CaP bone-like coating for fast osseointegration of dental implants
P5R2K ePOSTER BASIC RESEARCH
Background: Rapid osseointegration is crucial to improve stability of dental implants after placement. Calcium Phosphate (CaP) is a favourable element in bone-to-implant bonding process and state-of-the art titanium implants for trauma applications are already spray-coated with a thick Hydroxyapatite layer. However, due to delamination risks a thick coating is not suitable for dental implants with fine features and complex topography. A new method that allows CaP deposition on dental implants is then needed.
Aim/Hypothesis: Aim is to develop a thin bone-like CaP layer coating strongly anchored on the titanium implant surface to promote the osseointegration process. The coating shall not alter the implant SLA surface morphology and no delamination shall be visible after insertion.
Materials and Methods: The developed “NanoCoat” CaP coating is applied to sand blasted and acid etched surfaces. It consists of two steps process. First, a thin (˜1 μm) nanoporous grafting layer (GL), chemically bonded with the implant is created via the Kokubo method. This GL layer is based on a titanium oxide layer. Second, a CaP layer is deposited via wet-biomimetic route and anchored to the GL. The process does not modify but replicates the rough implant SLA-surface topography. The GL and CaP coating stability was assessed via SEM after insertion into predrilled Sawbone and cortical bovine bone (60 Ncm torque). In-vitro tests were conducted on ø14 mm discs with different surface treatments: maCHINAd, sandblasted and acid-etched, surface with grafting layer (GL) obtained by chemical and thermal treatments, and the final CaP NanoCoat surface. Biocompatibility was tested with human osteosarcoma MG63 cells. Cytotoxicity and alkaline phosphatase (ALP) activity for osteoblast differentiation were investigated.
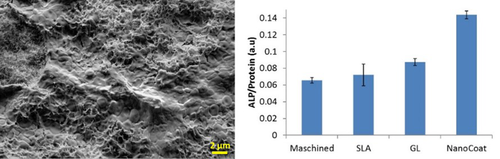
Results: Neither GL nor CaP modified surfaces were affected after insertion tests. The NanoCoat surface morphology can still be recognized and no delamination is visible. After extraction, biological material from the bone strongly adheres to the implant surface covering it (Fig 1 left). For the biocompatibility tests, MG63 cells proliferated on the NanoCoat surface at the same rate as on control SLA. However, the ALP assay results (Fig 1 Right) identify differences among the four substrates: the NanoCoat surface showed an osteoblastic differentiation with ALP activities double than the SLA gold-standard reference.
Conclusions and Clinical Implications: The presented results, together with the established coating process, show the potential of the NanoCoat method in promoting rapid osseointegration of dental implants. The tests results exclude as well possible coating delamination. Due to the peculiarity of the deposition process, NanoCoat CaP layer can be applied not only on dental implants but on Ti-implants of any geometry, i.e. for craniomaxillofacial, spinal or orthopaedic devices.
Acknowledgements: We thank the Swiss Nanoscience Institute and Medicoat AG for the financial support, and Hager & Meisinger for supplying the implants.
Left, SEM of NanoCoat surface after explantation from cortical bovine bone. Right, comparative ALP assay carried out on four different substrates. The error bar corresponds to ±SD.