by injecting effector proteins into host cells (Robinson & Aw, 2016). Given the importance of effector proteins, their functional characterisation provides profound insight into bacterial pathogenicity mechanisms as well as into cellular host processes. However, these proteins often act in concert with each other or have redundant functions (Agbor & McCormick, 2011; Ghosh & O'Connor, 2017), making defined functions difficult to unravel. Hence, numerous bacterial effector proteins were analysed in model systems, which contributed to a better understanding of their mode of action. Saccharomyces cerevisiae is the most widely used model system (Popa, Coll, Valls, & Sessa, 2016; Siggers & Lesser, 2008), but also Caenorhabditis elegans (Alegado, Campbell, Chen, Slutz, & Tan, 2003), Drosophila melanogaster (Botham, Wandler, & Guillemin, 2008), or plants such as N. benthamiana (Üstun, Muller, Palmisano, Hensel, & Bornke, 2012) have been successfully used to elucidate the molecular activity of bacterial effector proteins. Here, we also used N. benthamiana as a model system, which allowed us to confine the pathways to be analysed.
In this work, we provide novel insight into the molecular activity of the C. burnetii T4BSS effector protein CaeB. Initially, this 16.5 kDa effector was described to display mitochondrial localisation when ectopically expressed in mammalian cells leading to cell rounding and mitochondria aggregation (Carey et al., 2011). Thus, it is assumed that CaeB impairs cell viability. Contrary to this, CaeB was also shown to be a potent inhibitor of apoptosis by preventing mitochondrial outer membrane permeabilisation (Klingenbeck et al., 2013). In more recent reports, localisation of CaeB was re-evaluated and shown to co-localize with the ER (Rodriguez-Escudero et al., 2016). Similarly, we observed co-localisation of GFP-CaeB with the ER, but not with mitochondria (Figure S1). The reason for the different subcellular localisation observed for CaeB (ER versus mitochondria) is unknown, as similar cell lines and fusion-tags were used. However, this is not the first report about different subcellular localisation of an effector protein. Thus, the subcellular localisation of CBU0077 (MceA) was shown to be lysosomal, when ectopically expressed as a Flag-tag fusion protein (Carey et al., 2011). Similarly, the expression of HA-CBU0077 was shown to co-localize with the lysosomal marker protein LAMP-1, but expressed GFP-tagged CBU0077 showed mitochondrial localisation (Rodriguez-Escudero et al., 2016). Importantly, also Flag-CBU0077 produced and translocated by C. burnetii into the host cell associated with mitochondria (Fielden et al., 2017). One possibility for the different subcellular localisation of the effector proteins MceA and CaeB might be due to different folding states. Thus, the fusion tag as well as the place of expression, production within the eukaryotic cell versus bacteria, might influence the folding and/or post-transcriptional modification of the effector protein.
Since S. cerevisiae has been proven to be a powerful tool to investigate effector protein function, several C. burnetii effector proteins were analysed in yeast, but faced the problem of heterologous protein aggregation and proteostatic stress (Rodriguez-Escudero et al., 2016). In order to characterize the effector CaeB in more detail we utilised the plant N. benthamiana as a tool. Expression of CaeB in N. benthamiana leaves resulted in clear co-localisation with an ER marker protein, but not with a mitochondria marker protein (Figure 1a). In addition, 3xFlag-CaeB translocated by C. burnetii during infection into MEF cells localised to the host cell ER (Figure 1c), providing independent evidence that CaeB is targeted to the ER, where it prevented disruption of the ER under tunicamycin-induced ER stress conditions (Figure 3a). Furthermore, CaeB expression resulted in suppression of plant cell death induced by mammalian Bax expression or by Pto/AvrPto interaction (Figure 2). This indicates that CaeB, despite the evolutionary divergence of plants and animals, displays the same biological activity and subcellular localisation in plant cells as in mammalian cells.
Based on our observations that CaeB is an ER-localised protein with anti-apoptotic activity we investigated whether CaeB interferes with ER stress signalling. The ER is important for lipid and protein synthesis, calcium storage and the cellular stress response. Cells attempt to maintain their ER homeostasis under stress conditions by activating the UPR pathway (Almanza et al., 2019). The UPR aims to restore homeostasis by reducing protein synthesis, degradation of misfolded proteins and upregulation of target genes, which enhance the folding capacity (Hetz & Papa, 2018). Under prolonged stress conditions UPR signalling shifts towards apoptosis (Hetz & Papa, 2018). In mammalian cells the UPR depends on the signalling of PERK, ATF6 and IRE1 (Hetz, 2012). In plants, homologues for ATF6 and IRE1 have been described (Wan & Jiang, 2016), whereas in yeast only the IRE1 pathway has been identified (Wu, Ng, & Thibault, 2014), making IRE1 the most conserved UPR branch across eukaryotes.
Recent research uncovered that several pathogenic bacteria modulate ER stress response in both animal and plant hosts (Celli & Tsolis, 2015; Korner, Du, Vollmer, & Pajerowska-Mukhtar, 2015), supporting the idea that the establishment of the UPR plays an important role in plant and human immunity. For instance, infection of N. benthamiana with Pseudomonas cichorii resulted in upregulation of bZIP60 mRNA level (Tateda et al., 2008). Several mammalian pathogens also target ER stress signalling to subvert immune responses (Celli & Tsolis, 2015). Both Legionella pneumophila and Brucella spp., which create ER-like compartments in their host cells, interfere with ER stress signalling. The Brucella spp. T4BSS effector proteins TcpB and VceC induce ER stress signalling to support intracellular replication of Brucella (de Jong et al., 2013; Smith et al., 2013). L. pneumophila infection itself leads to ER stress induction, which in turn is inhibited by the effector proteins Lgt1 and Lgt2. This prevents XBP1 splicing to reduce ER stress caused by infection (Hempstead & Isberg, 2015; Treacy-Abarca & Mukherjee, 2015). In contrast to Brucella spp. and L. pneumophila, C. burnetii establishes a lysosome-derived vacuole (CCV), nevertheless the interaction between the CCV and the ER seems to be important for pathogenesis (Justis et al., 2017). Indeed chemical inhibition of the UPR antagonises the expansion of the CCV (Brann et al., 2020). Several C. burnetii effector proteins have been identified by a yeast-based interaction screen to target ER host proteins (Wallqvist et al., 2017). One ER localised C. burnetii T4BSS effector protein is ElpA (Graham, Winchell, Sharma, & Voth, 2015; Weber et al., 2013), which triggers disruption of ER structure (Graham et al., 2015). Other intracellular pathogens also utilise the T4BSS to target the ER or ER functions (Tsai, English, & Tsolis, 2019). Here, we showed that CaeB prevents tunicamycin-induced apoptosis and selectively modulates IRE1 signalling during tunicamycin treatment, while leaving PERK and ATF6 signalling unaltered (Figures 4 and 5). CaeB expression under ER stress inhibited the IRE1-mediated phosphorylation of p38 (Figure 5f), while RNase activity of IRE1 was increased, as shown by accelerated splicing of XBP1 mRNA (Figure 5c,d). Importantly, an early onset of XBP1 splicing is protective for the cell (Walter, Schmid, Dussmann, Concannon, & Prehn, 2015) and the concurrent reduction of p38 activation prevents pro-apoptotic activities (Sano & Reed, 2013). Thus, we reason that the CaeB-mediated shift in IRE1-activity contributes to promote cellular homeostasis under stress conditions.
IRE1 is a conserved ER stress sensor and the mechanism by which IRE1 mediates the unconventional cytoplasmic splicing of its target transcription factors are conserved between eukaryotes (Chen & Brandizzi, 2013). In plants, IRE1 catalyses the unconventional splicing of bZIP60 mRNA to generate the active transcription factor (Nagashima et al., 2011). Here, we showed that CaeB also altered the signalling of plant IRE1 (Figure 6a). Moreover, our data demonstrated that CaeB suppressed the ER stress-induced cell death in N. benthamiana and modulated the RNase activity of plant IRE1 by increasing splicing of bZIP60 mRNA (Figures 2 and 6a). It was previously shown that A. thaliana seedlings lacking ire1 or bzip60 are more sensitive to TM induced ER stress, with the ire1a/b double mutant being most affected (Mishiba et al., 2013). To analyse if the enhanced splicing of bZIP60 is essential for CaeB mediated ER stress-induced cell death suppression, we inhibited splicing of bZIP60 using the IRE1 class III inhibitor 4μ8C (Zhang et al., 2016). This led to increased cell death in N. benthamiana leaf discs independent of CaeB expression, indicating that CaeB activity relies on the modulation of IRE1 RNase signalling to suppress ER stress-induced cell death (Figure 6b). However, whether CaeB-mediated alteration of IRE1-signalling is also involved in CaeB-dependent inhibition of Bax-induced cell death is unclear and requires further research.
Studies analysing the signalling of IRE1 in eukaryotes revealed that components acting upstream of IRE1 activation are highly conserved (Zhang et al., 2016; Zhang, Chen, Brandizzi, Verchot, & Wang, 2015). This all together reveals that the IRE1 signalling pathway shows striking similarities across kingdoms.
About 150 T4BSS effector proteins have been identified for C. burnetii so far (Larson et al., 2016). Analysis of the function of C. burnetii effector proteins uncovered that these effectors modulate diverse host cell signalling pathways. Some of the C. burnetii effector proteins like CBU0372, CBU1576, CpeD, the pathotype-specific effector ElpA (CbuD1884) and CaeB were described to co-localize with the host cell ER (Graham et al., 2015; Rodriguez-Escudero et al., 2016; Voth et al., 2011; Weber et al., 2016). A recent study also showed that C. burnetii recruits the host protein ORP1L to the CCV to influence membrane dynamics of the CCV and interactions with the ER (Justis et al., 2017). During infection, C. burnetii establishes a huge CCV which largely expands into the host cell cytoplasm (Schulze-Luehrmann et al., 2016) suggesting a great demand on host membranes. The effector ElpA disrupts ER structure and secretory transport, and therefore influences ER function (Graham et al., 2015). Based on these data one could assume that C. burnetii infection induces ER stress signalling. Indeed, very recently, it was shown that C. burnetii induces PERK, but not ATF6 and IRE1 activation in THP-1 cells (Brann et al., 2020). Interestingly, we did not observe any activation of the host UPR during infection with C. burnetii in HeLa cells (Figure 8), suggesting that the induction and modulation of ER stress signalling is cell type specific. Therefore, it will be crucial in the future to determine the impact of C. burnetii on ER stress signalling in primary macrophages, their primary host cells. In HeLa cells infection with C. burnetii might either not induce ER stress or actively prevent ER stress induction. For the latter argues the fact that C. burnetii infection selectively modulated UPR signalling under ER stress conditions (Figure 8). While there were no alterations to ATF6 processing in infected cells compared to uninfected cells under ER stress, C. burnetii infection resulted in reduced expression of ATF4 under ER stress conditions, suggesting that C. burnetii interferes with PERK activation or signalling (Figure 8a and Figure S5d). This is very interesting in the light of recent findings, showing an increased level of phosphorylated eukaryotic initiation factor α (eIF2α), induced by PERK activation, is required for C. burnetii growth (Brann et al., 2020). Furthermore, C. burnetii infection led to reduced phosphorylation of p38 and increased splicing of XBP1 during ER stress, suggesting that C. burnetii modulates IRE1 signalling (Figure 7). While it is currently unknown how C. burnetii prevents ATF4 expression during ER stress, we provide evidence that the T4BSS effector protein CaeB is involved in modulation of IRE1 signalling during ER stress. Our data suggest that in addition to CaeB other C. burnetii factors are involved in modulating activation of p38, while modulation of the RNase activity of IRE1 seems to be solely dependent on CaeB (Figure 8). Some C. burnetii isolates lack or encode for a longer version of caeB. Thus, comparing these different isolates in their pathogenicity and in their ability to modulate ER stress-signalling might provide additional insight into the importance of the ER for C. burnetii infection.
Even though C. burnetii has a large repertoire of effector proteins in some instances there seem to be little functional redundancy between them, as the loss of a single effector can impair intracellular growth and CCV biogenesis (Weber et al., 2013). However, loss of CaeB did not impair bacterial growth, infection and formation of the CCV in vitro. Instead, C. burnetii lacking caeB exhibited reduced modulation of ER stress signalling, and is characterised by reduced anti-apoptotic activity. Importantly, the complemented strain showed restored anti-apoptotic activity, confirming the role of CaeB in preventing TM- and staurosporine-induced apoptosis. In addition, C. burnetii lacking caeB was attenuated in virulence in the G. mellonella infection model (Figure 9a–e), indicating that CaeB is important for C. burnetii pathogenicity. Whether this is mediated by modulation of IRE1 activity is yet to be determined. Thus, further research is necessary to clarify the role of CaeB during in vitro and in vivo infection. Especially the question, why CaeB only modulates IRE1-signalling under ER stress conditions, also need to be addressed in the future. One explanation might be that CaeB is only active under ER stress conditions; in that case, the mechanism(s) controlling the activity of CaeB need further study.
In addition, the unknown factor(s) involved in ER stress modulation, specifically in regulation of PERK activation and signalling have to be identified. Thereby, it will be possible to evaluate whether CaeB and these factor(s) act in concert and to dissect the role of ER stress modulation in C. burnetii pathogenesis.
4 EXPERIMENTAL PROCEDURES
4.1 Plant material and growth conditions
Nicotiana benthamiana plants were grown with 16 hr supplemental light and 8 hr dark cycle under greenhouse conditions with 25°C during light period and 22°C during the dark period. Light intensity was approximately 250 μmol * m−2 * s and relative humidity of 50–60%.
4.2 Cell culture
HEK293T cells stably expressing GFP and GFP-CaeB (Klingenbeck et al., 2013) were cultured at 37°C and 5% CO2 in DMEM (Life Technologies) with 10% heat inactivated fetal bovine serum (Biochrom), 1% penicillin–streptomycin (Invitrogen) and 1.5 mg/ml G418 (Roth). HeLa229, HeLa cells (obtained from ATCC) and mouse embryonic fibroblasts (MEFS) (Schulze-Luehrmann et al., 2016) were cultured in DMEM containing 5% FCS at 37°C and 5% CO2.
4.3 Bacterial culture and infection
Coxiella burnetii NMII strain (RSA439 clone 4) were cultured in ACCM-2, C. burnetii ΔcaeB in ACCM-D without lysine and C. burnetii ΔcaeB::3xFlag-CaeB in ACCM-D with citrulline, but without lysine and arginine at 37°C and 5% CO2, 2.5% O2. For infection, HeLa299 or HeLa cells were seeded in 6-well plates at a density of 2x105 and infected at a MOI of 100. One day post infection 1/10 of the cells in a 6-well were transferred in a 12-well plate and after 2 additional days ER stress was induced by TM treatment. MEF's were seeded on 10 mm coverslips in 24-well plates 1 day before infection at a density of 4x104 cells/well and infected with C. burnetii NM II strain (RSA439 clone 4) or C. burnetii ΔcaeB::3xFlag CaeB at a MOI of 200 for 40 hr.
4.4 Plasmid and strain construction
GFP-fusion constructs GFP-CaeB (caeB:pK7WGF2.0) and GFP-HopG1 (hopG1:pK7WGF2.0) were generated using the gateway cloning system and a CaeB specific primer set (5′-CACCGTAACAATGGCAGGCATAGCTGCAAC; 3′-CTTATTAAATTCGGGTATGTATG) or a hopG1 specific primer set (5′-CACCGTAACAATGCAAATAAAGAACAGTCATCT; 3′-GCCGTTGTAAAACTGCTTAGA) (Karimi, Inze, & Depicker, 2002). The IVD-mCherry marker (ivd:pRB-35S-GW-mcherry) was also generated using the gateway cloning system and a specific primer set for the IVD targeting sequence (5′-CACCGTAACAATGCAGAGGTTTTTCTCCGCC; 3′-ATCTTGCGCAAACTTGGATAC). Arabidopsis pto and avrPto from Pst were cloned in the pRB-35S-HA-Strep vector using pto (5′-GCACTAGTAACAATGGGAAGCAAGTATTCTAAG and 3′-GAATTCAATAACAGACTCTTGGAGACG) and avrPto (5′-ACTAGTAACAATGGGAAATATATGTGTCGGC and 3′-GAATTCTTGCCAGTTACGGTACGGGC) specific primer sets. For GFP expression the pRB-35S-GFP vector was used (Kraner, Muller, & Sonnewald, 2017). For construction of pJC-CAT::caeB-5′3′-lysCA the 5′ and 3′ regions of caeB were amplified by PCR from NMI genomic DNA using the specific primer sets (5′-CGGTACCCGGGGATCCGAAGCTGCACTTCCATGAG and 3′-CACCCATATGCGACGCGAGCGTCGAGAGCCCAAGCCGCCGTATC) and (5′-CGTCGCATATGGGTGCGCATGTACGTCATAAGTAAAGTAGCCCGCTGCGC and 3′-GAACCTGTTTGTCGACACGCCGGACAATTCTATC), respectively. The 5′ and 3′ PCR products were cloned into BamHI/SalI-digested pJC-CAT by In-Fusion, resulting in formation of an internal NdeI site between the 5′ and 3′ fragments and creation of pJC-CAT::caeB-5′3′. The 1169P-lysCA cassette was amplified from pJC-CAT::1169P-lysCA (Beare et al., 2018) by PCR with specific primers (5′-GCTCGCGTCGCATATGGAGCTCGGTACCCGGGGATCC and 3′-CATGCGCACCCATATGGATTAATTAGAGAACCTGTTTGTCGAC) and cloned by In-Fusion into NdeI-digested pJC-CAT::caeB-5′3′ to create pJC-CAT::caeB-5′3′-lysCA.
For transient, IPTG inducible expression of HA-tagged CaeB in C. burnetii ΔcaeB HA-tagged CaeB was introduced between the EcoRI and KpnI site into the vector pKM244mod (Schäfer et al., 2017). Thus, a seamless cloning strategy with the template pCMV-HA-CaeB and the specific primer pair pKM_ov_HA_fwd 5′-CAGGAAACAGAATTCATGTACCCATACGATGTTCCAGA and CaeB_pKM_ov_rev 3′-GCATCTAGAGGTACCTTACTTATTAAATTCGGGTATGTA was used according to the manufactures (Thermo Fisher) protocol, resulting in pKM 244mod-HA-CaeB.
4.5 Generation of C. burnetii ΔcaeB mutant and the complemented strain
Coxiella burnetii Nine Mile phase II was electroporated with 10 μg pJC-CAT::caeB-5′3′-lysCA as previously described (Beare & Heinzen, 2014). Co-integrants were selected by culturing the bacteria in ACCM-D media lacking lysine, but containing 2% sucrose for 4 days as previously described (Beare et al., 2018). Surviving transformants were expanded in ACCM-D media lacking lysine for 7 days. After spreading the diluted culture on 0.25% ACCM-D agarose without lysine clonal ΔcaeB mutants were picked after 7 days of culture. The picked clones were expanded in ACCM-D media without lysine. Clones were analysed via PCR for deletion of caeB with the specific primer sets (5′-CAGCCAACCAATGCCGGAG; 3′-CCTGGCGCCTTGACTCTAC and 5′-TTGGCAGGCATAGCTGCAACTTGT 3′-GCGGCAAAAATGTTGCCATATCAAATAATT), and for insertion of lysCA (5′-GCTAGATTCAGGTGACTGCTC; 3′-TCAGCCCGTTATAGTCTGTTCTGC). As control the IS1111 element was used (5′-AATTTCATCGTTCCCGGCAG; 3′-GCCGCGTTTACTAATCCCCA).
Coxiella burnetii ΔcaeB were electroporated with 10 μg pKM244mod-HA-CaeB as previously described (Beare & Heinzen, 2014). Bacteria were cultivated for 5 days in ACCM-D media lacking lysine, but containing 3 μg/ml chloramphenicol. The bacteria were diluted 1:500 in fresh ACCM-D media lacking lysine, but containing 3 μg/ml chloramphenicol. After 5 days of growth, the bacteria were diluted 1:100 and after 4 days of expansion, the bacteria were pelleted for 30 min at 4500×g and 4°C. C. burnetii ΔcaeB::HA-CaeB (Figure S5f) was resuspended in DMEM, 10% FCS and 5% DMSO, aliquoted and frozen at −70°C.
4.6 Agrobacteria-mediated transient expression
For transient protein expression in N. benthamiana, A. tumefaciens strain GV2260 was used. Agrobacteria were transformed with plasmids for the expression of GFP, GFP-CaeB, GFP-HopG1, ER:mCherry (Nelson et al., 2007), Pto-HA and AvrPto-HA. Agrobacteria GV2260 containing the dexamethasone inducible pTA7002:bax vector were provided by the group of H. Uchimiya from the University of Tokyo (Kawai-Yamada, Jin, Yoshinaga, Hirata, & Uchimiya, 2001). Approximately 8 weeks old fully expanded N. benthamiana leaves were inoculated with agrobacteria using a needleless syringe. For all experiments agrobacteria were mixed at equal ratios with agrobacteria containing the silencing suppressor p19 (Silhavy et al., 2002).
4.7 Confocal laser scanning microscopy
For analysis of protein localisation and ER network disruption, N. benthamiana leaves were infiltrated with the appropriate agrobacteria and imaged under a confocal laser scanning microscope (Leica SP5 II) 2 days after infiltration.
4.8 Plant cell death assays
Nicotiana benthamiana leaves were syringe-infiltrated using A. tumefaciens-mediated transient expression with a final density of 0.4 OD600. For co-expression of Bax and GFP-CaeB A. tumefaciens cultures were mixed at equal ratios and the expression of Bax was induced 24 hr post infiltration by spraying leaves with a 25 μM Dexamethasone solution. Cell death was analysed starting 1 day after Bax induction by either ion leakage measurement or visual symptom inspection. For co-expression of Pto/AvrPto and GFP-CaeB A. tumefaciens cultures were mixed at equal ratios. Cell death was analysed starting 4 days post infiltration by either ion leakage measurement or phenotype analyses.
4.9 Measurement of electrolyte leakage
Ion leakage was measured using a conductivity metre (Horiba B-771). N. benthamiana leaf discs of infiltrated tissue were taken with a cork borer no. 5 and floated on an aqueous solution. To monitor Bax-induced cell death; leaf discs were taken 1 day post Bax induction. Ion leakage was measured for a period of 48 hr at different time points. To obtain the absolute value of ions per leaf disc, leaf discs were incubated for 20 min at 99°C and conductivity was measured subsequently. For the Pto/AvrPto assay, leaf discs were taken 4 days post infiltration and ion leakage was monitored as described for Bax, for 4 days. For TM floating assays leaf discs expressing GFP or GFP-CaeB were sampled 1 day post infiltration and floated on an aqueous solution containing either 10 μg/ml tunicamycin (Sigma) or 0.1% DMSO as control or additionally 2.5 μM 4μ8C (Sigma) to inhibit IRE1 RNase activity. Increase in conductivity was measured for the indicated periods of time followed by determination of total conductivity. The increase in conductivity is presented either as percentage of the total conductivity (relative ion leakage) or as measured in μS/cm.
4.10 Apoptosis analysis of C. burnetii infected cells
HeLa cells on coverslips were infected with wild type, ΔcaeB and ΔcaeB::HA-CaeB C. burnetii at a MOI of 200 for 72 hr. At 24 hr post-infection cells were washed three times with PBS and supplemented with fresh medium. At 48 hr post-infection Isopropyl β D 1 thiogalactopyranoside (IPTG) was added to the media to induce expression of HA-CaeB in the complemented strain. The cells were either left untreated or were treated with 0.1 μM staurosporine (Cell Signalling) for 4 hr or with 5 μg/ml tunicamycin (Sigma) for 9 hr. The cells were fixed with 4% paraformaldehyde, permeabilised with ice-cold methanol and stained with antibodies against C. burnetii and LAMP-2. The cells were mounted using ProLong Diamond with DAPI (Thermo Fisher) to determine the percentage of infected cell with fragmented nuclei as described previously (Klingenbeck et al., 2013).
4.11 ER stress induction in mammalian cell lines
ER stress in stable GFP and GFP-CaeB HEK293T cells or HeLa229 and HeLa cells was induced by adding TM to the culture media at indicated concentrations and for indicated periods of time. HEK293T cells were seeded in 12-well plates with 1 × 105 cells per well. After 40 hr incubation TM was added.
4.12 XBP1 splicing analysis
mRNA of stable HEK293T or infected HeLa229 or HeLa cells was harvested and isolated using the RNeasy Plus Kit (Qiagen), cDNA synthesis was performed using SuperScript II (Invitrogen). XBP1 was amplified using dream tag polymerase (Thermo Fischer Scientific) and XBP1 specific primers (5′-CCTTGTAGTTGAGAACCAGGAG and 3′-CCATGGGGAGATGTTCTGGAG). The amplified products were analysed on a 3% agarose gel. Splicing of XBP1 was quantified using Image J software.
4.13 bZIP60 splicing analysis
Nicotiana benthamiana leaf discs expressing GFP or GFP-CaeB were floated on 10 μg/ml TM solution or as control on 0.1% DMSO. After 0, 2, 4 and 6 hr treatment total RNA was isolated using Z6-buffer (8 M guanidinium chloride, 20 mM MES, 20 mM EDTA, 50 mM 2-mercaptoethanol, pH 7.0). cDNA synthesis was performed using RevertAid Transcriptase (Thermo Fischer Scientific). bZIP60s was analysed with specific primers (5′-GGGGTTAGTTCTCCAGTGTTGTC and 3′-AGGGAACCCAACAGCAGACT) and either analysed via RT-PCR or via q-RT-PCR using the AriaMx Realtime PCR system (Agilent). Actin was used as control (5′-GGCTGGATTTGCTGGTGATG and 3′-TCCTTCTGTCCCATTCCGAC). Relative bZIP60s gene expression was analysed using the delta delta ct method as described by (Livak & Schmittgen, 2001).
4.14 Immunoblotting
For immunoblotting analysis cells were lysed in 2× SDS sample buffer, proteins were separated using 4–12% Bis-Tris Gels (Thermo Fischer Scientific) and transferred to a nitrocellulose membrane (GE Healthcare). Membranes were probed with the following antibodies: anti-cleaved PARP (cell signalling 5,625, 1:1,000), anti-XBP1s (cell signalling 12,782, 1:1000), anti-actin (Sigma-Aldrich A2066, 1:5,000), anti-Phospho-p38 MAPK (cell signalling 4,511, 1:1,000), anti-p38 MAPK (cell signalling 8,690, 1:1,000), anti-GFP (Thermo Fisher A6455, 1:1,000), anti-ATF6 (BioLegend 853,101, 1:500), anti-ATF4 (cell signalling 11,815, 1:1,000), anti-Flag M2 (MERCK F3165, 1:3,000) and anti-GAPDH (cell signalling 5,174, 1:5,000). Proteins were visualised by appropriate HRP-conjugated secondary antibodies. Densitometry analysis was performed using Image J software.
4.15 Immunofluorescence of infected MEF's and HeLa229
MEF's were fixed with 4% paraformaldehyde and permeabilised with 0.1% Trition X-100 before incubation with antibodies against Flag (staining tagged CaeB, labelled with Alexa Fluor 594) and Calnexin (staining the ER, labelled with Alexa Fluor 488). HeLa229 were transfected 18 hr prior immunostaining with GFP-CaeB using Lipofectamine 2000 (Thermo Fisher 11668019) according to the manufactures protocol. For mitochondrial staining the cells were incubated the last 45 min with Mitotracker CMXRos at 1:5,000 dilution of the stock according to the manufacture (Thermo Fisher). HeLa229 were fixed with 4% paraformaldehyde, permeabilised with 0.1% Triton X-100, and incubated with an antibody against Calnexin (staining the ER, labelled with Alexa Fluor 594). The cells were mounted using ProLong Diamond with DAPI to visualise cell nuclei and bacterial DNA using a Carl Zeiss LSM 700 Laser Scan Confocal Microscope and the ZEN2012 (black edition) software.
4.16 Infection of G. mellonella
Galleria mellonella larvae were purchased from TruLarv (UK). Larvae were infected by injection of 20 μl PBS, C. burnetii or C. burnetii ΔcaeB solutions (5 × 104/μl) into the uppermost right proleg. The larvae were incubated at 37°C for 10 days and survival was monitored every 24 hr.
4.17 Immunofluorescence of infected hemocytes
At day 1 and 5 post-infection hemocytes from 3 G. mellonella larvae infected with C. burnetii or C. burnetii ΔcaeB were seeded and centrifuged on Poly-L-lysine coated coverslips. The coverslips were incubated with antibodies directed against C. burnetii (labelled with Alexa Fluor 488) and actin (phalloidin labelled with Alexa Fluor 647). The cells were mounted using ProLong Diamond with DAPI to visualise cell nuclei and bacterial DNA. The percentage of infected cells was determined by analysing at least 100 cells each in three independent experiments using a Carl Zeiss LSM 700 Laser Scan Confocal Microscope and the ZEN2012 (black edition) software. For the area measurement of the CCV's combined tile scan and Z-stack pictures were taken by the LSM 700 and 100 CCV's each in four independent experiments were analysed. To measure the XY dimensions, the longest distance and the corresponding 90° angle were determined with the profile module of the confocal software Zen2012 (Zeiss). The XY dimensions of each CCV were multiplied and plotted as Vacuole size with GraphPad Prism 5 (GraphPad software, San Diego).
4.18 Quantification of bacterial load/larvae
Day 1 and 5 post infection two infected G. mellonella larvae were disrupted using a BeatRuptor and lysed with Proteinase K overnight. Genomic DNA of C. burnetii was isolated with the Qiagen DNeasy Blood and Tissue Kit. RT-PCR was performed to determine bacterial load as described (Schulze-Luehrmann et al., 2016). To calculate fold replication the genome equivalent of day 5 was compared to day 1.
ACKNOWLEDGEMENTS
We thank Stephan Reid and Martha Ölke for excellent technical assistance, Dr. Christina Müdsam for performing confocal microscopy of N. benthamiana leaves, Uwe Appelt (Core Facility for Cell Sorting and Immunomonitoring of the Universitätsklinikum Erlangen) for cell sorting, and Dr. Philipp Tripal (Optical Imaging Center Erlangen [OICE]) for support with confocal microscopy. We are particularly grateful to Prof. Uwe Sonnewald (Division of Biochemistry, Erlangen) and Prof. Christian Bogdan (Microbiology Institute, Erlangen) for advice and support. This work was supported by the Deutsche Forschungsgemeinschaft (DFG), project LU1357/5-1, and by the Bundesministerium für Bildung und Forschung (BMBF) under the project number 01KI1726A of ‘Q-GAPS’ as part of the research network zoonotic infectious diseases to AL.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Anja Friedrich, Sophia Sonnewald and Anja Lührmann conceived and designed the study. Anja Friedrich, Jan Schulze-Luehrmann, Arne Cordsmeier and Tobias Pazen performed the experiments. Anja Friedrich, Jan Schulze-Luehrmann, Arne Cordsmeier, Tobias Pazen, Sophia Sonnewald and Anja Lührmann analysed the data. Paul A. Beare provided reagents and resources. Sophia Sonnewald and Anja Lührmann supervised the study. Anja Friedrich, Sophia Sonnewald and Anja Lührmann wrote the paper. All authors read and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.