Volumetric analysis on the use of customized healing abutments with or without connective tissue graft at flapless maxillary immediate implant placement: A randomized clinical trial
Abstract
Objectives
To evaluate buccal volume change after using a customized healing abutment with or without connective tissue grafts (CTG) in flapless maxillary immediate implant placement (IIP).
Materials and Methods
The present study was designed as a randomized clinical trial (RCT). Patients treated with flapless maxillary IIP were allocated into two groups, both receiving a customized healing abutment, and additionally, the test group received a CTG. A cone-beam computerized tomography (CBCT) allowed to access the initial buccal bone thickness (BT). Digital impressions were taken prior to extractions (T0), 1 month (T1), 4 months (T2), and 12 months (T3) after implant insertion and superimposed with computer software allowing to compute variables related to buccal volume variation (BVv) and total volume variation (TVv) (ClinicalTrials.gov: NCT05060055).
Results
Thirty-two patients (mean age 48 ± 11 years), sixteen in each group, were evaluated after a 12-month period. After 1 year of treatment, no significant differences were found between groups, although in participants with BT ≤1 mm, control and test groups showed a BVv of −14.18 ± 3.49% and −8.30 ± 3.78%, respectively (p = .033). Regarding mucosa height variation variables, the control group showed approximately the triple vertical recession in both papillae.
Conclusions
The placement of a CTG was not capable of completely maintaining the initial peri-implant tissue architecture, although in thin-bone phenotypes, less dimensional changes are expected when a CTG was used.
1 INTRODUCTION
Immediate implant insertion has been found as a highly predictable option when a replacement of a hopeless tooth is needed (Ortega-Martínez et al., 2012; Vignoletti & Sanz, 2014). Although this treatment modality had highlighted several advantages either in single or multiple rehabilitations, such as the reduced treatment time, cost, and surgical trauma (Noelken et al., 2018), it requires some essential initial soft and hard tissue features in order to achieve a successful functional and esthetic outcome (Moraschini et al., 2015; Pieralli et al., 2018). The literature suggests that the immediate implant protocol is incapable of completely avoiding soft and hard tissue remodeling, both vertically and horizontally (Buser et al., 2017). It also considers the presence of intact bone walls, the thickness of the buccal bone plate, and the gingival biotype as crucial parameters to decide when a delayed approach might be favorable (Buser et al.).
Immediate implant placement (IIP) is frequently accompanied by marginal soft tissue recession, which can be followed by an esthetic failure (Blanco et al., 2019; Seyssens et al., 2021). Over the years, numerous surgical and prosthetic techniques have been elaborated to avoid possible IIP future complications (Blanco et al.). The preference for surgical procedures such as a flapless atraumatic approach and a palatal positioning of the implant (Raes et al., 2011), the filling of the gap between the implant shoulder and the bone walls with deproteinized bovine bone mineral (DBBM) (Zaki et al., 2021), the use of a customized healing abutment (Fernandes et al., 2021), and the application of an autologous connective tissue graft (CTG) (Seyssens et al.; Raghoebar et al., 2021) seem to counteract the shrinkage at the peri-implant area. Several studies' analyses have shown advantages of harvesting a CTG for buccal volume augmentation, reporting less midfacial recession, and a wider and thicker gingival biotype (Migliorati et al., 2015; Zuiderveld et al., 2021). Nevertheless, randomized clinical trials conducted by means of a digital methodology capable of interpreting peri-implant tissue behavior when a CTG is used or not are scarce. Three-dimensional imaging methodologies that assess profilometric measurements and volumetric changes promote an objective observer-independent evaluation at a predefined area during the timeline of treatment, reporting higher levels of reproducibility when compared with indices-based analyses.
Thus, the aim of this study was to evaluate buccal volume variation change after using a customized healing abutment with or without connective tissue grafts in flapless maxillary immediate implant placement.
2 MATERIALS AND METHODS
2.1 Study design
The present study was designed as a controlled clinical trial with a parallel-group design and balanced randomization (ratio 1:1) to document the peri-implant tissues buccal volume change in using customized healing abutments with connective tissue graft (test group) or without connective tissue graft (control group) as different treatment methods in flapless maxillary immediate implants. The protocol was reviewed and approved by the Institute of Bioethics of the Catholic University of Portugal (n°139/21), and the patients included were previously informed and agreed to participate in this investigation signing an informed consent considering the 1975 Declaration of Helsinki, revised in 2013. In addition, this investigation has been registered at the U.S. National Library of Medicine (ClinicalTrials.gov) website under the reference number NCT05060055. Group designation was computed and randomized after implant insertion by an investigator (D.F.) not involved in surgical procedures, randomly allocating participants to one of the two treatment groups. Thirty-two patients in need of a single implant restoration in the maxillary arch following tooth extraction were included in this study. Patients were treated between October 1, 2021, and November 4, 2022. Patients' inclusion criteria were as follows: (1) ≥18 years of age; (2) patients who had a failing tooth and needed an implant placing therapy in the esthetic zone (between 15 and 25); (3) the failing tooth has adjacent and opposing natural teeth; (4) sufficient mesial-distal and inter-occlusal space for placement of the implant and definitive restoration; (5) had an intact socket wall previously to the extraction; and (6) had sufficient apical bone to place an immediate implant with minimum primary stability of 30 N/cm. Exclusion criteria were as follows: individuals diagnosed with periodontal disease; medical and general contraindications for the surgical procedure; heavy smokers (>10 cigarettes/day); and an active infection at the implant site. A CONSORT 2010 checklist was performed in order to consider an appropriate guideline for the present randomized trial study (Schulz et al., 2010). No outcome data were excluded.
2.2 Surgical protocol
The surgical procedure was conducted under local anesthesia 4% articaine with adrenaline 1:100000 (Ubistesin™, 3 M-ESPE). In both groups, flapless tooth extractions were performed after sectioning the tooth, followed by the use of periotomes and elevators to separate the two parts of the tooth, avoiding damage to the buccal and palatal bone plates. All patients were treated with cylindrical shape implants (OsseoSpeed EV™, AstraTech Implant System, Dentsply Implants) with an internal connection platform following the surgical sequence protocol provided by the manufacturer. The implant was placed engaging the palatal and apical bone to achieve high primary stability. Implant diameter was selected in order to maintain a gap of at least 2 mm between the inner cortical buccal bone plate and the implant surface. This buccal void was filled with a deproteinized bovine bone mineral material (DBBM) (Symbios®, Dentsply Implants).
Both groups received a customized healing abutment with a polymethyl methacrylate (PMMA) material allowing them to close the socket without sutures (Figure 1). All customized healing abutments were manufactured in CAD/CAM software (Cerec in Lab MC XL, Sirona Dental Systems Gmbh) prior to surgery and milled by a specific milling machine (Sirona MCX5, Sirona Dental Systems Gmbh). The digital design of the customized healing abutments was adapted from the lost tooth anatomy, replicating the cervical area of the piece, ending in an abutment whose dimensions were a copy of the cervical area of the tooth. The test group was treated with a subepithelial connective tissue graft (CTG) harvested from the palate by the single-incision technique described by Hürzeler and Weng (1999) (Figure 1d–f), whereas control group did not receive any soft tissue augmentation (Figure 1a–c). Dimensions of each connective tissue grafted were adapted differently depending on the volume needed at the buccal peri-implant area. The distance between the adjacent papillae represented the width and changed with implant site, whereas the length was standardized for all connective tissue grafts (4 mm).
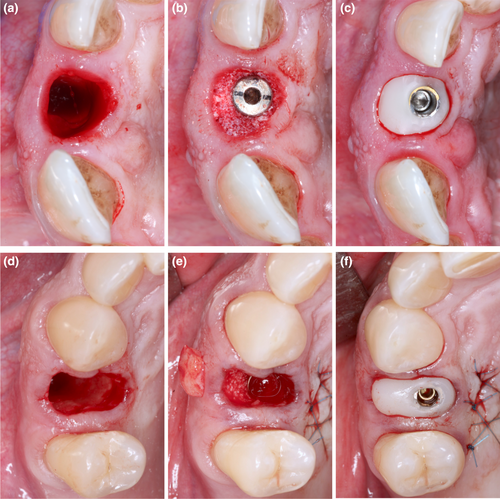
All surgical procedures were performed by one experienced surgeon (T.B.). The patients from both groups had provisional resin-bonded crowns to the adjacent teeth on the same day as the implant surgery, being removed after 16 weeks. Postoperative instructions were given to the patients, which included a soft diet, oral hygiene procedures, and chlorhexidine 0.12% rinsing twice per day for 2 weeks. Systemic antibiotics (amoxicillin 1 g twice per day for 7 days) and paracetamol 1000 mg, three times per day, for pain control, were prescribed. A screw-retained provisional crown was delivered after 4 months of healing and definitive restorations were inserted at the 6-month appointment, consisting of a screw-retained all-ceramic crown and a customized titanium abutment (Atlantis™, Dentsply Implants).
2.3 Clinical observation and data acquisition
Examination protocol and data collection were adapted from Borges et al. (2020) and consisted of four appointments: (1) T0 (before flapless tooth extraction and implant insertion); (2) T1 (1-month follow-up after implant placement); (3) T2 (4-month follow-up after implant insertion); and (4) T3 (1-year after implant placement). An intraoral optical scan (Cerec Primescan®, Sirona Dental Systems GmbH) of the upper arch and a cone-beam computer tomography (CBCT) evaluation (Ortophos XG 3D®, Sirona Dental Systems GmbH) were performed before tooth extraction and implant placement (T0). At this point, three clinical parameters were assessed with a periodontal probe (PCB 12; Hu-Friedy): KM (distance between the gingival groove and the mucogingival junction, to the nearest millimeter) and gingival thickness (GT), recorded in order to assess thin (Tn) and thick (Tk) soft tissue phenotypes. Immediately after implant placement, BID (distance between implant shoulder and the buccal bone plate) was assessed to the nearest millimeter in order to compute the dimension of the buccal gap created between the implant surface and the buccal bone plate. Intraoral scans were completed at 1 month (T1), 4 months (T2), and 12 months (T3) postimplant placement. Also, patient's perception of pain was registered using visual analog scale (VAS) on the 2nd day after surgery (VAS1) and on the 10th day after surgery (VAS2), with two endpoints representing 0 (“no pain”) and 10 (“pain as bad as it could possibly be”).
In all follow-up appointments, hygiene instructions were given to the patients and periodontal therapy was executed when necessary. Biologic complications such as peri-implant mucositis or peri-implantitis were recorded based on inflammation indicators such as bleeding on probing (BoP) and suppuration (Hashim et al., 2018). Radiographic examination was also conducted to observe possible interproximal bone loss. Technical complications were registered as prosthetic problems such as screw loosening, abutment fracture, ceramic chipping, or ceramic fracture.
2.4 Intra-observer agreement
One examiner (D.F.), blinded for the surgical procedure, was calibrated through an intra-examiner test (Dahlberg d-value), consisting of a double consecutive data collection of 10 randomly chosen patients included in this study. BVv variable was used for this assessment and an intra-class coefficient of 0.92 was obtained.
2.5 Matching digital models
All digital models were exported from the intraoral optical scanner software (Cerec Omnicam®, Sirona Dental Systems GmbH) in stereolithography (STL) format and were examined with a specially designed software (Geomagic Control X®, Geomagic, Inc.). The T0 and T1, T0 and T2, and T0 and T3 STL files were superimposed and a strict alignment was made into a common coordinate system. The final alignment was done through the best fit alignment algorithm for a perfect match of digital models.
2.6 Volumetric measurements
The digital analysis protocol was performed as described by Borges et al. (2020). After the superimposition of study models, a color map was created, allowing us to quantitatively analyze dimensional variations occurring in the surgical areas and surrounding tissues. Green color represents areas where no three-dimensional changes were found, while variations between yellow and red represent volume increase and variations between light blue and dark blue represent volume decrease. Moreover, the superimposed STL files were exported to another computer program (Materialise Magics®, Materialise) for volumetric assessment. A 3D volumetric ROI was manually selected with ‘Cut or Punch’ function considering interproximal areas as mesial and distal limits (Figure 2a–d). All cuts were performed in the same areas in all digital models ensuring that all measurements were carried out in the same regions. The use of ‘Boolean’ function was performed to create STL files related to volume reduction and volume increase that occurred at different time points (Figure 2d). Volumetric variation considering volume increase and volume reduction were represented as buccal volume variation (BVvT0-T1, BVvT0-T2, and BVvT0-T3) and total volume variation (TVvT0-T1, TVvT0-T2, and TVvT0-T3) in cubic millimeters (mm3) and relative percentages (%). The initial total volumes evaluated from each ROI at the buccal (BVt) and palatal (PVt) aspects were also computed for further comparison with volume variations at the different appointments. These calculations allowed to create relative percentages of volume variations which is essential to directly compare different patients due to anatomical variances. All measurements were recorded to the nearest 0.01 mm.
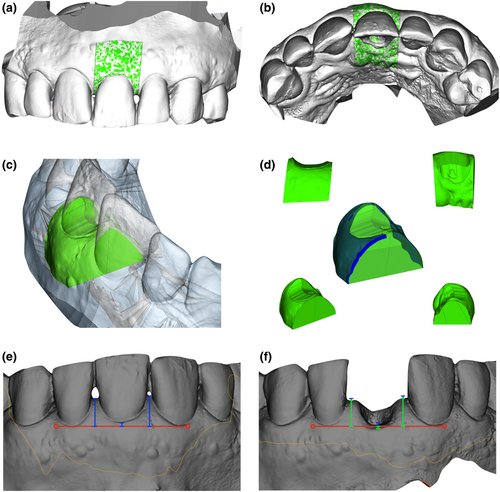
2.7 Midfacial mucosa and papillae outcomes
Midfacial mucosa and papillae height variation at the 1-year follow-up were analyzed using computer software (Materialise Magics®, Materialise). After precisely overlapping the T0 and T3 STL files in a common coordinate system, a standardized line (red) was created connecting the marginal gingiva's two most apical points of adjacent teeth, which served as a horizontal reference for the vertical measurements. Three measurements were computed in each STL file to calculate marginal gingiva mucosa and mesial and distal papilla height at T0 (Figure 2e) and T3 (Figure 2f). The mean differences in these measurements allowed us to calculate variables in mm representing the marginal gingiva height variation (MFHv), the mesial papilla height variation (MPHv), and the distal papilla height variation (DPHv). PHv (mm) variable was established as the mean difference considering both papillae.
2.8 Radiographic assessment
Radiographic examination was performed before surgery with a volumetric dimension of 8 × 8 centimeters for 14 s with the XG 3D tomography acquisition protocol, with a voxel size of 0.1 mm in high-definition mode. The obtained CBCT images were imported in a digital imaging and communications in medicine (DICOM) format to specific software for radiographic assessment (Materialise Mimics®, Materialise) in order to calculate buccal bone thickness (BT). All measurements were obtained through coronal slice reconstructions, using an adjacent line to the sinus/nasal plate as a reference as described by Arora and Ivanovski (2017). BT was measured 1 mm above the coronal bone margin using a central slice, as well as at the mesial and distal slices, ranging 1 mm from the central slice. Mean BT values were obtained as the average values of the three slices.
2.9 Statistical analysis
Sample size and power calculation were computed taking into consideration a significance value of α = .05 (type I error) and an effect size of 0.80 based on the BVv change as primary outcome, obtaining a sample size power of 81.2% for at least 16 patients per group. The mean value was considered as the reference parameter (difference between the two independent groups). The sample size computation for the present investigation was done using the sample size calculator G*Power version 3.1.9.6• taking into consideration the changes that were assessed between the initial situation and 1-year postimplant insertion.
The statistical analysis was performed using computer software (SPSS™, Statistical Package for the Social Sciences, version 21.0, IBM Corporation) by an independent statistician who was not involved in the surgical procedure or study design.
The established variables were presented as mean values, standard deviation, minimum, maximum, and 95% confidence interval. Normality of the data was assessed using Shapiro–Wilk test. Variables related to participant's characterization were analyzed to examine possible significant differences between the initial characteristics of the groups. Age, BT GT, BVt and PVt were assessed with independent sample t-test, whereas gender and implant site were evaluated with Chi-Square with Yates correction. Also, BID, KM, VAS1, and VAS were assessed with Mann–Whitney test. Volumetric variables at the different time points (T0, T1, T2, and T3) were evaluated with independent sample t-test, and the Mann–Whitney test was conducted to disclose differences for continuous nonpaired variables. The implant was defined as the statistical unit.
Moreover, a two-way ANOVA analysis with Bonferroni correction was computed to understand the buccal bone thickness effect on study volumetric variables, creating two classes of BT (BT ≤1 mm and BT >1 mm) for the comparison evaluation. Power calculation for this analysis was computed taking into consideration a significance value of α = .05 (type I error) and an effect size of 0.55, obtaining a sample size power of 82.3% for a total sample size of 32. All hypothesis tests were considered at the 5% level of significance.
3 RESULTS
3.1 Patients and implants
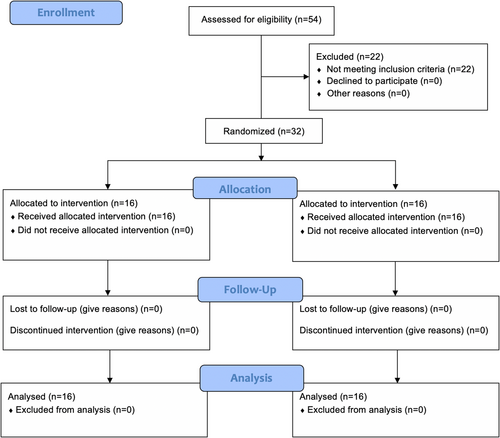
The patient distribution according to the CONSORT statement flow chart is reported in Figure 3. Fifty-four patients were initially enrolled in the study, however, after tooth extraction, 22 of 54 extraction sockets were not suitable for immediate implant placement, which led to them to be excluded from the trial. A total of 32 participants with a mean age of 48 ± 11 years (ranging from 23 to 69) were enrolled in this randomized clinical trial, with 16 individuals allocated to each experimental group. Participant's characteristics and distribution data are detailed in Table 1. All participants were healthy and nonsmokers. Also, no significant differences were found between groups in patient's demographic variables. In test group, 81% of the patients were men and 19% women, whereas control group had the same number of men and women. A buccal bone thickness mean value of 1.25 ± 0.47 mm was seen in control group, whereas test group showed a mean result of 1.15 ± 0.59 mm (p = .602). Regarding gingival thickness, control group showed the same number of participants with thick and thin biotypes, while in test group, 56% of patients had a thin biotype and 44% a thick biotype. Two days after surgery, control group reported a VAS score of 2.00 ± 0.7, and test group exhibited a VAS score of 2.38 ± 1.02 (p = .239). On the 10th day of follow-up, no significant differences (p = .780) were observed in control and test groups (0.13 ± 0.34 and 0.19 ± 0.40, respectively). No biological or technical complications were found in patients or implants within the 1-year follow-up, revealing a 100% implant success rate.
| Subject characterization | Group | N | Min | Max | SD | p–Value (Shapiro–Wilk)a | p–Value | |
|---|---|---|---|---|---|---|---|---|
| Patients | Control | 16 | – | – | – | – | – | |
| Test | 16 | |||||||
| Gender (male/female) | Control | 8♂/8♀ (50/50%) | – | – | – | – | – | .137b |
| Test | 3♂/13♀ (19/81%) | |||||||
| Age | Control | 16 | 37 | 69 | 51.25 | 9.125 | .926 | .061c |
| Test | 16 | 23 | 65 | 44.13 | 11.477 | .788 | ||
| Implant site incisive/premolar | Control | 7I/9PM (44/56%) | – | – | – | – | – | .457b |
| Test | 4I/12PM (25/75%) | |||||||
| BT (mm) | Control |
≤1 mm:6 >1 mm:10 |
0.10 | 1.95 | 1.25 | 0.471 | .627 | .602c |
| Test |
≤1 mm:8 >1 mm:8 |
0.10 | 2.42 | 1.15 | 0.588 | .359 | .273b | |
| BID (mm) | Control | 16 | 2 | 5 | 3.12 | 0.885 | <.05 | .305d |
| Test | 16 | 3 | 5 | 3.44 | 0.629 | <.01 | ||
| GT (Tk/Tn) | Control | 8Tn/8Tk (50/50%) | – | – | – | – | – | .999b |
| Test | 9Tn/7Tk (56/44%) | |||||||
| KM (mm) | Control | 16 | 3 | 5 | 4.13 | 0.806 | <.01 | .171d |
| Test | 16 | 3 | 5 | 3.63 | 0.957 | .065 | ||
| BVt (mm3) | Control | 16 | 136.54 | 457.03 | 246.85 | 90.832 | .916 | .625c |
| Test | 16 | 181.67 | 477.47 | 293.94 | 83.039 | .510 | ||
| PVt (mm3) | Control | 16 | 142.16 | 377.61 | 246.85 | 76.857 | .226 | .110c |
| Test | 16 | 195.77 | 428.64 | 288.64 | 67.455 | .528 | ||
| VAS1 | Control | 16 | 1 | 3 | 2.00 | 0.730 | <.01 | .239d |
| Test | 16 | 0 | 4 | 2.38 | 1.025 | .075 | ||
| VAS2 | Control | 16 | 0 | 1 | 0.13 | 0.342 | <.001 | .780d |
| Test | 16 | 0 | 1 | 0.19 | 0.403 | .001 |
- a Shapiro–Wilk test.
- b Qui-Square with Yates correction.
- c Independent sample t-test.
- d Mann–Whitney test.
- Abbreviations: , Mean; BID, Buccal Implant Distance (mm); BT, Buccal Thickness (mm); BVt, Buccal Volume Total (mm3); GT, Gingival Thickness; KM, Keratinized Mucosa (mm); Max, Maximum; Min, Minimum; PVt, Palatal Volume Total (mm3); SD, Standard Deviation; Tk, Thick; Tn, Thin; VAS, Visual Analogue Scale.
3.2 Digital assessment of volumetric variations
Volumetric peri-implant tissue variations from baseline to 1-year follow-up are shown in Table 2. None of the study volumetric variables exhibited statistically significant differences. At T1, BVv(%) of −5.59 ± 5.00% in control group and −4.35 ± 2.26% in test group was assessed, while TVv(%) showed a linear variation of −4.69 ± 4.46% at control group compared with −4.26 ± 2.87% at test group (p = .395 and p = .759, respectively). After 4 months of treatment, volumetric analysis revealed a change in BVv(%) of −6.98 ± 5.54% in control group and − 7.38 ± 4.02% in test group (p = .373) at T1, and TVv(%) showed values of −6.85 ± 4.37% for control group, whereas test group reported a TVv(%) of −5.38 ± 2.76% (p = .278). At T3, test group revealed less tissue variation than control group in all evaluated variables, yet no statistical significance was detected. A BVv(%) of −9.78 ± 4.59% in control group and − 9.69 ± 4.74% in test group were exhibited (p = .956), whereas TVv(%) showed a variation of −8.36 ± 3.08% in control group and −6.71 ± 3.47% in test group (p = .194).
| Variable | Group | N | Min;max | SD | CI (95%) lower;upper | t–Testa (p–Value) | |
|---|---|---|---|---|---|---|---|
| BVv T0–T1 (%) | Control | 16 | −18.40;−0.69 | −5.59 | 5.006 | −8.37;−2.82 | .395 |
| Test | 16 | −9.21;−0.84 | −4.35 | 2.257 | −5.66;−3.05 | ||
| BVv T0−T2 (%) | Control | 16 | −22.47;−1.06 | −6.98 | 5.544 | −10.05;−3.91 | .373b |
| Test | 16 | −19.08;−2.32 | −7.38 | 4.021 | −9.61;−5.16 | ||
| BVv T0−T3 (%) | Control | 16 | −19.18;−2.99 | −9.78 | 4.594 | −12.33;−7.24 | .956b |
| Test | 16 | −19.97;−3.68 | −9.69 | 4.744 | −12.55;−6.82 | ||
| TVv T0−T1 (%) | Control | 16 | −15.90;0.85 | −4.69 | 4.461 | −7.16;−2.22 | .759 |
| Test | 16 | −9.77;−0.29 | −4.26 | 2.869 | −5.91;−2.60 | ||
| TVv T0−T2 (%) | Control | 16 | −17.18;−2.01 | −6.85 | 4.371 | −9.27;−4.43 | .278 |
| Test | 16 | −12.46;−0.47 | −5.38 | 2.761 | −6.90;−3.85 | ||
| TVv T0−T3 (%) | Control | 16 | −13.24;−3.59 | −8.36 | 3.080 | −10.07;−6.66 | .194 |
| Test | 16 | −12.99;0.52 | −6.71 | 3.470 | −8.81;−4.62 | ||
| MFHv (mm) | Control | 16 | −1.68;0.51 | −0.60 | 0.723 | −0.98;−0.21 | .356 |
| Test | 16 | −1.08;0.80 | −0.38 | 0.446 | −0.62;−0.14 | ||
| MPHv (mm) | Control | 16 | −0.88;0.73 | −0.29 | 0.369 | −0.49;−0.10 | .157 |
| Test | 16 | −0.63;1.34 | −0.07 | 0.544 | −0.36;0.22 | ||
| DPHv (mm) | Control | 16 | −1.45;0.53 | −0.38 | 0.498 | −0.64;−0.11 | .706 |
| Test | 16 | −0.96;1.99 | −0.13 | 0.688 | −0.50;0.24 | ||
| PHv (mm) | Control | 16 | −0.96;0.44 | −0.34 | 0.358 | −0.53;−0.15 | .345 |
| Test | 16 | −0.66;1.51 | −0.10 | 0.554 | −0.40;0.19 |
- a Independent sample t-test.
- b Mann–Whitney test.
- Abbreviations: , Mean; BVv, Buccal Volume Variation (%); CI, Confidence Interval; DPHv, Distal Papilla Height Variation (mm); Max, Maximum; MFHv, Midfacial Mucosa Height Variation (mm); Min, Minimum; MPHv, Mesial Papilla Height Variation (mm); PHv, Mean Papillae Height Variation (mm); SD, Standard Deviation; TVv, Total Volume Variation (%).
3.3 Midfacial mucosa and papillae height variation
Midfacial mucosa and papillae height variation after the 1-year follow-up were computed and outcome data are shown in Table 2. No statistical differences were found in any variable related to midfacial mucosa and papilla height variation. At the final observation follow-up, test group showed a more coronal position of midfacial mucosa compared to control group (−0.38 ± 0.45 mm and −0.60 ± 0.72 mm, respectively). Both papillae sites had approximately three times less height variation in test group participants when compared with the control group. A height variation of −0.07 ± 0.54 mm at mesial site and −0.13 ± 0.69 mm at the distal site at the test group implants was in contrast with a height variation at the mesial and distal papilla of −0.29 ± 0.37 mm and −0.38 ± 0.50, respectively, at the control group implants. When considering both papillae height variations, PHv did not showed statistical significance between the two groups (p = .345).
3.4 Buccal bone thickness influence on volumetric variations
The two-way ANOVA analysis was conducted to understand the effect of buccal bone thickness (BT) on peri-implant tissues volumetric alterations, creating two classes of BT (BT ≤1 mm and BT >1 mm) for the comparison evaluation (Table 3). The interaction between group and BT factors proved to be significant on BVv(%) and TVv(%) variables at the different time points.
| Group | BT | Two-way ANOVA | |||
|---|---|---|---|---|---|
| BT (≤1 mm) | BT (>1 mm) | ||||
| BVv T0−T1 (%) | Control | −10.26 | −3.90 |
Group factor p = .053 BT factor p = .095 Interaction group/BT p = .006b (ƞ2 = .264) |
|
| SD | 5.179 | 3.662 | |||
| Test | −3.51 | −5.20 | |||
| SD | 1.978 | 2.333 | |||
| BVv T0−T2 (%) | Control | −11.55 | −5.31 |
Group factor p = .535 BT factor p = .226 Interaction group/BT p = .030a (ƞ2 = .169) |
|
| SD | 7.479 | 3.854 | |||
| Test | −6.39 | −8.26 | |||
| SD | 2.871 | 4.838 | |||
| BVv T0−T3 (%) | Control | −14.18 | −8.19 |
Group factor p = .364 BT factor p = .331 Interaction group/BT p = .020a (ƞ2 = .205) |
|
| SD | 3.490 | 3.917 | |||
| Test | −8.30 | −10.88 | |||
| SD | 3.777 | 25.435 | |||
| TVv T0−T1 (%) | Control | −9.12 | −3.08 |
Group factor p = .163 BT factor p = .148 Interaction group/BT p = .004b (ƞ2 = .292) |
|
| SD | 4.923 | 3.150 | |||
| Test | −3.15 | −5.36 | |||
| SD | 1.824 | 3.417 | |||
| TVv T0−T2 (%) | Control | −10.81 | −5.41 |
Group factor p = .040a BT factor p = .130 Interaction group/BT p = .014a (ƞ2 = .212) |
|
| SD | 5.233 | 3.159 | |||
| Test | −4.64 | −6.02 | |||
| SD | 1.692 | 3.432 | |||
| TVv T0−T3 (%) | Control | −10.85 | −7.46 |
Group factor p = .059 BT factor p = .473 Interaction group/BT p = .062 (ƞ2 = .137) |
|
| SD | 2.217 | 2.90 | |||
| Test | −5.88 | −7.43 | |||
| SD | 2.259 | 4.306 | |||
- a Statistically significant changes at the 5% level.
- b Statistically significant changes at the 1% level.
- Abbreviations: , Mean; BVv, Buccal Volume Variation (%); CI, Confidence Interval; ƞ2, Cohen's d formula; SD, Standard Deviation; TVv, Total Volume Variation (%).
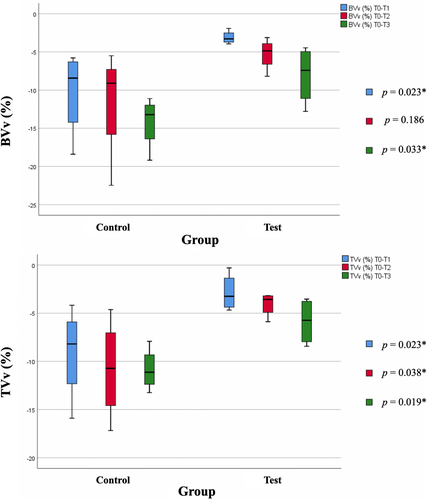
After the first year of treatment, when BT ≤1 mm, control group showed a buccal volume variation of −14.18 ± 3.49 mm, whereas test group exhibited a variation of −8.30 ± 3.78 mm. When considering participants with BT >1 mm, a buccal volume variation of −8.19 ± 3.91 mm in control group and a variation of −10.88 ± 25.43 mm in test group (p = .020) was observed. Cohen's d formula showed a significant interaction between group factor and BT factor on BVv(%) at T1, T2, and T3 revealing a moderate-effect size (ƞ2 ≥ .13).
Also, when comparing both groups of participants with BT ≤1 mm, a significant difference was found at T1 and T3 in both BVv(%) and TVv(%) variables (Figure 4).
4 DISCUSSION
This study comprised a randomized clinical trial of 32 dental implants that was conducted in order to evaluate soft and hard peri-implant tissues volumetric variation after maxillary flapless IIP and customized healing abutments with or without a connective tissue graft. All surgical procedures were performed by an experienced surgeon (T.B.) aiming to reduce the impact that the surgeon's skills might have had on the final esthetic outcome. Also, the digital analysis that was carried out in this investigation was done entirely by an independent investigator (D.F.) previously trained with the required tools and not involved in the surgical procedure.
Both groups presented a 100% success rate after 1 year of treatment, which is comparable to implant success rates reported in other investigations when choosing IIP as the treatment option (Vignoletti & Sanz, 2014). The existence of a three-dimensional bone structure with an adequate buccal bone plate and a sufficient band of keratinized mucosa is mandatory to achieve a future functional and esthetic outcome (Buser et al.). When these strict criteria are applied and when a correct restorative protocol is used, we are able to reach high percentages of postextraction implant survival rates. In fact, the period from the surgical stage to the final restoration step represents a critical time lapse for tissue healing and the restoration option (Vignoletti & Sanz, 2014).
Some literature states that after tooth removal, a horizontal and vertical resorption pattern of the surrounding mucosa will inevitably take place and the complete maintenance of initial tissue structures is not achievable (Araújo & Lindhe, 2005). Nevertheless, the clinician should take into consideration different approaches to minimize volumetric tissue contraction. In this investigation, two treatment approaches were evaluated in order to minimize and compensate for the changes that occur in the peri-implant tissues. Since our investigation aimed to test the influence of different surgical approaches in IIP, BVv was adopted as the main variable, a factor that intended to access the changes that may be present after the surgical implant insertion. The use of a CAD/CAM technique to fabricate a perfectly adapted PMMA healing abutment is a growing subject but is almost limited to description of techniques and case reports in terms of scientific evidence (Ruales-Carrera et al., 2019; Thoma et al., 2019). This approach has been described as a beneficial solution that permits to reproduce precisely the contours of the root cervical area, maintaining and personalizing peri-implant tissues architecture during the healing phase (Menchini-Fabris et al., 2020). Also, they allow to reduce postoperative discomfort and morbidity by avoiding a second stage surgery for dental implant exposure. When comparing patient morbidity by means of a numerical rate scale of pain after the use of standard healing screws (control group) and customized healing abutment (test group), Beretta et al. (2019) found that control group participants were associated significantly with more postoperative pain. An RCT from Perez et al. (2020), comparing customized healing abutments and standard healing abutments at IIP sites, showed favorable results in terms of papilla presence and marginal bone loss at the sites treated with customized abutments. Fernandes et al. (2021) also noticed statistically significant less volume variation at the first-month follow-up after the use of a customized healing abutment when compared to a collagen matrix as socket sealing option in IIP, although after the 1-year follow-up, no significant differences were found. It must be highlighted that in the control group, it was used a matrix that will clearly influence the healing cascade and enhance re-epithelialization.
Recently, different studies have been conducted in order to better understand the role of an autogenous connective tissue graft placed at IIP (Valles et al., 2022). The use of a connective tissue graft in a postextractive implant seems to promote beneficial results regarding soft tissue thickness. On the contrary, Ferrantino et al. (2021) concluded that the use of a CTG is not mandatory to achieve a satisfactory esthetic result when compared to not using a CTG. A randomized controlled trial with 60 single implants conducted by Nimwegen et al. (2018) under a digital analysis protocol also did not find less mucosa volume loss after a 12-month follow-up period after the use of a CTG, however, a more coronally position of the midfacial mucosa margin was observed. Other studies have also reported less facial mucosa recession after a soft tissue autogenous graft placement over 6 months (Jiang et al., 2020), 1 year (Yoshino et al., 2014; Zuiderveld et al., 2021), and 2 year of treatment (Migliorati et al.). In the present investigation, when analyzing midfacial and papillae height changes, a less vertical recession was assessed when a CTG was used. Despite the fact that no significant differences were found in midfacial mucosa and papillae height variation, it should be taken into account that control group mesial and distal papillae showed approximately three times more vertical recession when compared to test group, and midfacial mucosa recession was slightly higher at control group. The systematic review conducted by Valles et al. (2022) did not find statistical differences in papillae height variation between grafted and nongrafted implant sites as well.
In the present clinical trial, a similar volumetric variation was seen after the 1-year follow-up in both groups. Nevertheless, when conducting a two-way ANOVA analysis in order to investigate buccal bone plate thickness influence on volumetric variation variables, some interesting outcomes were observed. The interaction between group and BT values presents itself as statistically significant on buccal and total volume variation. In thin bone phenotypes, a statistically significant volume variation value when a CTG was not used was observed. In addition, it seems that participants with thin bone phenotypes that underwent a CTG placement exhibited similar results to those with a thick bone phenotype without receiving an autogenous graft. These results are in line with those stated by Seyssens et al. (2021) and Migliotori et al. (2015), reporting the importance of the use of a CTG in the presence of thin bone phenotypes.
Also, the gingival thickness seems to play an influencing role in peri-implant tissue dimensional changes after IIP (Kan et al., 2011). Nonetheless, Kan et al. (2009), when conducting a consecutive investigation over 2 years, observed that when soft tissue grafting procedures are applied to IIP, facial soft tissues maintain their stability irrespective of the gingival biotype. Other factors may be more crucial in order to avoid an increased buccal volume reduction and midfacial recession like the buccal bone thickness, a palatal positioning of the implant, and the filling of the gap (Sanz et al., 2017). The use of DBBM material appears to give long-term stability to the buccal bone wall since bone substitute granules have a low resorption rate over time (Sanz et al.).
An important concern related to the use of an autogenous graft is postoperative discomfort and patient morbidity. An alternative to this is the use of a collagen-based matrix (De Angelis et al., 2021). Different studies have reported fine functional and esthetic outcomes, nonetheless, CTG remains the gold standard to increase mucosal thickness around dental implants (Cosyn et al., 2021; Jung et al., 2022; Schmitt et al., 2021). In addition, less postoperative discomfort can be seen with the use of collagen matrices. In our study, no significant differences were exhibited in patient's perception of pain between groups. Also, Jung et al. (2022) did not find any significant difference regarding patient morbidity after single implants with or without an autogenous graft. These findings may be related to the graft size, surgeon's expertise, or patient. On the contrary, Thoma et al. (2022), when conducting a systematic review with meta-analysis that evaluated the patient-reported outcome measures after the use of autologous soft tissue grafts or soft tissue substitutes, stated that less pain perception was experienced at the soft tissue substitute group. Another alternative to a CTG might be the socket shield technique (SST), which some authors have considered capable of counteracting peri-implant tissue shrinkage. Recently, Gómez-Meda et al. (2022) stated that the performance of a CTG and SST allows maintaining peri-implant tissue architecture after 1 year of treatment. Notwithstanding, SST has been associated with postoperative complications such as infection and bone loss caused by the exposure of the root shield, thus, it is mandatory that only experienced surgeons perform this treatment option.
We may consider as a limitation of the present investigation the fact that no separation was made between soft and hard tissue variations over time. Chappuis et al. (2015) found that after tooth removal, in thin bone phenotypes patients, a natural compensation of soft tissue thickness gain would happen in order to counteract bone loss. It would be recommended to superimpose STL files from the soft and hard tissue separately to better understand peri-implant alveolar tissues behavior in future studies. Despite every participant maintaining a successful esthetic result from the surgeon's perspective after the 1-year follow-up, no esthetic evaluation like PES was conducted in this study. It is important to outline that this type of analysis was not the goal of our study, although it could be considered in future investigations. Another aspect that should be noticed is that premolars were also allocated in this protocol. Regardless of the fact that we obtained a normal distribution for BT, it is well known that premolars have a three-dimensional bone structure more favorable to operate and traditionally are less technique sensitive in comparison to incisive teeth. The effect of the placement of a connective tissue graft may also be less important when placing implants in these sites when compared to central and lateral incisors. We also believe that a future investigation with a larger sample and a longer follow-up may lead to different results.
In conclusion, the placement of a subepithelial connective tissue graft was not capable of completely maintaining the initial peri-implant tissue architecture and counteract effectively the initial reduction in the peri-implant tissues after IIP and CA insertion. Nevertheless, in individuals with thin bone phenotypes, significantly less dimensional changes were found when a CTG was used in comparison with participants who only received a customized healing abutment.
AUTHOR CONTRIBUTIONS
DF: Data collection; data analysis; and manuscript writing. TM: Data interpretation and manuscript writing. TB: Clinical treatment of cases; data collection; manuscript writing; and study coordination. JM: Revision of statistical analysis; manuscript revision; and study coordination.
ACKNOWLEDGMENTS
The authors thank Dr. Celeste Morais, Professor of Statistics, for the support with the statistical analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest in relation to this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




