Formation of oxidised (OX) proteins in Entamoeba histolytica exposed to auranofin and consequences on the parasite virulence
Funding information: Israel Ministry of Health ERA-NET Infect-ERA (AMOEBAC project), Grant/Award Number: 031L0004; Israel Science Foundation, Grant/Award Number: 260/16; Rappaport Family Institute for Research in the Medical Sciences; US-Israel Binational Science Foundation, Grant/Award Number: 2015211
Abstract
Metronidazole (MNZ), the first line drug for amoebiasis and auranofin (AF), an emerging antiprotozoan drug, are both inhibiting Entamoeba histolytica thioredoxin reductase. The nature of oxidised proteins (OXs) formed in AF- or MNZ-treated E. histolytica trophozoites is unknown. In order to fill this knowledge gap, we performed a large-scale identification and quantification of the OXs formed in AF- or MNZ-treated E. histolytica trophozoites using resin-assisted capture coupled to mass spectrometry (MS). We detected 661 OXs in MNZ-treated trophozoites and 583 OXs in AF-treated trophozoites. More than 50% of these OXs were shared, and their functions include hydrolases, enzyme modulators, transferases, nucleic acid binding proteins, oxidoreductases, cytoskeletal proteins, chaperones, and ligases. Here, we report that the formation of actin filaments (F-actin) is impaired in AF-treated trophozoites. Consequently, their erythrophagocytosis, cytopathic activity, and their motility are impaired. We also observed that less than 15% of OXs present in H2O2-treated trophozoites are also present in AF- or MNZ-treated trophozoites. These results strongly suggest that the formation of OXs in AF- or MNZ-treated trophozoites and in H2O2-treated trophozoites occurred by two different mechanisms.
1 INTRODUCTION
The intestinal protozoan parasite Entamoeba histolytica is the causative agent of amoebiasis. This disease is a substantial risk in developing countries with poor socioeconomic and sanitary conditions. It is estimated that amoebiasis accounted for 55,500 deaths and 2.237 million disability-adjusted life years (the sum of years of life lost and years lived with disability) in 2010 (Turkeltaub, McCarty, & Hotez, 2015). Despite the high prevalence in developing countries, in recent years, amoebiasis can also be seen in travellers from developed countries who return from endemic regions.
The major clinical manifestations of amoebiasis are colitis and liver abscess. Amoebiasis is initiated by the ingestion of food contaminated with cysts, the infective form of the parasite. Following excystation, the trophozoites migrates to the gastrointestinal tract where they can either colonise the gut asymptomatically, accounting for almost 90% of all infections or cause lesions and intestinal symptoms including bloody diarrhoea. It is hypothesized that the host's genetic susceptibility, age, immune condition, and the composition of the individual's gut microbiota contribute and can be used as a prediction to the manifestation and severity of the disease (Kantor et al., 2018).
No vaccines or prophylactic medications for amoebiasis have been approved for human clinical trials. Metronidazole (MNZ) has been used for decades as a forefront treatment for invasive amoebiasis (Powell, MacLeod, Wilmot, & Elsdon-Dew, 1966). MNZ's mode of action relies on the fact that it is reduced to a nitro radical anion or to a nitrosomidazole by thioredoxin reductase (TrxR) inside the parasite. Subsequently, this nitro group reduces O2, resulting in the generation of reactive oxygen species that are highly detrimental to the parasite. Alternatively, the nitroso imidazole can react with different proteins such as thioredoxin (Trx), TrxR, superoxide dismutase, purine nucleoside phosphorylase, and nonproteins such as cysteine, to form covalent adducts, resulting in the depletion of nonprotein thiols and the modification of the proteins listed above. All these actions of MNZ render the cells vulnerable to oxidative stress (OS) and as a result kill the parasite (Leitsch, Kolarich, Wilson, Altmann, & Duchene, 2007). MNZ has different adverse effects such as nausea, vomiting, headaches, and a metallic or bitter taste in the mouth and more serious effects such as anorexia, ataxia, and skin rashes/itching (Andersson, 1981; Roe, 1977). MNZ has a mutagenic effect in Salmonella typhimurium strains (Dobias, Cerna, Rossner, & Sram, 1994). Despite the fact that only sporadic cases of MNZ resistance have been reported, reluctance of amoebiasis to MNZ is an increasing concern as it was shown that in vitro, resistance to the drug has been induced by exposure to increasing amounts of the drug (Samarawickrema, Brown, Upcroft, Thammapalerd, & Upcroft, 1997; Wassmann, Hellberg, Tannich, & Bruchhaus, 1999). Trophozoites that have been selected to grow in four times the concentration of MNZ that is generally tolerated by regular trophozoites are overexpressing the iron containing superoxide dismutase and peroxiredoxin and have a decreased expression of flavin reductase and ferredoxin 1 (Wassmann et al., 1999). Indeed, trophozoites that overexpress superoxide dismutase or peroxiredoxin are more resistant to MNZ than control trophozoites (Wassmann et al., 1999). Thus, these observations have led to the search for new antiamebic drugs. A high throughput screen of more than 900 Food and Drug Administration-approved and unapproved bioactive compounds has identified AF, a gold containing compound that has been therapeutically used since 1985 for treatment of rheumatoid arthritis, as an antiamebic drug which is 10 times more potent than MNZ (Debnath et al., 2012).
The exact antiamebic mechanism of the drug is not completely understood; however, Debnath et al. have found that AF by targeting E. histolytica thioredoxin reductase (EhTrxR) prevents its reduction and consequently enhances the sensitivity of trophozoites to reactive oxygen-mediated killing (Parsonage et al., 2016). Genes encoding proteins that are involved in mitosis and nucleotide metabolism and genes encoding adenosine diphosphate ribosylation factor, Ras1p, and arsenite-inducible RNA-associated protein were found to be, respectively, up- and down-regulated in AF-treated trophozoites (Debnath et al., 2012).
Despite these informative data on the mode of action of AF and on the transcriptional response of the parasite to this drug, our knowledge on the effect of AF on the redox-status of the parasite is incomplete. Here, we report the results of a study whose aim was to identify and to determine the biological relevance of oxidised proteins (OXs) in E. histolytica exposed to AF using resin-assisted capture (RAC) coupled with mass spectrometry (MS). The result of this analysis revealed that the formation of F-actin that is linked to the virulence potential of E. histolytica is impaired in the AF-treated parasites. We also report that AF-treated trophozoites and MNZ-treated trophozoites are sharing more than 50% of their OXs. In contrast, only few of these OXs are shared with H2O2 treated trophozoites (Shahi et al., 2016). These results strongly suggest that the formation of OXs in AF- or MNZ-treated trophozoites and the formation of OXs in H2O2 treated trophozoites occurred by two different mechanisms. We have also shown that some of the OXs identified in AF- or MNZ-treated trophozoites like peroxiredoxin and pyruvate:ferredoxin oxidoreductase are crucial components of the antioxidant stress system of E. histolytica.
2 RESULTS
2.1 Characterisation of OXs ins in AF-treated E. histolytica trophozoites
We determined that the lethal dose 50 (LD50) of AF in E. histolytica trophozoites is 1.46 μM (Table S1) as determined by probit analysis (rounded up to 1.5 μM in our various assays).
We then used detection of OX proteins by RAC (OX-RAC) coupled to label-free quantification (liquid chromatography) MS for the detection and quantification of OXs in the lysate of AF-treated trophozoites (Figure 1(a)). The purification of OXs by OX-RAC, which has been previously described in detail (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016), has three steps: (a) blocking by N-ethylmaleimide of nonoxidised cysteine residues present in E. histolytica proteins; (b) reduction of oxidised cysteine residues with dithiothreitol (DTT); and (c) binding of the cysteine residues reduced by DTT to a thiopropyl sepharose resin. The OXs are then eluted from the thiopropyl sepharose resin and identified by MS. We identified 583 OXs in those trophozoites that were exposed to AF (Table S2). These 583 OXs were then classified using the Protein ANalysis THrough Evolutionary Relationships (PANTHER) sequence classification tool (Mi et al., 2017; Figure 1b). The five most abundant OXs families belong to hydrolase (exemplified by cysteine proteinase 5 [EHI_168240]), enzyme modulator (exemplified by Rho family GTPase [EHI_197840]), transferase (exemplified by tRNA (Cytosine-5-)-methyltransferase [EHI_098500]), nucleic acid binding (exemplified by 60S ribosomal protein L7 [EHI_010650]), and oxidoreductase (exemplified by Pyruvate:ferredoxin oxidoreductase [EHI_051060]). According to the PANTHER statistical overrepresentation test that compares classifications of multiple clusters of lists to a reference list, very significant enrichment (fold enrichment >2 and p > 0.05) was found for proteins belonging to chaperonin (exemplified by chaperonin containing TCP-1 [EHI_125800]), reductase (exemplified by nicotinamide adenine dinucleotide phosphate [NADP]-dependent alcohol dehydrogenase [EHI_023110]), metalloprotease (exemplified by cell surface protease gp63 [EHI_200230]), dehydrogenase (exemplified by Glyceraldehyde-3-phosphate dehydrogenase [GADPH; EHI_008200]), oxidoreductase (exemplified Superoxide dismutase [EHI_159160]), microtubule family cytoskeletal protein (exemplified by Tubulin alpha chain [EHI_010530]), G protein (exemplified by Ras family GTPase [EHI_124610]), translation initiation factor (exemplified by elongation factor-1 alpha [EHI_044230]), transfer/carrier protein (exemplified by Ubiquitin-activating enzyme [EHI_098550;ortholog]), small GTPase (exemplified by GTP-binding nuclear protein [EHI_148190]), ligase (exemplified by Long-chain-fatty-acid–CoA ligase [EHI_127830]), and cytoskeletal protein (exemplified by Actophorin, [EHI_197480]; Figure 1c).
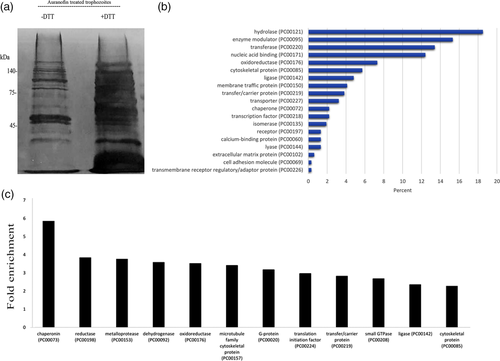
2.2 Comparison of OXs in trophozoites exposed to AF and in trophozoites exposed to MNZ
We have determined that the LD50 for MNZ is 5 μM (Table S1). We used OX-RAC coupled to MS to detect and quantify OXs in MNZ-treated trophozoites (Figure 2(a)). We identified 661 OXs in the MNZ-treated trophozoites (Table S2). These 661 OXs were then classified using the PANTHER sequence classification tool. We found that the most abundant OX families identified belong to nucleic acid binding, enzyme modulator, cytoskeletal proteins, oxidoreductase, and transporter proteins (Figure 2(b)). According to the PANTHER statistical overrepresentation test, very significant enrichment (fold enrichment >2 and p > 0.05) was found for proteins belonging to chaperonins, translation elongation factors, vesicle coat proteins, chaperones, translation initiation factors, actin family cytoskeletal proteins, translation factors, oxidureductases, G-proteins, cytoskeletal proteins, ribosomal proteins, small GTPases, membrane traffic proteins, and dehydrogenases (Figure 2(C)). We then compared OXs in AF- and MNZ-treated trophozoites and found that 309 of these OXs are shared (Table S3). Interestingly, 7 out of the 12 enriched OX families found in AF-treated trophozoites (Figure 1c) are also enriched in MNZ-treated trophozoites (Figure 2c), namely; chaperonins, dehydrogenases, oxidoreductases, G-proteins, translation initiation factors, small GTPases, and cytoskeletal proteins.

2.3 Comparison of OXs in H2O2-treated trophozoites and in AF- or MNZ-treated trophozoites
We have previously identified 140 OXs in H2O2-treated trophozoites (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). These OXs were compared with OXs in AF- or MNZ-treated trophozoites. We found that 85 OXs are shared between H2O2-treated trophozoites and AF-treated trophozoites (Table S4). We also found that 81 OXs are shared between H2O2-exposed trophozoites and MNZ-exposed trophozoites (Table S5). Whereas OXs in H2O2 exposed trophozoites are specifically enriched in ribosomal proteins and actin cytoskeletal proteins (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016), OXs in AF-treated trophozoites are specifically enriched in chaperonin, mettaloprotease, reductase (oxidoreductase), dehydrogenase, microtubule family cytoskeletal protein, and G-proteins (Figure 1c). OXs in MNZ-treated trophozoites are specially enriched in membrane traffic proteins, small GTPases, ribosomal proteins, cytoskeletal proteins, G-proteins, translation factors, oxidoreductases, actin family cytoskeletal proteins, dehydrogenases, and translation initiation factors.
2.4 Comparison of OXs in trophozoites exposed to AF or to MNZ and TrxR target proteins
Sixteen EhTrxR substrates were identified by using Trx affinity chromatography (Schlosser, Leitsch, & Duchene, 2013). Interestingly, 7 of these 16 Trx substrates, namely NADP-dependent alcohol dehydrogenase (EHI_023110), purine nucleoside phosphorylase, (EHI_200080), pyruvate ferredoxin oxidoreductase (EHI_051060), radical sterile alpha motif domain protein (EHI_068170), peroxiredoxin, (EHI_121620; EHI_201250; EHI_145840; EHI_114010; EHI_061980; EHI_001420; EHI_123390; EHI_122310), Trx (EHI_021560), and aminoacyl-histidine dipeptidase (EHI_042170) were also OXs in AF-treated trophozoites. In addition, we found that Hsp70 (EHI_052860) and Hsp70 (EHI_148990) are, respectively, a TrxR substrate and an OX in AF-treated trophozoites. Interestingly, seven OXs identified in MNZ-treated trophozoites, namely NADP-dependent alcohol dehydrogenase (EHI_023110), purine nucleoside phosphorylase, putative (EHI_200080), pyruvate:ferredoxin oxidoreductase (EHI_051060), radical sterile alpha motif domain protein (EHI_068170), Trx, putative (EHI_021560), and aminoacyl-histidine dipeptidase, putative (EHI_042170) were also TrxR substrates. In comparison, only two OXs present in H2O2-treated trophozoites, namely NADP-dependent alcohol dehydrogenase (EHI_023110) and peroxiredoxin, putative (EHI_121620) were TrxR substrates.
2.5 Effect of AF on the actin cytoskeleton
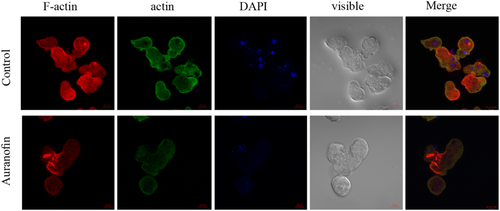
Fifty-two genes are related to the amoebic cytoskeletal organisation in AF-treated trophozoites. The enrichment of actin family of cytoskeletal proteins among AF-treated trophozoites motivated us to investigate the presence of F-actin in control and AF-treated trophozoites by immunofluorescence microscopy with phalloidin, a molecule that binds selectively to F-actin. The F-actin signal was quantified by densitometry. The intensity of the F-actin signal in AF-treated trophozoites was around six times less than the control trophozoites (Figure 3). These results show that AF impairs the formation of F-actin in AF-treated trophozoites. Because actin polymerisation is an essential process during migration of the parasite (Emmanuel, Nakano, Nozaki, & Datta, 2015), we compared the migration of control trophozoites and AF-treated trophozoites using the transwell migration assay. We found that the number of AF-treated trophozoites that passed through the pores was five times less of that of the control trophozoites (Figure 4a).
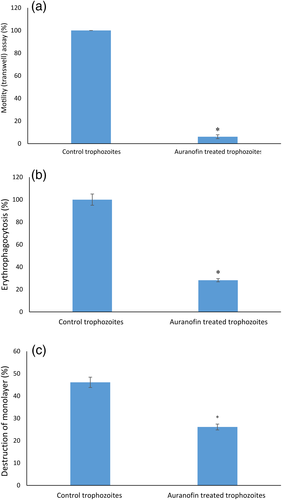
Actin polymerisation is essential for the phagocytic and cytopathic activities of E. histolytica trophozoites (Godbold & Mann, 1998). A comparison between control trophozoites and AF-treated trophozoites was carried out using the erythrophagocytosis assay. The extent of erythrophagocytosis by AF-treated trophozoites was five times less of that of the control trophozoites (Figure 4b). Cytopathic activity of AF-treated trophozoites was around twice less than that of control trophozoites (Figure 4c).
2.6 Protein synthesis in AF-treated trophozoites
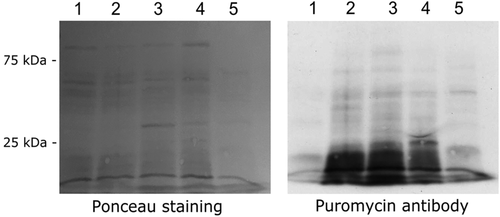
We have previously reported that OS induced by H2O2 inhibits protein synthesis in E. histolytica (Shahi et al., 2016; Trebicz-Geffen et al., 2017). The presence of proteins that are involved in translation among OXs, such as the elongation factor-1 alpha and elongation factor 2, suggests that AF regulates the translation of proteins in the AF-treated trophozoites. In order to test this hypothesis, we used the surface sensing of translation (Schmidt, Clavarino, Ceppi, & Pierre, 2009) to determine the amount of puromycin that was incorporated into nascent peptide chains. As previously described (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016), we found that protein synthesis is strongly inhibited in H2O2-treated trophozoites compared with that in untreated trophozoites (Figure 5). In contrast, we found that protein synthesis in AF-treated trophozoites is comparable with that in untreated trophozoites (Figure 5).
3 DISCUSSION
In the present work, we measured the response of E. histolytica to AF and MTZ by determining the LD50, which is defined as the amount of a toxic agent that is sufficient to kill 50% of a population within a certain time. Previous studies on this parasite have determined the half maximal effective concentration (EC50) for AF and MTZ (Debnath et al., 2012; Ehrenkaufer, Suresh, Solow-Cordero, & Singh, 2018) that is defined as the concentration of a compound that is necessary to reduce the culture density to 50% of that of an untreated culture. Indeed, some difference between our experimental conditions and those of these previous studies (time of incubation with the drugs, tubes vs. plates, etc.) make a direct comparison difficult.
E. histolytica lacks both glutathione reductase activity and glutathione synthetic enzymes; therefore, it relies on TrxR to prevent, regulate, and repair the damage caused by OS (Jeelani & Nozaki, 2016). AF inhibits EhTrxR and consequently prevents the reduction of Trx. As a result, proteins that are redox-regulated will be blocked in an oxidised state leading to the death of the parasite (Parsonage et al., 2016). Based on the present mechanism of action of AF, we assumed that most of the 583 OXs identified in AF-treated trophozoites are target proteins of the TrxR/Trx system. This assumption is supported by the fact that some target proteins of the E. histolytica TrxR/Trx system that have been previously identified (Schlosser et al., 2013) have also been identified in this work. This assumption is also supported by the fact that out of the 22 genes encoding Trxs in the parasite (Jeelani & Nozaki, 2016), 6 were identified as OX Trx proteins in AF and 9 in MNZ-treated trophozoites whereas only 3 were identified in H2O2-treated trophozoites (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). Moreover, more than 50% of the OXs identified in AF-treated parasites are also found in MNZ-treated parasites. MNZ like auranofin inhibits E. histolytica TrxR activity (Leitsch et al., 2007) that overall leads to the oxidation of the same target proteins of the TrxR/Trx system. Some of these proteins like peroxiredoxin and pyruvate:ferredoxin oxidoreductase are crucial components of the antioxidant stress system of E. histolytica (Jeelani & Nozaki, 2016).
Based on the above-mentioned information about the role of TrxR in E. histolytica, it is almost certain that this enzyme is essential for E. histolytica as it is for the erythrocytic stages of Plasmodium falciparum (Krnajski, Gilberger, Walter, Cowman, & Muller, 2002). Consequently, it would have been very difficult to demonstrate by knocking down EhTrxR expression that the 583 OXs identified in AF-treated trophozoites are target proteins of the TrxR/Trx system.
This study revealed a strong overlap between OXs in AF- and MNZ-treated trophozoites. In contrast, only a weak overlap was found between OXs in AF- or MNZ-treated trophozoites and OXs in H2O2-treated trophozoites. These results strongly suggest that the formation of OXs in AF-treated trophozoites or in MNZ-treated trophozoites occurs through a mechanism that is different from the one occurring in H2O2-treated trophozoites. Thiol oxidation by H2O2 is a direct event that begins by the attack of the reactant thiolate to an oxygen atom of H2O2 (Zeida et al., 2012). It is reasonable to assume that OXs formed in the parasite exposed to an acute OS (2.5 mM H2O2 for 1 hr; Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016) are the ones that are the most susceptible to oxidation. In contrast, the formation of reactive oxygen species in AF- or MTZ-treated trophozoites is a secondary event that results from the inhibition of TrxR activity. Therefore, the formation of OXs in AF or MTZ trophozoites is probably the result of a progressive accumulation of ROS that occurs during the 24 hr of treatment with these drugs. This point of view is supported by the following observation: peroxiredoxin, an enzyme directly involved in the reduction of peroxides, was oxidised in H2O2-treated parasites (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016) but not found as an OX in AF- or MTZ-treated trophozoites (this work).
We observed some similarities but also some difference between OXs in AF-treated trophozoites and in H2O2-treated trophozoites that will be discussed here.
A common group of enriched OXs that are represented in AF-, MTZ- and H2O2-treated trophozoites is the cytoskeletal proteins. E. histolytica's virulence depends on an intact cytoskeleton (Gautam, Ali, Bhattacharya, & Gourinath, 2019; Kumar, Dutta, Maiti, & Gourinath, 2014; Manich et al., 2018), and our previous work has shown that oxidation of its cytoskeletal proteins has a direct effect on the mobility of the parasite and on its virulence (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). It has also been demonstrated that MTZ has a direct effect on E. histolytica motility (Nesthus, Glette, Bjorvatn, & Solberg, 1987). Interestingly, one of the OX-chaperonin family of proteins that is only found in AF-treated E. histolytica is also linked to cytoskeletal organisation. This family is represented by different subunit of the T-complex protein 1 (TCP1). TCP1 is distinctive among the chaperonins in its arrangement of eight distinct subunits (Lopez-Fanarraga, Avila, Guasch, Coll, & Zabala, 2001). A main role of TCP1 is to bind actin and tubulin monomers and help them to reach their native states and therefore contributes to the cytoskeletal organisation of the cell. Even if it is not clear at that stage how the AF-mediated oxidation of TCP1 affects its activity, it is probable that the oxidation of crucial cytoskeletal proteins is a main mechanism that affects the virulence of AF-treated parasites (Tejman-Yarden et al., 2013). This hypothesis is also in agreement with the results of this investigation that shows an impairment in the motility, erythrophagocytosis, cytopathic activity, and in the formation of F-actin of the AF-treated parasite.
Another common family of OXs related to the cytoskeletal organisation belongs to the G-protein family. Heterotrimeric G-protein signalling is thought to possibly modulate amebic motility and as a result, affects the ability of the parasite to attach to and kill host cells, making it an important factor in the pathogenesis of the amoeba (Bosch & Siderovski, 2013). This group of OXs is mainly represented by the heterotrimeric G-protein subunits Ras, Rho, and Rab GTPases. Redox regulation of G-proteins have been well documented (Heo & Campbell, 2005; Hobbs, Zhou, Cox, & Campbell, 2014; Mitchell, Hobbs, Aghajanian, & Campbell, 2013). It is therefore possible that the AF induced oxidation of G-proteins in the parasite contributes to an impairment of its motility and to its death.
In this OX-RAC analysis of AF-treated parasites, we identified a group of OXs that belongs to the oxidoreductase, reductase, and dehydrogenase family that has not been identified among OXs in H2O2-treated trophozoites (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). One of these enzymes is GAPDH. We have previously reported that GAPDH is detrimental to the parasite exposed to nitrosative stress (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). It is also known that oxidation of GAPDH leads to its nuclear translocation in mammalian cells and induces apoptosis (Chuang, Hough, & Senatorov, 2005). Based on this information, it is tempting to speculate that OX-GAPDH is involved in the AF-mediated death of the parasite. Another reductase of interest is the aldose reductase (EHI_039190). This enzyme is an nicotinamide adenine dinucleotide phosphate-dependent oxidoreductase that catalyses the reduction of a variety of aldehydes and carbonyls, including monosaccharides. Aldose reductase shows increased expression in E. histolytica HM-1:IMSS compared with the nonvirulent Rahman strain both in culture and during contact with the human colon. This observation suggests that this enzyme is involved in the virulence of the parasite probably for its adaptation to the low glucose environment present in the human colon (Baumel-Alterzon & Ankri, 2014; Baumel-Alterzon, Weber, Guillen, & Ankri, 2013; Thibeaux et al., 2013). In the human aldose reductase, the oxidative modifications surrounding the sulfur atom of Cys-298 are anticipated to impair cofactor binding, conformational rearrangements, and enzymatic activity (Balendiran et al., 2011). Interestingly, this Cys-298 is conserved in E. histolytica's aldose reductase (equivalent to Cys-286). Based on this data, it is tempting to speculate that Cys-286 in E. histolytica's aldose reductase is crucial for its activity and that its oxidation induced by AF contributes to the lower virulence observed in AF-treated E. histolytica trophozoites (Debnath et al., 2012).
Metalloprotease is a family of OXs present in AF-treated trophozoites but that was not present in H2O2-treated trophozoites. One of these metalloprotease is the cell surface protease gp63 (EHI_200230) also called EhMSP-1. EhMSP-1 belongs to the M8 metalloproteases that are defined by the occurrence of a zinc-binding catalytic site motif (HEXXH), a third zinc-binding His residue located further towards the C terminus, and a highly conserved Met residue C terminal to the third His (Rawlings & Barrett, 1993). Interestingly, at the N-terminal of these metalloprotease is a prodomain region that caps the catalytic site as a result of “thiol interaction” between the cysteine in the prodomain with the Zn2+ in the catalytic domain, thereby keeping the enzyme inactive. For activation of the enzyme, the prodomain can be cleaved by various proteases or the thiol interaction can be perturbed by oxidation of the Cys residue present in this prodomain (Gomis-Ruth, 2009; Kar, Subbaram, Carrico, & Melendez, 2010; Koch et al., 2009). This mechanism of activation that is shared by most M8 metalloproteases probably applies to EhMSP1. It is possible that the activity of this metalloprotease that is involved in the binding of the parasite to mammalian cells and in its mobility (Teixeira, Sateriale, Bessoff, & Huston, 2012) is specifically redox-regulated by AF, and this regulation may contribute to the impairment of the parasite's virulence in presence of AF (Debnath et al., 2012).
As previously reported, we found that protein synthesis is strongly inhibited in H2O2-treated trophozoites and that this inhibition is associated with the oxidation of ribosomal proteins (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). AF inhibits protein synthesis in human lymphocytes (Finkelstein, Burrone, Walz, & Misher, 1977). However, at the concentration of AF and experimental conditions used in this study, we did not find that AF inhibits protein synthesis in E. histolytica. Ribosomal proteins in AF-treated parasites are not particularly enriched among OXs, which may explain why protein synthesis was not affected. OX proteins of the translation initiation factor family were found in the AF-treated trophozoites but not in the H2O2-treated trophozoites (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). One representative of this group is the Elongation factor 1-alpha (eEF1A; EHI_011210). During the elongation stage of translation, the GTP dependent eEF1A delivers aminoacyl-tRNA to the A-site of the 80S ribosome. Elongation factors are prone to oxidation due to the presence of cysteine residues that are essential for their translational activity (Liu et al., 2015; Nagano et al., 2012; Yutthanasirikul et al., 2016). According to the results of protein synthesis in AF-treated parasites, we can exclude that oxidation of eEF1A in E. histolytica inhibits protein synthesis. eEF1A is a moonlighting protein that is involved in the reorganisation of the actin cytoskeleton by interacting with F-actin (Stapulionis, Kolli, & Deutscher, 1997). Although we do not know if this interaction takes place in the parasite, it is possible that the oxidation of eEF1A in the parasite contributes to the inhibition of F-actin formation in the AF-treated trophozoites.
To conclude, we inform on the presence of many novel OXs in AF-treated trophozoites that have not been previously identified in H2O2-treated trophozoites. We proposed that this difference results from the different mode of action between AF and H2O2 to induce OS in the parasite. Whereas hydroxylradicals resulting from the H2O2 are directly causing oxidative damage, AF acts indirectly through the inhibition of TrxR. Although we assumed that most of the OXs identified in the AF-treated trophozoites are resulting from the inhibition of TrxR by AF, we cannot rule out that some of them result from the inhibition of other proteins. For example, it has been found recently that E. histolytica adenosine 5'-phosphosulfate kinase, an essential enzyme in sulfolipid metabolism, is directly inhibited by AF (Mi-Ichi et al., 2019). Such direct inhibition may explain why adenosine 5'-phosphosulfate kinase protein was not found among the OXs in AF-treated trophozoites.
Of the oxidised proteins in AF-treated trophozoites, we discovered that the oxidation of cytoskeletal proteins impairs the formation of actin cytoskeleton and consequently the motility of the parasite. TrxR and its substrates have been considered as interesting targets for cancer chemotherapy (Arner & Holmgren, 2006). We envisage that this study will pave the way for further studies on OXs identified in AF-treated trophozoites and their potential as new targets for antiamebic drugs.
4 EXPERIMENTAL PROCEDURES
4.1 E. histolytica culture
E.histolytica trophozoites HM-1:IMSS strain were grown under axenic condition at 37°C in a trypticase yeast extract iron serum medium prepared according to a previously reported protocol (Diamond, Harlow, & Cunnick, 1978). The trophozoites were harvested during the logarithmic phase of growth by chilling the culture tubes at 4°C and pelleted by centrifugation at 500 g for 5 min. The pellet was washed twice with ice-cold phosphate-buffered saline.
4.2 Viability of E. histolytica exposed to AF or MTZ and determination of the median lethal dose (LD50)
E. histolytica trophozoites were first cultivated in the standard trypticase yeast extract iron serum medium in 7-ml culture tubes for 24 hr at 37°C. The culture medium was then replaced with fresh medium and no AF (control) or different concentrations of AF (a kind gift of Prof. Benhar, Faculty of Medicine, Technion; 0.2, 0.5, 2, and 3 μM) were added to the tubes. The culture was continued for an additional 24 hr. The viability of the trophozoites untreated or treated with AF was determined by the eosin dye exclusion method (Shahi, Trebicz-Geffen, Nagaraja, Hertz, et al., 2016). The experiment was performed in duplicates and repeated three times. The value of AF required to kill half of the trophozoites population after 24 hr of incubation (LD50) was calculated by probit analysis (Baten & Stafseth, 1956). The plot of mortality in probits against log10 doses was made by using the LD50 probit analysis calculator module in Excel.
The same experimental procedure was followed to determine the LD50 for MTZ except the different concentrations of the drug that were used (5, 8, and 10 μM) and the incubation time with the drug (24 hr).
4.3 Detection of OX proteins by RAC (OX-RAC)
The detection of OXs by OX-RAC was performed using a previously described protocol (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). Captured proteins were eluted with 30-μl elution buffer which contained 10 mM HEPES, 0.1 mM EDTA, 0.01 mM neocuproine, 0.1% sodium dodecyl sulfate (SDS) and 100 mM 2-mercaptoethanol for 20 minutes at room temperature. Proteins in a 10-μl aliquot of each eluent were resolved on a 12.5% SDS–polyacrylamide gel electrophoresis (PAGE) gel. Each gel was then stained with silver (Pierce Silver Stain), and each gel slice was independently analysed by MS. A protein was considered to be oxidised when its relative amount in the DTT-treated lysates was significantly less than that in the DTT-untreated lysates (p < .05 according to the results of an unpaired t-test).
4.4 In gel proteolysis and MS analysis
In gel proteolysis by trypsin and analysis by liquid chromatography–tandem mass spectrometry on the Q Exactive Plus (Thermo Fisher) and data analysis with MaxQuant 1.5.2.8 (Cox & Mann, 2008) and the UniProt database as the reference were done using a previously described protocol (Shahi, Trebicz-Geffen, Nagaraja, Alterzon-Baumel, et al., 2016). The data was quantified by LF analysis using the same software. The identifications are filtered for proteins identified with a false discovery rate of <0.01, and at least two identified peptides in the project. The intensities are presented as raw intensities without normalisation and as label-free quantification with normalisation, both presented as log2 intensities.
4.5 Classification of OXs according to their protein class
The OXs were classified according to their protein class using PANTHER Classification System software (http://www.pantherdb.org/) Mi et al., 2017).
4.6 Determination of E. histolytica motility using the transwell migration assay
Trophozoite motility was determined using a previously described protocol (Trebicz-Geffen et al., 2017).
4.7 Determination of protein synthesis by surface sensing of translation
Protein synthesis in untreated trophozoites (control), trophozoites exposed to H2O2 (2.5 mM for 30 min) and trophozoites exposed to AF (1.5 μM for 24 hr) was determined by using the surface sensing of translation protocol (Schmidt et al., 2009). Briefly, trophozoites (2 × 106 ml−1) were incubated with 10-μg ml−1 puromycin (Sigma-Aldrich, St. Louis, MO, USA), a structural analogue of tyrosyltRNA, for 20 min at 37°C. The trophozoites were then lysed using 1% Igepal (Sigma-Aldrich) in phosphate-buffered saline (PBS). Whole proteins were resolved on a 10% SDS-PAGE in SDS-PAGE running buffer. The proteins were electrotransferred in protein transfer buffer to a nitrocellulose membrane. Loading equivalency was determined by Ponceau staining of the membrane before immunostaining. Puromycin was detected by immunoblotting using a 1:5,000 monoclonal puromycin antibody (12D10 clone, Millipore). After incubation with the primary antibody, the blots were incubated with 1:5,000 secondary antibody for 1 h at RT (Jackson ImmunoResearch, Enco Diagnostics, Israel), and then developed using enhanced chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate, ThermoFisher Scientific, USA). Protein quantification/synthesis was estimated from the intensity of the immunoreactive blots (densitometry) using Fiji software (Schindelin et al., 2012).
4.8 Immunofluorescence microscopy
E. histolytica trophozoites (1.5 105 trophozoites per millilitre) were suspended in a complete Diamond's trypticase yeast extract iron serum medium at 37°C and transferred onto acetone-cleaned glass coverslips that were placed in the bottom of each well of a 24-well plate. Trophozoites were incubated for 15 min at 37°C in order to allow them to adhere to the coverslip surface. The attached trophozoites were fixed with prewarmed (37°C) 3.7% paraformaldehyde for 30 min at RT. After fixation, the attached trophozoites were permeabilized with 0.1% Triton X-100/PBS for 1 min at RT. The coverslips were washed three times with PBS and quenched with PBS containing 50 mM NH4Cl for 30 min at RT. The coverslips were then blocked with 1% bovine serum albumin (BSA) in PBS (BSA/PBS) for 30 min at RT. The samples were then probed with 1:500 monoclonal actin antibody (clone C4, MP Biomedicals, Solon, Ohio, USA) overnight. This monoclonal actin antibody was successfully used to detect E. histolytica actin (Perdomo et al., 2013) The next day, the samples were first washed three times in PBS, followed by two washes in 1% BSA/PBS, and then incubated with 1:250 Alexa Flour 488 (Jackson ImmunoResearch, PA, USA) and 1:1,000 4',6-diamidino-2-phenylindole (DAPI; MP Biomedicals, Solon, Ohio, USA) for 3 hr at 4°C. At the end of the incubation, coverslips were incubated overnight at 4°C with 20 μM phalloidin [1 μM] conjugated to rhodamine (phalloidin conjugated to rhodamine was generously given by Prof. Adi Salzberg, Rappaport institute of Medicine, Technion, Haifa, Israel).
After incubation, the coverslips were washed three times in 1% BSA/PBS, and then with PBS. The samples were then mounted onto microscope slides with Fluoromount G (SouthernBiotech, Birmingham, AL, USA). The specimens were then examined under a confocal immunofluorescence microscope (ZEISS-LSM700 Meta Laser Scanning System confocal imaging system) with a 63× oil immersion objective.
Fluorescent quantification of control trophozoites and trophozoites treated with AF was performed using Fiji software (Schindelin et al., 2012).
4.9 Erythrophagocytosis assay
Erythrophagocytosis was assayed using a previously described protocol (Trebicz-Geffen et al., 2017).
4.10 Measurement of cytopathic activity
Cytopathic activity was assayed using a previously described protocol (Trebicz-Geffen et al., 2017).
ACKNOWLEDGMENTS
We would like to thank the staff of the Microscopy Imaging Laboratory in the Faculty of Medicine, Technion for help with their confocal microscopy resources, and the excellent support in image recording and analysis. We would also like to thank the staff of the Smoler Proteomics Center, Technion for help with the analysis of proteomics data.
The work was supported by the Israel Ministry of Health within the framework European Research Area NETwork Infect-ERA (031L0004; AMOEBAC project), the Israel Science Foundation (260/16), the Rappaport Institute, and the US–Israel Binational Science Foundation (2015211).
CONFLICT OF INTERESTS
The authors declare that they have no financial or other competing interests.