Perspectives on mycobacterial vacuole-to-cytosol translocation: the importance of cytosolic access
Summary
Mycobacterium tuberculosis, the infectious agent of human tuberculosis is a master player in circumventing the defense mechanisms of the host immune system. The host-pathogen interaction in the case of an infection with M. tuberculosis is highly complex, involving dedicated mycobacterial virulence factors as well as the action of the innate and adapted immune systems, which determine the outcome of infection. Macrophages play a key role in this process through internalizing the bacterium in a phagosomal vacuole. While this action has normally the function of eliminating invading bacteria, M. tuberculosis employs efficient strategies to prevent its extermination. The question on how-and-where the bacterium succeeds in doing so has interested generations of scientists and still remains a fascinating and important research subject focused on mycobacterial lipids, secretion systems and other contributing factors. This topic is also central to the longstanding and partially controversial discussion on mycobacterial phagosomal rupture and vacuole-to-cytosol translocation, to be reviewed here in more detail.
Historic insights and techniques during the study of the intracellular niche of mycobacteria.
Electron-microscopy
Forty-five years ago, Armstrong and D'Arcy Hart published their pioneering work using cultured mouse peritoneal macrophages for infection experiments with viable and non-viable M. tuberculosis and Mycobacterium bovis BCG, followed by electron-microscopy (EM). One of the take-home messages was that phagosomes containing intact (viable) mycobacteria, showed only infrequent signs of phagosome-lysosome fusion (Armstrong & Hart, 1971). From this landmark study and follow-up investigations, a scenario of exclusive phagosomal containment of mycobacteria emerged, in which M. tuberculosis resides and multiplies within a vacuole whose maturation/acidification is blocked or retarded by the bacterium. However, in later EM studies in the 1980's, phagosomal egress of M. tuberculosis was observed with rabbit alveolar macrophages at a high multiplicity of infection (MOI) of 20-25:1 (Leake et al., 1984; Myrvik et al., 1984). In the 1990's, infection experiments of J774 mouse macrophages with M. tuberculosis H37Rv, H37Ra and BCG at an MOI of 10:1 identified differences between the used strains (McDonough et al., 1993). Whereas BCG was nearly always found surrounded by a distinct vacuolar membrane at 4 days after infection, M. tuberculosis H37Rv was seen in clearly membrane-enclosed vacuoles only about half of the time, and H37Ra had an intermediate phenotype (McDonough et al., 1993). These results were different from data obtained soon after by EM analysis of Mycobacterium avium and M. tuberculosis-infected mouse bone marrow-derived macrophages (BMDM) (Xu et al., 1994), for which no such extra-phagosomal intracellular location of mycobacteria was observed. As a possible explanation for the discrepancy, the use of organic solvents in EM sample preparations of the earlier study was evoked, which might influence the preservation of phagosomal membranes, whereas in the latter study cryopreservation and immuno-EM were applied (Xu et al., 1994). Hence, the question of vacuole-to-cytosol translocation during mycobacterial infection remained subject of intense scientific debate.
Novel insight into this matter came about ten years later from experiments involving Mycobacterium marinum in RAW264.7 and BMDM cells (Stamm et al., 2003). M. marinum is a natural pathogen of fish and frogs causing a systemic tuberculosis-like disease marked by the presence of a granulomatous host response. M. marinum is also an opportunistic human pathogen and has become a mycobacterium model organism (Stinear et al., 2008; Tobin & Ramakrishnan, 2008). In 2003, it was shown by EM that M. marinum was able to escape the phagosome (Stamm et al., 2003). The same study also found through confocal microscopy that M. marinum forms comet-tail like actin protrusions to promote bacterial movements in the cytoplasm (Stamm et al., 2003) similar to Listeria monocytogenes (Cossart, 2011) or Shigella flexneri (Ehsani et al., 2012). This recently confirmed feature of M. marinum inducing actin-tail formation (Singh et al., 2016) seems to be unique among mycobacteria. Indeed, it was not observed for M. tuberculosis, although more and more evidence for its cytosolic presence at certain stages of infection accumulated in recent years. In particular, it was the study by the group of Peters, which challenged again the paradigm of exclusive phagosomal containment in 2007, as images obtained by sophisticated cryo-immunogold EM showed a substantial percentage of intracellular M. tuberculosis bacteria that were clearly not contained in a membrane-enclosed compartment inside dendritic cells, nor could they be labeled with the lysosomal-associated membrane protein LAMP-1 (van der Wel et al., 2007). Such extra-phagosomal location was not observed for the attenuated BCG vaccine strain (van der Wel et al., 2007), which is deleted for the ESX-1 type VII secretion (T7S) system (Pym et al., 2002; Hsu et al., 2003; Brodin et al., 2006; Abdallah et al., 2007). This finding emphasized the implication of an intact ESX-1 T7S system in vacuole-to-cytosol translocation, a feature that was also previously evoked for M. marinum (Stamm et al., 2003). Cytosolic M. tuberculosis was also observed in another EM study, which reported loss of phagosomal membrane for approximately 25% of the viewed bacteria 5 days after infection of human macrophages (Lee et al., 2008). Finally, EM-generated observations on vacuole-to-cytosol translocation were recently extended to a range of clinical M. tuberculosis isolates, which showed that between 25 to 50 % of these bacteria were cytosolic at day 2 post infection of human macrophage-like THP-1 cells, whereas reference strains CDC1551 and H37Rv showed similar percentages at day 3 and 4, respectively (Houben et al., 2012), suggesting that strain-specific characteristics also have an impact on the egress phenomenon.
An alternative model: The amoeba Dictostelium discoideum
In parallel to EM studies that used different cell lines, BMDMs, and/or primary human phagocytes, the group of Soldati established an alternative infection model by using the social amoeba Dictostelium as a genetically tractable host for pathogenic mycobacteria. Amoeba naturally feed on different soil bacteria and have developed efficient phagocytosis and defense mechanisms. Experimental infection of Dictyostelium with M. marinum or M. tuberculosis revealed phagosome-to-cytosol translocation in an ESX-1 T7S system-dependent manner for both mycobacterial species. Moreover, bacteria spread from intact hosts to neighbouring cells using an actin-based structure termed the ejectosome (Hagedorn et al., 2009; Friedrich et al., 2012; Gerstenmaier et al., 2015).
Beta-lactamase-driven Fluorescence Resonance Energy Transfer (FRET) screen for detection of cytosolic bacteria
The development of a vacuolar rupture assay applicable to slow-growing mycobacteria has been an important advance for the study of cytosolic access of mycobacteria (Simeone et al., 2012; Simeone et al., 2015; Wang et al., 2015). This method, which was originally developed to monitor Shigella-induced vacuole rupture in single cells for the identification of the involved factors (Ray et al., 2010; Mellouk et al., 2014), takes advantage of the endogenous, surface-anchored mycobacterial ß-lactamase BlaC (Flores et al., 2005) to cleave a fluorescent substrate (CCF4), composed of a cephalosporin core linking 7-hydroxycoumarin and fluorescein (Fig. 1). This approach together with automated microscopy revealed that during infection of THP-1 macrophages M. tuberculosis and recombinant ESX-1-reconstituted BCG strains displayed phagosomal rupture 3 to 4 days post-infection, which was followed by host cell death (Simeone et al., 2012). Using a highly sensitive flow-cytometric readout, the FRET-based method showed a weak but reproducible signal of phagosomal rupture and cytosolic access by ESX-1-proficient M. tuberculosis as early as 3 h after infection, whereas the maximal values were obtained after 48 hours (Simeone et al., 2015). This latter adaptation of the method also allowed to observe a phagosome-to-cytosol connection in vivo in different M. tuberculosis-infected phagocyte subtypes inside the lung parenchyma, granuloma and spleen of mice at the chronic phase of infection (Simeone et al., 2015). Moreover, the FRET approach together with the use of mouse macrophage cell lines harbouring functional or non-functional alleles of the Natural resistance-associated macrophage protein (Nramp)-1 established a link between the inhibition of phagosomal acidification and phagosomal membrane rupture. It was noticed that functional Nramp-1R associated with the acidification of the phagosomal lumen weakens the ESX-1-mediated induction of phagosomal rupture. Together, this suggests that both the ability for blocking of maturation/acidification (Russell, 2011) and the induction of phagosomal rupture are necessary for full virulence of pathogenic mycobacteria (Simeone et al., 2015) .
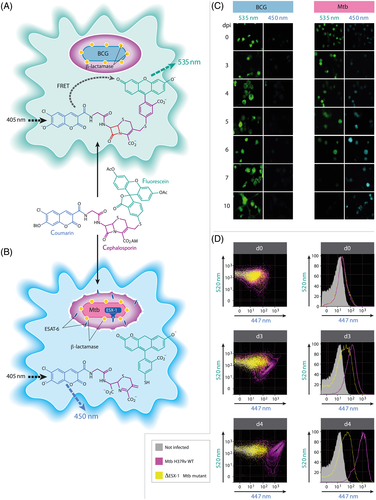
Cartoon of the CCF4 based Fluorescence Resonance Energy Transfer (FRET) assay in infected phagocytes.
A. CCF4-AM (Life Technologies) represents a lipophilic, esterified form of the CCF4 substrate, which allows it to readily enter host cells. Endogenous cytoplasmic esterases rapidly convert CCF4-AM into a negatively charged form, which is trapped in the cytosol. Following excitation at 405 nm, a green fluorescence FRET signal (535 nm) is emitted. Situation found for infection with BCG.
B. In case of phagosomal rupture induced by ESX-1-proficient M. tuberculosis (Mtb) and cytosolic access, the mycobacterial β-lactamase (shown as yellow dots linked to the bacterium) gets in contact with the CCF4 substrate trapped in the cytosol, and is inducing cleavage of the substrate and inhibiting FRET, thereby leading to emission of blue fluorescence at ~ 450 nm.
C. Comparison of fluorescence emission by automated microscopy of THP-1 cells infected with BCG or M. tuberculosis over time (days post infection; d.p.i.); figure adapted from reference (Simeone et al., 2012).
D. Comparison of the ability to induce phagosomal rupture subsequent to infection with wild-type (WT) M. tuberculosis H37Rv or ΔESX-1 M. tuberculosis, as determined by the number of bone marrow derived dendritic cells (MOI = 1) emitting green and/or blue CCF4 derived signals at different time points; figure adapted from reference (Simeone et al., 2015).
Galectin-3/ubiquitin assays to detect vacuolar disruption
Paz and co-workers have developed an approach taking advantage of the faculty of members of the galectin protein family, namely galectin-3, which binds host N-acetyllactosamine-containing glycans in a Type III-secretion (T3S) system-dependent manner. Fluorescently labelled galectin-3 or galectin-8 containing structures around entering bacteria visualize the remnants of ruptured vacuoles, thus serving as a potential novel tool to spot vacuole lysis induced by extraphagosomal pathogens, such as Shigella or Salmonella (Paz et al., 2010; Thurston et al., 2012). In 2011, Wong and Jacobs adapted this technique for mycobacterial infection experiments and showed that in M. tuberculosis-infected THP-1 cells at 2 days post infection, phagosomes containing ESAT-6-expressing M. tuberculosis recruited galectin-3 and ubiquitinated protein aggregates, whereas ESAT-6-deletion mutants did not (Wong & Jacobs, 2011). In contrast, a previous EM study showed galectin-3 accumulation also around vacuoles containing ESX-1-deficient BCG (Beatty et al., 2002). This could suggest temporary damage of the BCG-containing vacuoles, as can be observed during epithelial cell infection with Salmonella (Kreibich et al., 2015), and/or background activity. It would be interesting to compare the situation for M. tuberculosis under the same experimental conditions.
Mycobacterial factors that mediate perturbation and disruption of phagosomal membranes
Among the currently known mycobacterial factors implicated in phagosomal rupture, the aforementioned ESX-1 T7S plays a primordial role. It is widely distributed among actinobacteria (Bottai et al., 2016; Dumas et al., 2016) and it has gained an important role as virulence factor in pathogenic mycobacteria. Importantly, strains lacking a functional ESX-1 system (BCG, Mycobacterium microti) are attenuated (Brodin et al., 2002; Pym et al., 2002) and ESX-1 deletion mutants are unable to spread from cell to cell (Gao et al., 2004; Guinn et al., 2004). In virulent strains this latter feature has been linked to the membrane-damaging activity of ESAT-6 (Hsu et al., 2003; Gao et al., 2004; de Jonge et al., 2007; De Leon et al., 2012), which is also essential for disruption of phagosomal membranes (Stamm et al., 2003; van der Wel et al., 2007; Hagedorn et al., 2009; Houben et al., 2012; Simeone et al., 2012; Simeone et al., 2015). However, differences for ESX-1 systems and/or substrates from different mycobacterial species have been noted. While ESX-1-proficient slow-growers, such as M. marinum, Mycobacterium kansasii or M. tuberculosis were able to cause phagosomal rupture (Simeone et al., 2012; Wang et al., 2015), the non-virulent species Mycobacterium smegmatis which contains a more distantly related ESX-1 system (Converse & Cox, 2005; De Leon et al., 2012; Dumas et al., 2016,) was unable to induce this phenomenon (Simeone et al., 2012). These findings also suggest that additional mycobacterial factors might potentially contribute to phagosomal rupture and research into this question is ongoing. However, recent investigations on phospholipases C (PLCs) of M. tuberculosis showed that they were not essential for induction of phagosomal rupture or virulence (Le Chevalier et al., 2015), unlike the PLCs of L. monocytogenes (Cossart, 2011).
Comparison of mycobacterial cytoplasmic access with other pathogens
Cytoplasmic access is a widely used strategy of bacterial pathogens that occurs in professional phagocytes and in invaded epithelial or endothelial cells. The most studied examples have been S. flexneri and L. monocytogenes. Mechanistically, specialized pore-forming toxins, such as Listerolysin-O or the translocon complex of Shigella, have been portrayed as the main machinery capable of damaging the endocytic vacuole. A similar process could be imagined for the ESX-1 T7S components of mycobacteria, however its mode of operation requires more biophysical analysis. More recently, the involvement of host proteins for vacuolar membrane damage has been investigated highlighting a prominent role of host pathways, such as Rab GTPases Rab5 and Rab11 in the case of Shigella (Mellouk et al., 2014), or the regulatory proteins gamma-interferon-inducible lysosomal thiol reductase (GILT) and cystic fibrosis transmembrane conductance regulator (CFTCR) for Listeria (reviewed in (Fredlund & Enninga, 2014)).
Our changing understanding of intracellular localization of mycobacteria portrayed here is also taking place for other “historically” phagolysosomal pathogens, such as Salmonella enterica or Legionella pneumophila species (Fredlund & Enninga, 2014). Within the first two hours of host cell invasion, Salmonella damages its endocytic vacuoles in 10-20% of the cases subjecting the pathogen either to autophagy or it starts to hyper-replicate within the cytoplasm (Knodler et al., 2010; Thurston et al., 2012). In the case of Legionella, injected type 4 secretion (T4S) system effector, namely SdhA and PlaA are required to maintain the Legionella containing vacuole, similarly to Salmonella SPI-2 effectors (Creasey & Isberg, 2012). The subversion of host pathways for the control of cytoplasmic access remains to be investigated in more detail for mycobacteria. Interestingly, vacuolar damage can also be repaired during bacterial invasion, as has been shown prominently during Salmonella infection of epithelial cells (Kreibich et al., 2015). It is likely that similar mechanisms might take place in mycobacteria-infected macrophages.
Cell biological and immunological consequences of cytosolic access of mycobacteria
In the controversy about the exclusive phagosomal containment versus cytosolic presence of M. tuberculosis, McDonough and colleagues evoked that the major histocompatibility complex (MHC) class I-restricted presentation of mycobacterial antigens could arise either from bacteria escaping directly from the tightly apposed membrane vesicles into the cytoplasm or from bacilli enclosed within a peculiar vesicular membrane that allowed export of mycobacterial components into the cytoplasm (McDonough et al., 1993). This prediction made in 1993 was rather visionary. Indeed, a few years later the ESX-1 T7S system was discovered, and since then shown to be essential for the induction of a wide range of innate and adaptive immune responses mostly linked to the cytosolic presence of mycobacteria and/or their bacterial products (Ferwerda et al., 2005; Stanley et al., 2007; Simeone et al., 2015; Watson et al., 2015). ESX-1-dependent cytosolic contact is also needed for IL-18-mediated noncognate IFN-γ secretion by M. tuberculosis-antigen-independent memory CD8+ T-cells and NK cells during infection with M. tuberculosis (Kupz et al., 2016). The cytosolic detection of mycobacterial dsDNA occurs partly via the key innate sensor AIM2 (Absent in Melanoma 2), which activates the inflammasome and ultimately the secretion of active IL-1β (Mishra et al., 2010) with largely admitted protective effects (Fremond et al., 2007). Moreover, signalling via the mammalian cytosolic DNA sensor cGAS (cyclic GMP-AMP (cGAMP) synthase) is also an ESX-1-dependent process (Collins et al., 2015; Majlessi & Brosch, 2015; Wassermann et al., 2015; Watson et al., 2015). Following infection with ESX-1-proficient mycobacteria, host cGAS forms intracytosolic punctate structures, which co-localize with DNA. This event is concomitant with the synthesis of the second messenger cGAMP that engages the ER-associated adaptor STING (Stimulator of IFN Genes) as a secondary receptor (Manzanillo et al., 2012; Sun et al., 2013), which in turn recruits the TBK-1 (TANK-Binding Kinase-1) to phosphorylate IRF3 (Interferon regulatory factor). Activation of this axis triggers transcription of IFN-β and interferon-stimulated genes including CXCR10 (IP-10). IFN-β is believed to have detrimental effects during M. tuberculosis infection, comprising the triggering of IL-10 production (Kaufmann & Dorhoi, 2016).
Conclusions
As outlined above, numerous arguments point into the direction that virulent M. tuberculosis strains indeed gain access to the cytosolic compartment of host phagocytes. Whether this access is happening due the complete disassembly of the phagosomal membrane, as observed in certain images generated by EM and/or cryo-immunogold EM, or whether cytosolic access may also represent a dynamic process that is associated with membrane rupture and subsequent membrane repair, is difficult to define, as such conclusions require time-resolved data at ultrastructural resolution that are not readily obtainable. However, several other features on cytosolic access of M. tuberculosis and selected other mycobacterial pathogens are very clear-cut. First of all, FRET techniques can reliably distinguish between strains that establish cytosolic contact from strains that remain vacuolar-bound, although method-related limitations for defining the subcellular location of the pathogen exist (Simeone et al., 2012). Moreover, it is clear that the ESX-1 T7S system is required for such cytosolic access, even if mechanistic insights into the interactions of ESX-1 effectors with phagosomal membranes and their dynamics are still scarce. Moreover, the link between type I interferon responses and ESX-1-mediated cytosolic access of M. tuberculosis is clear, same as for MHC-I-mediated immune responses. Similarly, the link between NLRP3 inflammasome activation and a functional ESX-1 T7S system appears obvious.
All these features are clearly different between ESX-1-proficient M. tuberculosis and the ESX-1-deficient BCG, which is important to remember in the context of infection biology. It seems that quite often, experiments undertaken with BCG or the attenuated M. tuberculosis H37Ra were considered as representative for virulent wild-type (WT) M. tuberculosis. However, for the study of intracellular location and/or ex vivo/in vivo growth experiments BCG and H37Ra are both inadequate model organisms, as they either have deleted the ESX-1 system (BCG) or have a regulatory mutation blocking ESAT-6 secretion (H37Ra) (Frigui et al., 2008). Caution should indeed be taken for the interpretation of cell biological and immunological results obtained with such attenuated strains, as they might not at all reflect the characteristics seen with WT M. tuberculosis.
Finally, it is intriguing that all strains used in large-scale vaccination programs throughout the history of human anti-tuberculosis vaccination (BCG and M. microti) (Sula & Radkovsky, 1976; Hart & Sutherland, 1977; Mangtani et al., 2014) were ESX-1 mutants, unable to access the cytosol of phagocytes and to induce cytosol-associated immune responses. However, there is growing evidence that such responses are important for achieving superior protection relative to BCG in experimental models (Pym et al., 2003; Brodin et al., 2004; Bottai et al., 2015; WMCVfTS-Group, 2015; Kupz et al., 2016), a feature that shall be considered for the development of future improved human anti-tuberculosis vaccines.
Acknowledgements
The authors acknowledge support from the EC grants 643381 and 261166, the Agence National de Recherche (ANR-14-JAMR-001-02) and the Fondation pour la Recherche Médicale FRM (DEQ20130326471). R.B. and J. E. are members of the LabEx consortium IBEID at the Institut Pasteur.