The role of the unfolded protein response in dengue virus pathogenesis
Summary
Symptomatic dengue virus (DENV) infections range from mild fever to severe haemorrhagic disease and death. Host-viral interactions play a significant role in deciding the fate of the infection. The unfolded protein response (UPR) is a prosurvival cellular reaction induced in response to DENV-mediated endoplasmic reticulum stress. The UPR has complex interactions with the cellular autophagy machinery, apoptosis, and innate immunity. DENV has evolved to manipulate the UPR to facilitate its replication and to evade host immunity. Our knowledge of this intertwined network of events is continuously developing. A better understanding of the UPR mediated antiviral and proviral effects will shed light on dengue disease pathogenesis and may help development of anti-DENV therapeutics. This review summarizes the role of the UPR in viral replication, autophagy, and DENV-induced inflammation to describe how a host response contributes to DENV pathogenesis.
1 INTRODUCTION
Dengue virus (DENV) causes a reemerging arthropod-borne viral infection transmitted by the Aedes mosquito. Approximately half of the global population is at risk of infection from this Flavivirus, which poses a significant health burden (Gubler, 2011). Infection by DENV serotypes 1–4 causes symptoms ranging from asymptomatic illness to severe haemorrhagic fever and death. Severe dengue disease is characterized by increased vascular permeability leading to hypovolaemic shock and multiorgan failure (Basu & Chaturvedi, 2008). Pathogenesis of severe manifestations is poorly understood, and the variable presentation is presumed to be due to complex host-viral interactions modulating viral replication and the host immune response. There have been significant advances in vaccine development against DENV, but an effective antiviral is still not available.
DENV utilizes the host cellular machinery for the production of viral proteins. Endoplasmic reticulum (ER) stress caused by DENV infection mounts a prosurvival cellular reaction known as the unfolded protein response (UPR). This cascade of events, important not just in viral but also bacterial and nonpathogenic conditions, maintains cell survival and facilitates eradication of the virus. The UPR has complex interactions with the cellular autophagy machinery, apoptosis mediators, innate immunity, and proinflammatory reactions. DENV selectively modifies this host process to enhance viral replication and has the ability to evade host innate immunity. As such, host-viral interactions play an important role in deciding the fate of the infection in affected individuals. Our understanding of the highly complex intersection of and interplay between the UPR, induction, and effector mechanisms of innate immunity and autophagy with the DENV life cycle in human host cells is continuously evolving. It is important to explore the role of the UPR and its multiple cellular effects during DENV infection for a better understanding of its pathophysiology and to explore potential dengue therapeutic targets. This review summarizes the current knowledge of the role of the UPR in DENV pathogenesis with special emphasis on its effects on viral replication, autophagy, and DENV-induced inflammation.
2 THE UPR AND ITS PATHWAYS OF ACTIVATION
The UPR is a host cellular reaction that counteracts and alleviates ER stress. Viral or bacterial infections, changes in cellular redox status, changes in calcium homeostasis, and nutrient starvation all cause accumulation of unfolded and misfolded proteins, which exceed the capacity of ER protein handling (Celli & Tsolis, 2015; Smith, 2014). This results in ER stress, which leads to activation of the UPR for cell survival. Once activated, the ensuing cascade of events results in attenuated messenger RNA (mRNA) translation, which limits protein load in the ER, an increase in the ER protein folding capacity and ER-associated degradation (ERAD) of misfolded proteins. However, increased synthesis of ER chaperones BiP/GRP78, GRP94, protein disulphide isomerase, and CHOP/GADD135 amidst inhibition of global protein production highlights the specificity of this process (Diehl, Fuchs, & Koumenis, 2011). Furthermore, activation of the UPR is known to potentiate the host antiviral response creating an inflammatory milieu (Diwaker, Mishra, & Ganju, 2015; Smith, 2014). Inability of these mechanisms to restore cellular homeostasis results in apoptosis.
The UPR initiation molecules, inositol requiring kinase 1 (IRE1 or ERN1), protein kinase R (PKR)-like ER kinase (PERK or EIF2AK3), and activating transcription factor 6 (ATF6) reside in the ER membrane and are maintained in an inactive state by the folding chaperone immunoglobulin heavy-chain binding protein (BiP or GRP78; Smith, 2014; Figure 1). BiP, a member of the Hsp70 chaperone family, is an inhibitory regulator of the three ER membrane sensors and is bound to the lumenal domains of these molecules. When ER stress occurs, BiP is competitively sequestered by misfolded proteins in the ER and released from the initiation molecules. This triggers homodimerisation and phosphorylation of IRE1 and PERK with subsequent activation of the downstream signaling cascades (Smith, 2014). Other potential mechanisms of activation are direct sensing of misfolded proteins by IRE1 (Ron & Walter, 2007) and dissociation of stable BiP-ATF6 complex by an undefined mechanism (Shen, Snapp, Lippincott-Schwartz, & Prywes, 2005).
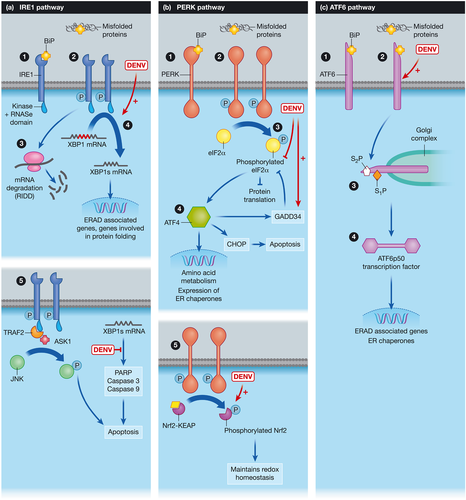
IRE1 has a stress sensing ER-lumenal N-terminal and an effector C-terminal tail (protein kinase domain and RNase domain) in the cytoplasm (Bhattacharyya, 2014; Diehl et al., 2011). Activation of IRE1 cleaves XBP1 (Chan, 2014; Diehl et al., 2011; Iranpour et al., 2016), activates the regulated IRE1-dependent decay (RIDD) pathway, and promotes expansion of the ER membrane (Iranpour et al., 2016). Activation of c-Jun N-terminal kinase (JNK) and the effect of IRE1 on apoptosis is shown in Figure 1a (Chen et al., 2016; Peña & Harris, 2011; Smith, 2014; Upton et al., 2012). PERK phosphorylates eukaryotic translation initiation factor 2α (eIF2α), which inhibits global protein translation except that of the activated transcription factor 4 (ATF4) reading frame (Figure 1b). ATF4 is a transcription factor that regulates amino acid metabolism and oxidative stress and modulates expression of ER chaperones and foldases (Iranpour et al., 2016). Phosphorylation and activation of eIF2α by PKR, general control nonderepressible-2-kinase, and heme-regulated eIF2α kinase are alternative pathways of eIF2α activation (Janssens, Pulendran, & Lambrecht, 2014). Activated ATF6 is cleaved to produce the activated transcription factor ATF6p50, which translocates to the nucleus to regulate the expression of genes in the ERAD pathway (Grootjans, Kaser, Kaufman, & Blumberg, 2016). Overlap of the branches of the UPR is demonstrated by activation of XBP-1 by ATF6, enhancing the prosurvival factors and upregulation of ER chaperones (Yoshida, Matsui, Yamamoto, Okada, & Mori, 2001). Both ATF6 and IRE1-XBP1s cascade independently and in combination help to relieve ER protein burden by enhancing ERAD. Low level of ER stress is presumed to be handled by the ATF6 pathway, medium to high levels by both ATF6 and IRE1-XBP1, and extremely high levels by multiple rounds of activation of the XBP1 cycle (Yoshida et al., 2001). However, prolonged ER stress results in activation of the cell death pathways.
3 UPR POTENTIATES VIRUS-INDUCED INFLAMMATION
3.1 The inflammatory pathways activated in response to viral infections
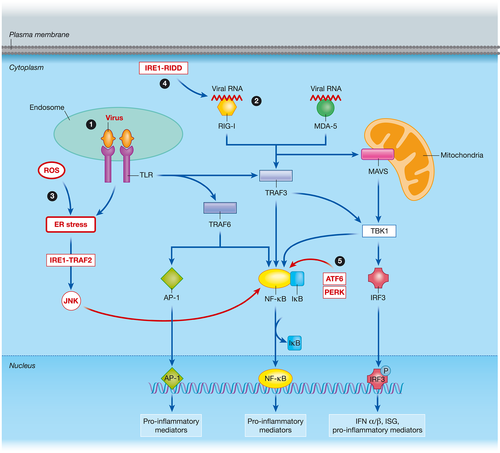
Viral infections induce a potent inflammatory response in the infected cells. The complex network activates type 1 interferons (IFNs) and other antiviral mediators to eradicate the offending virus and limit its dissemination (Chang, Liao, & Lin, 2006). Proinflammatory mediators such as tumour necrosis factor α (TNFα), interleukin (IL) 6, IL-8, and RANTES recruit other cell types, induce oxidative stress, and contribute to inflammation (Valadao, Aguiar, & de Arruda, 2016). In addition, several cell death pathways are activated in the infected cells to limit infection. Viral components are detected by toll-like receptors (TLRs) and cytoplasmic helicases (Nasirudeen et al., 2011; Olagnier et al., 2014; Valadao et al., 2016). The ensuing cascade of events (Dalrymple, Cimica, & Mackow, 2015; Green, Beatty, Hadjilaou, & Harris, 2014) is shown schematically in Figure 2.
3.2 The role of UPR in the inflammatory response against viral infection
There is evidence that the UPR plays a crucial role in inflammatory mediator production during viral infections (Grootjans et al., 2016). It potentiates the production of inflammatory mediators, which limit viral replication and facilitate cell survival. The UPR and its downstream inflammatory cascade is activated by virally induced TLR signaling, NADPH oxidase 2 (NOX2)-derived reactive oxygen species (ROS) produced during viral infection, and ER stress related to misfolded and unfolded proteins. Type 1 IFN and IFN-stimulated gene (ISG) produced in response to viral infection play a major role in limiting dissemination of the virus. Here, we have summarized the role of the UPR in activating inflammatory pathways in response to viral infections. We also highlight how certain viruses utilize the UPR in a proviral manner to inhibit IFN signaling.
Viral infection-induced TLR signaling can play a role in UPR activation. TLR signaling activates the IRE1 pathway leading to splicing of XBP1 (Figure 1a). XBP1s is crucial in inducing the production of TNFα, IFN-β, and IL-6 in macrophages following TLR stimulation (Kim et al., 2015; Martinon, Chen, Lee, & Glimcher, 2010). Macrophages deficient in XBP1 have an impaired cytokine response to pathogens (Martinon et al., 2010). In addition, IRE1 is able to increase IL-1β directly by inducing glycogen synthase kinase 3β (Kim et al., 2015). The IRE1-TRAF2 complex activates JNK (Figure 1a) and recruits IκB kinase to induce nuclear factor-κB (NF-κB) activation (Walter & Ron, 2011; Figure 2). Translational activation of retinoic acid-inducible gene I (RIG-I) by the IRE1-RIDD pathway further activates NF-κB-induced inflammation (Lencer, DeLuca, Grey, & Cho, 2015; Figure 2). Activation of the PERK pathway and the subsequent attenuation of protein translation increases the ratio of NF-κB/IκB resulting in increased NF-κB activity (Tam, Mercado, Hoffmann, & Niwa, 2012). Both JNK and NF-κB lead to induction of cytokines and inflammatory mediators. ER stress potentiates induction of TNFα, IL-1, IL-6, and chemokines such as CXCL1 and CXCL2. ATF6 also plays a role in activating NF-κB (Grootjans et al., 2016; Figure 2). GADD34 is important in overcoming the translation inhibition induced by phosphorylated eIF2α (Figure 1). The recovery of protein synthesis by expression of GADD34 plays a crucial role in producing IFNβ in response to viral double-stranded RNA sensing (Clavarino et al., 2012; Dalet et al., 2017). GADD34 deficiency inhibited IFNβ production and increased chickungunya viral replication in mouse embryonic fibroblasts. Despite such evidence on the role of GADD34 in enhancing innate immunity, reversing the translation attenuation could be hijacked by viruses to produce viral proteins. This will be further discussed in subsection 4.1 in the context of DENV.
In addition, viral infections produce NOX2-derived ROS. The oxidative stress cause accumulation of misfolded and unfolded proteins resulting in ER stress and activation of UPR. ROS are essential for XBP1-induced cytokine production (Grootjans et al., 2016). Concomitant activation of TLR and UPR pathways has the potential to amplify the cytokine response and determine cell survival during viral infections.
Although the UPR facilitates innate immune responses against viral infections, the literature reveals that viruses inhibit type 1 IFN signaling in a PERK-dependent manner (Liu et al., 2009). Activation of the PERK arm due to ER stress phosphorylates IFN-α/β-receptor 1 leading to its degradation. Liu et al. revealed that vesicular stomatitis virus and hepatitis C virus (HCV) infections cause PERK-dependent ubiquitination of IFN-α/β-receptor 1 and subsequent reduced type 1 IFN signaling.
4 MODULATION OF UPR BY DENV FOR ITS PATHOGENESIS
4.1 The role of the UPR in facilitating viral replication in DENV infection
Increased production of viral proteins in dengue infection leads to accumulation of unfolded and misfolded proteins in the ER (Diwaker et al., 2015). This results in ER stress and thus activation of the UPR as a host response. The ensuing cascade of events described above relieves ER stress, determines cell survival, and mounts an inflammatory response to eliminate the virus. DENV, similar to many other viruses, has been found to manipulate the host UPR pathways to enhance its survival. In this section, we describe how DENV facilitates viral protein production and inhibits apoptosis to ensure enhanced viral replication.
Dengue infection induces the three arms of the UPR (Klomporn, Panyasrivanit, Wikan, & Smith, 2011; Peña & Harris, 2011; Umareddy et al., 2007). Activation of this host response is observed in immune (Klomporn et al., 2011) and nonimmune cells (Peña & Harris, 2011; Umareddy et al., 2007; Yu, Hsu, Liao, & Lin, 2006) infected with DENV. Activation of the UPR is time dependent (Peña & Harris, 2011) and also strain and serotype specific (Umareddy et al., 2007). The PERK arm is activated early in DENV infection followed by IRE1-XBP1 in midinfection and ATF6 later in the infection (Peña & Harris, 2011). Serotype-specific differences in UPR activation are seen in dengue-infected A549 cells and similarly in antibody-dependent enhancement (ADE) infection of THP-1 monocytic cells (Paradkar, Ooi, Hanson, Gubler, & Vasudevan, 2011; Umareddy et al., 2007). DENV 1 and 3 serotypes cause a stronger induction of BiP, phosphorylation of eIF2α, higher GADD34, and CHOP mRNA levels than DENV 1 and 4 (Paradkar et al., 2011). In addition, ADE infection of monocytes using different strains of DENV 2 clinical isolates (Indonesian outbreak) showed higher levels of BiP, CHOP, and GADD34 in strains causing severe disease (Paradkar et al., 2011). Fast replicating viral strains demonstrated higher ER stress. This suggests a possible role for UPR in DENV pathogenesis and severity.
Activation of the PERK arm is antiviral due to attenuation of protein translation. Peña et al. demonstrated that DENV transiently activated PERK-dependent eIF2α phosphorylation at 6 hr postinfection, which reversed rapidly causing suppression of eIF2α activation thereafter. DENV ensures continuous production of its proteins and facilitates virion production by rapidly reversing PERK activation (Figure 1b). This is presumed to be due to an increased expression of GADD34, which acts in a feedback loop to inhibit further phosphorylation of eIF2α (Peña & Harris, 2011; Umareddy et al., 2007). Pharmacological activation of the PERK arm (via nuclear transporter 4-HPR) or removing the GADD34-mediated dephosphorylation of eIF2α (using Salubrinal) results in significant inhibition of DENV viral replication (Fraser et al., 2014; Umareddy et al., 2007). It is apparent that DENV modulates the PERK pathway to overcome translation attenuation for survival, and pharmacological upregulation of this process results in enhanced antiviral activity.
Infection with DENV activates the IRE1-XBP1 pathway and a subset of XBP1-activated genes involved in ERAD, which helps to alleviate ER stress (Yu et al., 2006). XBP1 pathway activation is induced by ER-associated DENV glycoproteins (prM, E, and NS1) and smaller hydrophobic ER-anchored proteins (NS2A, NS2B, and NS4B). In addition, DENV is able to inhibit apoptosis mediators downstream of IRE1-XBP1 (Figure 1a), which results in increased cell survival and enhanced viral replication (Peña & Harris, 2011). Inhibition of XBP1 using small interfering RNA, in conjunction with DENV infection, results in reduced ER expansion, increased cytopathic effects of the virus, and increased levels of the apoptosis marker procaspase 3 (Yu et al., 2006). This is further evidence that activation of the IRE1-XBP1 pathway in dengue infection protects the cells against apoptosis and alleviates ER stress contributing to DENV pathogenesis.
4.2 Modulation of autophagy via the UPR facilitates DENV production and inhibits cellular apoptosis
Autophagy is a cellular catabolic process which promotes formation of autophagic vacuoles to degrade and recycle cellular constituents. It plays an important role in regulating cell growth and development and is implicated in certain disease pathologies (Lee et al., 2008). Dengue NS4A is an important mediator of autophagy (McLean, Wudzinska, Datan, Quaglino, & Zakeri, 2011). Activation of this antiviral response promotes removal of viral constituents and facilitates viral antigen presentation. However, DENV is known to modulate the process in a proviral manner. DENV induces autophagy resulting in protection of infected cells against apoptosis (Datan et al., 2016; Fischl & Bartenschlager, 2011; Lee et al., 2008). Lee et al. demonstrated that activation of autophagy during dengue infection results in an increase in intracellular and extracellular viral load. Double membrane vesicles formed during autophagy provide a platform for viral replication (Jain, Chaturvedi, & Jain, 2014). In addition, autophagy promotes viral transmission by evasion of antibody-dependent neutralisation (Wu et al., 2016). DENV-induced autophagy is able to facilitate β-oxidation of cellular lipids to provide adenosine triphosphate for viral replication (Heaton & Randall, 2011). It is evident that autophagy is utilised by DENV for its advantage.
Two of the mediators activating autophagy in DENV infection are ER stress and the UPR (Datan et al., 2016). The PERK pathway activated in response to ER stress upregulates ROS production and increases autophagy turnover during DENV infection. Although the contribution of ROS to autophagosome formation is modest, PERK plays an important role in maintaining high autophagy levels and contributes to production of mature and infective viral particles (Datan et al., 2016). Thus, the UPR plays an important role in mediating and activating autophagy, which facilitates viral replication.
4.3 The role of the UPR in DENV-induced inflammation
DENV, similar to many viruses, activates signaling pathways leading to production of antiviral mediators. These proinflammatory cytokines and chemokines aid eradication of DENV. Evasion of these defenses by DENV will result in enhanced viral replication. Simultaneously, overexpression of these mediators can cause harm to the host (Jaiyen, Masrinoul, Kalayanarooj, Pulmanausahakul, & Ubol, 2009; Valadao et al., 2016) and possibly contribute to the cytokine storm, which is associated with increased vascular permeability seen in severe dengue disease. The UPR is an important mediator of virus-induced inflammatory signaling. However, the role of the UPR in DENV-mediated inflammation is underinvestigated. The literature supporting this area is complicated to interpret due to the different cell systems used and the pathways investigated. We bring together what is currently understood to summarize for the field how interactions between DENV and the UPR can affect, with both proviral and antiviral outcomes, innate immune pathways such as IFNα/β, NF-κB, IFN regulatory factor 3 (IRF3), and TNF-α (Table 1).
| Study type | Cell type | Main findings | Citation |
|---|---|---|---|
| In vitro | RAW (murine macrophages), human monocytes | DENV NS2B3 induces ER stress to cause PERK-regulated Nrf2 activation, CLEC5A expression, and TNF-α production | Cheng et al. (2016) |
| In vivo (mice) | Mouse brain tissue | Pharmacological inhibition of Nrf2 reduced CLEC5A expression and production of TNF-α, IL-6, and IP-10 in brain tissue of DENV-infected mice | Cheng et al. (2016) |
| In vitro | THP-1 (human monocytic cells) | Pharmacological inhibition of GRP78 (BiP) using VER treatment activated PERK, IRE1, and ATF6 arms of UPR and led to increased expression of NF-κB, IRF3, PKR, and IL-1β in DENV-infected cells | Diwaker et al. (2015) |
| VER treatment significantly reduced DENV viral load compared to mock treated cells | |||
| In vitro | Huh 7 (human hepatoma cells) | UPR activates autophagy, which downregulates PAMP-mediated IFNβ promoter activation in HCV and DENV infection | Ke and Chen (2011) |
| UPR-autophagy downregulates IFN mediated downstream signaling (ISRE promoter activity to IFNα stimulation and ISG56 expression) in HCV and DENV infection |
- Note. UPR = unfolded protein response; DENV = dengue virus; ER = endoplasmic reticulum; PERK = protein kinase R-like ER kinase; TNF = tumour necrosis factor; IL = interleukin; BiP = immunoglobulin heavy-chain binding protein; IRE1 = inositol requiring kinase 1; ATF6 = activating transcription factor 6; NF-κB = nuclear factor-κB; IRF = interferon regulatory factor; PKR = protein kinase R; VER = VER-155008; HCV = hepatitis C virus; ISRE = IFNα-stimulated ISG response element; ISG = interferon-stimulated gene; IFN = interferon; PAMP = pathogen-associated molecular pattern.
Activation of the UPR by VER-155008 (VER) treatment in DENV infection activates innate immune factors such as PKR, IRF3, IL-1β, and NF-κB (Diwaker et al., 2015). VER causes pharmacological inhibition of GRP78 (BiP) and the enhanced innate immune activation achieved by upregulation of IRE1, PERK, and ATF6 results in increased DENV clearance. This suggests that the UPR can potentiate innate immune responses in DENV to facilitate viral clearance.
A critical antiviral mechanism against DENV is the type 1 IFN response. Patients with severe disease have lower serum IFNα levels and suppression of ISG during early DENV infection, which highlights the role of type 1 IFN in dengue pathogenesis (De La Cruz Hernandez et al., 2014; Medina et al., 2015; Singla et al., 2016). DENV, similar to many viruses, has developed strategies to evade IFN activation, signaling, and effector mechanisms. DENV inhibits IFN induction and its signaling pathways to facilitate its replication and survival (Green et al., 2014). Both TLR-induced inflammatory pathways and RIG-I/MDA-5-induced pathways are inhibited by DENV in an attempt to reduce type 1 IFN and cytokine production (Borsa et al., 2015; Chang et al., 2012; Dalrymple et al., 2015). One host response exploited by DENV to evade innate immunity is the UPR-autophagy pathway. The role of UPR-autophagy in suppressing innate immunity is well studied in infection with HCV, another Flavivirus. Activation of the UPR is required for upregulating autophagy in HCV-infected cells, and this activation significantly reduces the HCV pathogen-associated molecular pattern-mediated cytoplasmic RIG-I signaling. RIG-I activation leads to phosphorylation, dimerization, and nuclear translocation of IRF3 (Figure 2). which induces IFNβ promoter activation. Ke et al. demonstrated that UPR-autophagy mediators (CHOP, ATG5, LC3B, IRE1, PERK, and ATF6) negatively regulated HCV pathogen-associated molecular pattern-mediated IFNβ mRNA levels and IFNβ promoter activation. Interference with the UPR-autophagy pathway by stable gene knockdown (ATG5 and CHOP gene silencing) in human hepatoma cells led to increased IFNβ promoter activation and IFNβ mRNA levels in HCV. Furthermore, IFN-mediated downstream signaling detected by IFNα-stimulated ISG response element promoter activity and IFN-induced protein with tetratricopeptide repeats 1 (IFIT1/ISG56) expression was upregulated in ATG5 and CHOP gene knockdown cells in HCV infection (Ke & Chen, 2011). In the same study, the authors showed that the negative effect of UPR-autophagy on innate immune reponses is not limited to HCV but extended to DENV. IFNβ promoter activity and ISG56 expression were enhanced in cells following ATG5 and CHOP gene silencing and in Atg5−/− mouse embryonic fibroblasts in DENV infection (Ke & Chen, 2011). It is evident that UPR-autophagy plays a crucial role in inhibiting type 1 IFN activation and its downstream signaling cascades in DENV infection. Further investigation of the role of UPR-autophagy pathway in DENV-mediated inflammation is useful as repression of this cascade is an attractive antiviral therapeutic option.
DENV infection induces production of proinflammatory mediators, such as TNFα, implicated in causing severe disease (Cardier et al., 2005; Chakravarti & Kumaria, 2006). Activation of the PERK arm of the UPR in DENV infection was recently shown to upregulate Nrf2-CLEC5A-mediated TNFα production (Cheng et al., 2016). Pharmacological inhibition of Nrf2 in vivo results in reduced TNFα production in the brain of DENV-infected mice and a better clinical outcome.
The UPR has complex interactions with the anti-inflammatory and proinflammatory cascades during DENV infection. Manipulation of induction of UPR pathways during DENV infection shows that they can mediate suppression and induction of innate immune and inflammatory pathways, affecting both viral replication and immunopathology. Further investigation of these interactions would lead to a better understanding of DENV pathogenesis and help explore antiviral therapeutic targets.
5 MANIPULATION OF ER STRESS AND THE UPR AS THERAPEUTIC TARGETS
Utilising the antiviral effects of the UPR provides attractive options for developing antiviral therapy. We herein discuss four such examples in the literature. Salubrinal inhibits the protein phosphatase 1-GADD34 complex and thereby maintains eIF2α-induced protein attenuation in DENV infection. Treatment with salubrinal causes an antiviral effect in DENV-infected cells. The BiP inhibitor, VER, activates all three arms of the UPR causing reduced viral replication in DENV (Diwaker et al., 2015). A nuclear transporter activating the PERK pathway, 4-HPR, inhibits DENV replication in monocytes and in a lethal ADE mouse model (Fraser et al., 2014). Celgosivir is an antiviral iminosugar, which is undergoing phase 2 clinical trials for DENV. It exerts antiviral effects by interfering with N-linked glycosylation of glycoproteins in the ER. In addition, celgosivir modulates the UPR to reduce phosphorylation of eIF2α, CHOP mRNA expression, and increases EDEM-1 transcripts to repress ER stress and facilitate cell survival (Rathore et al., 2011). These are some examples of how manipulation of the UPR and its pathways could be utilised for antiviral therapy. Additional investigation into UPR-autophagy and UPR-inflammatory pathways could provide further options of developing anti-DENV therapeutics.
6 CONCLUDING REMARKS
DENV infection causes ER stress, which activates the cellular UPR as a host response. The overall antiviral effect of the UPR pathways against DENV is highlighted by evidence that pharmacological activation of the UPR is antiviral. The UPR has complex interactions with apoptosis, autophagy, and viral-mediated inflammatory pathways. DENV exploits this host response to enhance viral replication by inhibiting apoptosis, upregulating autophagy, and suppressing innate immune responses. These proviral outcomes are due to both the time-dependent activation of different pathways and to modulation of different downstream regulators, such as DENV-induced upregulation of GADD34. The UPR exerts both proinflammatory and anti-inflammatory effects in DENV infection. These are mostly in vitro studies or in vivo data in animal models. It is unclear how the overall effects add up to disease pathogenesis in humans during DENV infection. We can assume that the variable disease severity seen in DENV could be due to the individual balance between these different effects. Further studies are needed to understand the complex nature of these interactions, which would provide useful targets for antiviral therapy and expand our knowledge on DENV pathogenesis.
ACKNOWLEDGEMENTS
We are grateful to Beatrice Tyrrell for her comments and feedback on the manuscript. NP is funded by the Commonwealth Scholarship Commission. J. L. M. is supported by Oxford Glycobiology Institute Endowment and N. Z. is a Fellow of Merton College, Oxford.
CONFLICT OF INTEREST
The authors do not declare any conflicts of interest.