Surface decontamination protocols for surgical treatment of peri-implantitis: A systematic review with meta-analysis
Funding information
This study was funded solely by the institutions of the authors.
Abstract
Objective
To answer the following PICO question: “In patients requiring surgical treatment of peri-implantitis (P), is any implant surface decontamination protocol (I) superior to others (C) in terms of clinical and radiographic parameters (O)?”
Methods
Randomized clinical trials (RCTs) comparing two or more decontamination protocols as part of the surgical treatment of peri-implantitis were included. Two authors independently searched for eligible studies, screened titles and abstracts, did full-text analysis, extracted data, and performed the risk-of-bias assessment. Whenever possible, results were summarized through random effects meta-analyses.
Results
Twenty-two manuscripts reporting on 16 RCTs were included, testing mechanical, chemical and physical decontamination protocols. All of them resulted in an improvement in clinical parameters; however, the superiority of specific protocols over others is mainly based on single RCTs. The use of titanium brushes and implantoplasty showed favorable results as single decontamination methods. Meta-analyses indicated a lack of added effect of Er:Yag laser on probing pocket depth (PPD) reduction (n = 2, WMD = −0.24 mm, 95% confidence interval [CI] [−1.10; 0.63], p = .59); while systemic antimicrobials (amoxicillin or azithromycin) showed an added effect on treatment success ([PPD ≤5 mm, no bleeding or suppuration, no progressive bone loss]; n = 2, RR = 1.84, 95% CI [1.17;2.91], p = .008), but not in terms of PPD reduction (n = 2, WMD = 0.93 mm, 95% CI [−0.69; 2.55], p = .26), even if with substantial heterogeneity.
Conclusions
No single decontamination method demonstrated clear evidence of superiority compared to the others. Systemic antibiotics, but not Er:Yag laser, may provide short-term clinical benefits in terms of treatment success (CRD42020182303).
1 INTRODUCTION
Peri-implantitis is a plaque-associated pathological condition affecting tissues around dental implants characterized by the inflammation of the peri-implant mucosa and the progressive resorption of supporting bone (Berglundh, Armitage, et al., 2018). Its estimated prevalence has been reported between 1 and 47%, depending on the employed case definitions (Derks & Tomasi, 2015; Romandini, Berglundh, et al., 2021; Romandini, Lima, et al., 2021). Peri-implantitis has a bacterial etiology, and therefore the success of treatment mostly depends on arresting the inflammatory process through efficient control of infection and removal of dysbiotic biofilm from the implant surface (Lindhe & Meyle, 2008).
Despite no clinical guidelines being available yet, a stepwise therapeutic approach similar to the one used for periodontitis is employed in the management of peri-implantitis (Sanz et al., 2020). After an initial phase, including oral hygiene instructions, risk factor control and supra-mucosal instrumentation, implants affected by moderate–severe peri-implantitis undergo surgical treatment, which comprehends access, resective or reconstructive procedures (Heitz-Mayfield & Mombelli, 2014). Although those surgical approaches demonstrated favorable treatment outcomes in terms of probing pocket depth (PPD) reduction (Roccuzzo et al., 2018), in most cases the composite criteria employed for defining treatment success are not achieved (Carcuac et al., 2016; Khoury et al., 2019).
Incomplete implant decontamination represents the main reason for this limited predictability (Meyle, 2012), due to the complex micro- and macro-topography of titanium interfaces and bony defects anatomies (Koo et al., 2019). Several mechanical (curettes, ultrasonic, irrigations with saline, air powder abrasion, titanium brushes, implantoplasty), chemical (citric acid, chlorhexidine - CHX, enamel matrix derivatives - EMD, topical or systemic antimicrobials) and physical (laser, photodynamic therapy) decontamination methods have been proposed either alone or in combination (Carcuac et al., 2016; Klinge et al., 2002; Louropoulou et al., 2014). Despite some reviews having tried to comprehensively assess the efficacy of adjunctive measures for the treatment of peri-implantitis (Ramanauskaite et al., 2021; Schwarz et al., 2015), a focused synthesis of the effect of the proposed decontamination protocols during surgical treatment of peri-implantitis, as well as the identification of the eventual superiority of specific methods over others is currently lacking.
Therefore, the primary aim of the present systematic review was to answer the following PICOS question: ‘In patients requiring surgical treatment of peri-implantitis (P), is any implant surface decontamination protocol (I) superior to others (C) in terms of clinical [changes in probing pocket depth (PPD) – primary outcome] and radiographic parameters (O) in randomized clinical trials (RCTs) (S)?’ Moreover, the present systematic review aimed at comprehensively analyzing the longitudinal effects of the implant surface decontamination protocols tested in RCTs.
2 MATERIALS AND METHODS
This systematic review is reported according to the PRISMA statement (Moher et al., 2009), and the protocol was registered on PROSPERO (CRD42020182303).
2.1 Eligibility criteria
- (P) Population. Patients in good general health requiring surgical treatment of peri-implantitis.
- (I) Interventions. Any type of local or systemic (i.e., systemic antimicrobials) implant surface decontamination protocol used during surgical treatment of peri-implantitis.
- (C) Comparisons. Any possible comparison between different protocols for intra-surgical decontamination (including placebo).
-
(O) Outcomes of interest:
- Primary outcome: changes in PPD.
- Other considered outcomes: changes in marginal bone level (MBL), treatment success (possibly adhering to the definition to Carcuac et al., 2016 - residual PPD ≤5 mm, no bleeding/suppuration on probing [BoP/SoP], no progressive marginal bone loss after treatment), BoP/SoP, soft tissue level changes, clinical/relative attachment level (CAL/RAL), implant survival, need of retreatment, patient-reported outcome measures (PROMs) and adverse events.
- (S) Types of studies. RCTs with at least 6-months follow-up and a minimum of 10 patients (5 per group). RCTs not directly comparing different decontamination protocols were excluded.
2.2 Search methods for the identification of studies
Four electronic databases were independently searched for relevant articles using the following search algorithms by two authors (GB and FC).
2.2.1 [MEDLINE] (via PUBMED) (2021-12-20)
(“peri-implantitis”[MeSH Terms] OR “peri-implantitis”[All Fields] OR “peri implantitis”[All Fields]) AND (“therapy”[All Fields] OR “treatment”[All Fields] OR “Decontamination”[Mesh] OR Antiinfective agents OR antimicrobials OR antibiotics OR “Therapeutics”[Mesh] OR “therapeutics”[All Fields]) NOT (retrospective OR review OR in vitro OR case report OR orthopedic OR animal OR experimental)
Filter: English.
Search 2: [EMBASE] (via ELSEVIER) (2021-12-20)
(‘periimplantitis’/exp OR periimplantitis OR (perimplant AND [‘disease’/exp OR disease])) AND (‘therapy’/exp OR ‘decontamination’/exp OR ‘debridement’/exp OR antimicrobials OR antibiotics) NOT (([retrospective OR review OR in] AND vitro OR case) AND report OR orthopedic OR animal OR experimental)
Filter: English.
Search 3: [SCOPUS] (2021-12-20)
periimplantitis AND (therapy OR decontamination) AND NOT (review OR in AND vitro OR animal).
Search 4: [CENTRAL (Cochrane central register of controlled trials)] (2021-12-20)
(peri-implantitis OR periimplantitis OR peri implantitis) AND (surgical treatment OR surgery OR surgical)
In addition, duplicate (GB and NB) hand-searching was performed from January 2010 to June 2021 on the following journals: Journal of Clinical Periodontology, Journal of Periodontology and Clinical Oral Implants Research. Reference lists and previous systematic reviews were also screened.
2.3 Study selection
The titles and abstracts of all identified studies were screened independently and in duplicate by two calibrated reviewers (NB and GB). Initial calibration of investigators was achieved by online discussion sessions. Any disagreement was resolved by discussion with a third reviewer (FC). Full text of studies of possible relevance were assessed in duplicate by two reviewers (NB and FC), in order to make a final decision about their inclusion. Percentage of agreement and kappa statistics were employed to score inter-rater agreement (yes/no) of the screening and full-text analysis processes. Disagreements were again resolved by a joint discussion with a third review author (GB). The reasons for study exclusion after full text analysis were recorded.
2.4 Data extraction and management
Data from included studies were extracted in duplicate by two reviewers (GB and FC) using predefined data extraction forms. If necessary, corresponding authors of the included studies were contacted for clarification of any missing information. Data on general information (first author, year of publication and setting); methods (study design, diagnostic criteria for peri-implantitis, follow-up period); participants (inclusion criteria, number of randomized participants and implants, drop-outs, number of analyzed participants and implants, age, gender, smoking, history of periodontitis, implant surface), interventions and controls (pre-surgical procedures, type of surgery, decontamination protocols, biomaterials, post-surgical care, frequency of supportive peri-implant care) and outcome/results of interest (for each outcome considered: collected or not, definition, time-points, results). The type of surgery was categorized as access flap, resective, reconstructive or combined (Appendix S1).
2.5 Assessment of risk of bias in the included studies
The risk of bias in the included studies was assessed independently and in duplicate by two review authors (GB and FC) according to the RoB2 tool, considering PPD reduction as the main outcome of interest (Sterne et al., 2019).
2.6 Data synthesis
In the presence of at least 2 studies, random-effects meta-analyses were carried out using specific softwares (OpenMeta [Analyst], Brown University, RI, USA; RevMan v5.4, The Cochrane Collaboration, 2020), using the Mantel–Haenszel method for dichotomous data, and the inverse of variance method for continuous data. In order to account for within-patient correlation in studies which failed to adjust for it, an intracluster correlation coefficient of 0.07 was assumed for the calculation of the effective sample size and CIs (Campbell et al., 2012; see Appendix S2). Statistical significance was set in advance to p < 0.05. Only subgroup analyses according to the employed surgical approach (access, resective or reconstructive) were performed.
Two different sets of analyses were conducted. First, the effect of each implant surface decontamination protocol was assessed by comparing baseline values with values at follow-up. Continuous data were combined in weighted mean effects (WME) and 95% confidence intervals (CIs), while binary data were pooled as weighted mean percentage (WMP) and 95% CIs. Second, when possible, pairwise comparisons were carried out to compare different decontamination protocols. The estimates of the effect were expressed as weighted mean differences (WMD) and 95% CIs for continuous outcomes and as risk ratio (RR) and 95% CIs for dichotomous outcomes.
Statistical heterogeneity among studies was explored using the I2 index and the Cochrane's Q statistic (p < 0.1). Network meta-analysis was not possible due to the lack of common comparators.
2.7 Certainty of evidence
The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) tool has been used to summarize the overall quality of the evidence for the questions for which pairwise meta-analyses were available (Guyatt et al., 2011). The certainty of the body of evidence was not evaluated for comparisons for which meta-analyses were not possible.
3 RESULTS
3.1 Study selection
The electronic search yielded 1835 records and hand searching identified 6 additional studies (Figure S1). After removal of duplicates, the total number of screened articles was 1497. Twenty-six records were selected for full-text analysis (agreement: 98.6%; Kappa = 0.68, 95% CI: 0.55–0.81), which resulted in the exclusion of 4 of them (agreement: 96.1%; Kappa = 0.83, 95% CI: 0.52–1.14 - reasons for exclusion reported in Table S1) and the final inclusion of 22 manuscripts reporting on 16 RCTs (Albaker et al., 2018; Carcuac et al., 2016; Cha et al., 2019; de Waal et al., 2013; de Waal et al., 2015; Hallström et al., 2017; Isehed et al., 2016; Isehed et al., 2018; Isler et al., 2018; Lasserre et al., 2020; Papadopoulos et al., 2015; Romeo et al., 2004; Romeo et al., 2007; Schlee et al., 2019; Schwarz et al., 2011; Schwarz et al., 2012; Schwarz et al., 2013; Schwarz et al., 2017; Tapia et al., 2019; Toma et al., 2019; Wang et al., 2020).
3.2 Characteristics of the included studies
Table 1 shows details about the characteristics of the included studies.
| Study | Study design | Groups | Randomized patients | Randomized implants | Follow- up (mo) | Diagnosis of peri-implantitis | Pre-treatment phase at affected implants | Systemic ABX | Mechanical decontamination | Chemical decontamination | Physical decontamination | Biomaterials | Post-op | Frequency of SPT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lasserre et al. (2020) | RCT parallel (test/ control) | Implantoplasty | 16 | 22 | 6 | MBL ≥2 mm, PPD ≥5 mm, BOP and/or SoP | OHI and supragingival instrumentation with scalers, polishing paste, and rubber cups 4 w before surgery | No | Plastic curettes, diamond burs | Sterile saline | NA | NA | 0.2% CHX x 10 d; Ibuprofen 3 x 600 mg for 2 d, paracetamol 1 g | 1w, 3 m, 6 m |
| Glicine air polishing | 15 | 20 | Plastic curettes, air powder device | |||||||||||
| Cha et al. (2019) | RCT parallel (test/control) | Minocycline ointments | 25 | 25 | 12 | MBL ≥3 mm, PPD ≥6 mm, and BOP | Supragingival instrumentation, and standardized OHI | Amoxicillin 3 x 500 mg for 3 d | Titanium curettes, ultrasonic scaler, titanium brush, air-powder device | Minocycline ointment | NA | NA | Ibuprofen 3 x 600 for 3 d | 1w, 1 m, 3 m, 6 m (Minocycline or placebo administered at 1 and 3 mo) |
| Placebo | 25 | 25 | Placebo ointment | |||||||||||
| Toma et al. (2019) | RCT parallel (2 test/1 control) | Perio Flow | 16 | 22 | 6 | MBL ≥2 mm, PPD ≥5 mm, BOP and/ or SoP | 2 w before surgery, OHI and professional supragingival instrumentation, using a rubber cup with polishing paste | No | Air powder device | Sterile saline | NA | NA | 0.2% CHX and paracetamol 3 g for 10 d | 1w, 3 m, 6 m |
| Titanium brushes | 16 | 23 | Titanium brushes | |||||||||||
| Plastic curettes | 15 | 25 | Plastic curettes | |||||||||||
| Albaker et al. (2018) | RCT parallel (test/control) | Photodynamic Therapy | 11 | 11 | 12 | MBL ≥2 mm, PPD ≥5 mm, BOP and/ or SoP | Full mouth SRP using ultrasonic scaler and hand instruments | Amoxicillin + Clavulanic Acid 3 x 625 mg for 7 d | Curettes plus saline soaked cotton gauzes | Sterile saline | Photodynamic therapy (methylene blue + diode laser) | NA | Ibuprofen 3 x 600 mg for 7 d, 0.2% CHX for 2 weeks | 1w, 3 m, 6 m, 9 m, 12 m |
| Curettes | 13 | 13 | Curettes plus saline soaked cotton gauzes | Sterile saline | NA | |||||||||
| Hallström et al. (2017) | RCT parallel (test/control) | Access flap + Systemic antimicrobials | 20 | 20 | 12 | MBL ≥3 mm, PPD ≥5 mm and BOP/SoP | NR | Azithromycin (2 x 250 mg at surgery, and 1 x 250 mg for 4 d) | Titanium curettes plus gauze soaked in saline | NA | NA | NA | CHX 0.12% twice x 10 d | 2w, 6w, 3 m, 6 m, 12 m |
| Access flap + placebo | 19 | 19 | No | Titanium curettes plus gauze soaked in saline | NA | NA | ||||||||
| Carcuac et al. (2016) also reported in: Carcuac et al. (2017) | RCT parallel (2 test/2 control) | Antibiotic + Antiseptic + | 27 | 47 | 12 | MBL ≥2 mm, PPD ≥6 mm, BOP and/or SoP | Supragingival instrumentation using rubber cups, polishing paste, and OHI | Amoxicillin 2 × 750 mg for 10 d commenced 3 d prior to surgery | Titanium curettes | Gauze soaked in 0.2% CHX | NA | NA | 1 min 0.2% CHX twice daily for 14 d | 2w, 3 m, 6 m, 9 m, 12 m |
| Antibiotic + Antiseptic - | 25 | 46 | Titanium curettes | Sterile saline | ||||||||||
| Antibiotic Antiseptic + | 24 | 49 | No | Titanium curettes | Gauze soaked in 0.2% CHX | |||||||||
| Antibiotic -Antiseptic - | 24 | 37 | Titanium curettes | Sterile saline | ||||||||||
| Isehed et al. (2016) also reported in: Isehed et al. (2018) | RCT parallel (test/control) | Access flap + EMD | 15 | 15 | 12 | MBL ≥3 mm, PPD ≥5 mm BOP and/or SoP | Periodontitis was treated with mechanical debridement and OHI | No | Ultrasonic scaler, titanium curettes | Sterile saline plus EMD | NA | NA | 2 x 10 ml CHX for 6 w and not chew or brush on the treated side for 2 weeks | 2w, 6w, 3 m, 6 m, 9 m, 12 m |
| Access flap | 14 | 14 | Sterile saline | |||||||||||
| Papadopoulos et al. (2015) | RCT parallel (test/control) | Access flap + Diode laser | 9 | 9 | 6 | MBL ≥2 mm, PPD ≥5 mm BOP and/or SoP | Mechanical debridement using ultrasonics and hand instruments to the whole dentition | No | Plastic curettes, plus gauzes soaked in saline | NA | Diode laser | NA | 2 x 0.12% CHX for 2 w and a careful tooth brushing with a soft toothbrush | 2w, 3 m, 6 m |
| Access flap | 10 | 10 | NA | |||||||||||
| Wang et al. (2020) | RCT parallel (test/control) | Er:Yag Laser | 12 | 12 | 6 | MBL >2 mm, PPD ≥5 mm BOP and/or SoP | Full mouth prophylaxis performed with piezo-instruments and stainless-steel hand scalers | Amoxicillin 3 x 500 mg for 10 d | Ultrasonic scaler, stainless-steel curettes | NA | Er:Yag Laser | Alloplastic bone graft + acellular dermal matrix | 2 x CHX for 1 w and Ibuprofen 600 mg as needed | 2w, 1 m, 3 m, 6 m |
| Placebo | 12 | 12 | Sham laser application | |||||||||||
| deTapia et al (2019) | RCT parallel (test/control) | Titanium brushes | 15 | 15 | 12 | MBL >30%, PPD ≥6 mm and BOP and/or SoP | Subgingival scaling with plastic curettes and irrigation with 0.12% CHX | Amoxicillin 3 x 500 mg and Metronidazole 3 x 500 mg for 7 d | Plastic ultrasonic scaler, titanium brushes (infraosseous) | 3% H2O2 | NA | Alloplastic bone graft + collagen membrane | 2 x 0.12% CHX for 2 w | 1w, 2w, 3w, 1 m, 3 m, 6 m, 9 m, 12 m |
| Ultrasonic scalers | 15 | 15 | Plastic ultrasonic scaler (infraosseous) | |||||||||||
| Schwarz et al., 2011 also reported in: Schwarz et al. (2012, 2013, 2017) | RCT parallel, single blind (test/control) | Er:Yag Laser | 16 | 16 | 6 | PPD of ≥5 mm and an intrabony component of >3 mm as estimated clinically | Non-surgical instrumentation using plastic curettes, combined with 0.2% CHX solution and CHX gel 0.2%. | No | Plastic curettes + cotton pellets soaked in saline (infraosseous part) | NA | Er:Yag Laser (infraosseous part) | Xenogenic bone graft + collagen membrane | 2 x 0.12% CHX for 2 w | 2w, 4w, 6w, 8w, 4 m, 6 m |
| Plastic curettes | 16 | 16 | NA | |||||||||||
| Schlee et al. (2019) | RCT parallel (test/control) | Electrolytic method (EC) | 12 | 12 | 6 | MBL ≥3 mm, PPD ≥6 mm, and BOP and/ or SoP | Suprastructures removed 14 days before surgery, implants cleaned by powder spray and CHX. Cover screw was placed | No | Curettes and/or ultrasonic devices | Pilot electrolytic approach for 120 s, then sterile saline. | Autogenous bone graft and xenograft 50:50 + collagen membrane | NR | 2w, 6w, 6 m | |
| Powder spray plus EC | 12 | 12 | Curettes and/or ultrasonic devices plus powder spray | |||||||||||
| Isler et al. (2018) | RCT parallel (test/control) | Ozone therapy | 23 | 42 | 12 | MBL ≥2 mm, deepening PPD, and BOP and/or SoP | Non-surgical treatment provided. In test group, ozone therapy was initiated | Amoxicillin 3 x 500 mg and Metronidazole 3 x 500 mg for 1 week | Titanium curettes | Sterile saline plus ozone delivery | NA | Xenogenic bone graft + growth factors | 2 x 0.12% CHX for 2 weeks, anti-inflammatory and analgesic drugs for the first 3 d | 1w, 1 m, 3 m, 6 m, 9 m, 12 m |
| Saline irrigation | 23 | NR | Sterile saline | |||||||||||
| de Waal et al. (2015) | RCT parallel, double blind (test/control) | 2% CHX | 22 | 49 | 12 | MBL ≥2 mm, PPD ≥5 mm and BOP and/or SoP | Mechanical debridement of implants, suprastructures, and remaining dentition | No | Gauze soaked in saline | 2% CHX | NA | NA | 0.12% CHX + 0.05% CPC without alcohol (two times daily x 2 weeks) | 2w, 3 m, 6 m, 9 m, 12 m |
| 0.12% CHX + 0.05% CPC | 22 | 59 | 0.12% CHX + 0.05% CPC x 1 min | |||||||||||
| de Waal et al. (2013) | RCT parallel, single blind (test/control) | 0.12% CHX + 0.05% CPC | 15 | 31 | 12 | MBL ≥2 mm, PPD ≥5 mm and BOP and/or SoP | Mechanical debridement of implants, suprastructures, and remaining dentition | No | Gauze soaked in saline | 2% CHX x 1 min | NA | NA | 0.12% CHX + 0.05% CPC without alcohol (two times daily x 2 weeks) | 2w, 3 m, 6 m, 9 m, 12 m |
| Gauze soaked in saline | 15 | 48 | NA | |||||||||||
| Romeo et al (2005) also reported in: Romeo et al. (2007) | RCT parallel, single blind (test/control) | Implantoplasty | NR | 19 | 12 | PPD ≥5 mm, radiographic evidence of horizontal peri-implant radiolucency and BOP and/or SoP | NR | Amoxicillin 50 mg/kg/die for 8 d per os | Implantoplasty (burs) | Gel of metronidazole plus a solution of tetracycline hydrochloride | NA | NA | 0.2% CHX (10 mL for 1 min at interval of 8 h for 2 w) | NR |
| No implantoplasty | NR | 16 | None |
- Abbreviations: BOP, bleeding on probing; CHX, chlorhexidine; CPC, cetylpyridinium chloride; EMD, enamel matrix derivatives; MBL, marginal bone level; NR, not reported; PPD, probing pocket depth; SoP, suppuration on probing.
3.2.1 Design and settings
All RCTs had a parallel arm design, and all of them except 3 were carried out in Europe. Fourteen studies included one experimental and one control group, while 2 studies adopted multiple arms. Six included studies had a 6-months follow-up, while the remaining 12-months. Four trials were also reported at longer follow-up periods, such as 2 years (Schwarz et al., 2012), 3 years (Carcuac et al., 2017; Isehed et al., 2018; Romeo et al., 2007), 4 years (Schwarz et al., 2013), 5 years (Isehed et al., 2018) and 7 years (Schwarz et al., 2017).
3.2.2 Patients and implants
Diagnostic criteria for peri-implantitis were consistent across most included studies except for minor differences (either bone loss/level ≥2 or 3 mm, together with PPD ≥5 or 6 mm, and BoP/SoP). In total, 849 implants were treated in 604 patients. Percentage of current smokers ranged from 14.2% to 50%, whereas in 5 studies smokers were excluded. Only 5 studies included implants with a machined surface, with proportions ranging from 1.3 to 35%.
3.2.3 Interventions
Eight studies performed a non-surgical sub-mucosal instrumentation prior to the surgical treatment, while the remaining 8 only performed supra-mucosal instrumentation or did not report this information. Eight trials reported decontamination protocols during access surgery, 3 in resective surgery, 2 in reconstructive and 3 in combined procedures.
Curettes were the most used method for local mechanical decontamination (25 arms of 12 RCTs), either alone or in combination with other devices. Plastic curettes were employed in 6 arms of 4 RCTs, titanium curettes in 12 arms of 5 RCTs, only one study employed stainless steel curettes in 2 arms, while two studies did not report this information (Albaker et al., 2018; Schlee et al., 2019). Gauzes soaked in saline were employed in 16 arms of 7 studies; ultrasonic scalers were adopted in 10 arms of 5 studies; air-powder devices in 5 arms of 4 studies; titanium brushes in 4 arms of 3 studies; while implantoplasty was carried out in 6 arms of 4 studies. Among local chemical decontamination agents, CHX-based formulations were applied in 4 arms of 3 studies, either in 2% gel formulation or in solution (at 0.2% concentration or at 0.12% in combination with 0.05% cetylpyridinium chloride - CPC). Other local chemical decontamination employed were a gel of metronidazole, a solution of tetracycline hydrochloride, minocycline ointments, 3% hydrogen peroxide (H2O2), and EMD (each one in 1 arm of 1 study). Among local physical decontamination, lasers were used in 3 arms of 3 different studies (2 studies employed a Er:Yag laser, while the remaining one a diode laser); electrolytic current was employed in 2 arms of one study; while ozone therapy and photodynamic therapy were each one part of 1 arm of 1 study.
Six studies utilized systemic chemical decontamination agents (i.e., antibiotics) in all treatment groups, 2 only in test groups as part of the studied comparisons, while the remaining 8 did not administer them. Of the 8 studies which utilized systemic antibiotics, 4 trials employed amoxicillin alone, 3 amoxicillin in combination with either clavulanic acid or metronidazole, while the remaining one azithromycin. Systemic antibiotics were administered prior to the surgical treatment in two RCTs, while in 6 studies they were prescribed on the day of the surgery.
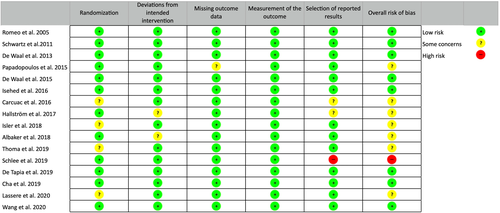
3.3 Risk of bias within studies
The risk of bias assessment for the included RCTs is summarized in Figure 1. Seven trials resulted at low risk of bias, eight trials with some concerns and the remaining one at high risk.
3.4 Effect of different decontamination protocols
3.4.1 Results of individual studies
The results of the included studies in terms of effect of different decontamination protocols are reported in the Appendix. Moreover, a summary of the results on the main outcomes considered in the present systematic review is reported in Table 2, while on the other outcomes is reported in Table S2 (“Effect of studied decontamination protocols” columns).
| Study | Type of surgery | Tested methods | Common methods | Analyzed patients | Analyzed implants | Success criteria | Effect of studied decontamination protocols | Comparison of the studied protocols (effect of test procedure) |
|---|---|---|---|---|---|---|---|---|
| Lasserre et al. (2020) | Access flap | Test: Implantoplasty (diamond burs) | Plastic curettes, sterile saline | 15 | 20 | PPD ≤5 mm, no BoP/SoP, and no further bone loss ≥0.5 mm from baseline |
ΔPPD: −4.00 ± 1.83 mm ΔMBL: −0.26 ± 1.08 mm Success: 15.0% |
ΔPPD: 0.61 mm ΔMBL: 0.27 mm (in favour of control group) |
| Control: Glycine air polishing | 14 | 18 |
ΔPPD: −3.29 ± 1.73 mm ΔMBL: −0.53 ± 0.94 mm Success: 26.0% |
|||||
| Cha et al. (2019) | Access flap | Test: Repeated application of minocycline ointments | Titanium curettes, ultrasonic scaler, titanium brush, air-powder device, systemic amoxicillin | 24 | 24 | PPD <5 mm, no BoP, and no further bone loss |
ΔmPPD: −2.68 ± 1.73 mm ΔwPPD: −3.58 ± 2.32 mm ΔMBL: −0.72 ± 0.56 mm Success: 66.7% |
ΔmPPD: 1.13 mma ΔwPPD: 1.13 mma ΔMBL: 0.41 mma Success: RR = 2a |
| Control: Repeated application of placebo ointments | 22 | 22 |
ΔmPPD: −1.55 ± 1.86 mm ΔwPPD: −2.45 ± 2.13 mm ΔMBL: −0.31 ± 0.49 mm Success: 36.3% |
|||||
| Toma et al. (2019) | Access flap | Test A: Air powder device | Sterile saline | 16 | 22 | PPD ≤5 mm, no BoP/SoP, and no further bone loss ≥0.5 mm from baseline |
ΔPPD: −2.39 ± 1.12 mm ΔMBL: −0.95 ± 0.24 mm Success: 29.0% |
A versus B ΔPPD: 0.2 mma ΔMBL: 0.17 mma |
| Test B: Titanium brushes | 16 | 23 |
ΔPPD: −2.41 ± 0.48 mm ΔMBL: −1.12 ± 0.24 mm Success: 33.0% |
A versus control ΔPPD: 1.02 mm ΔMBL: 0.44 mm |
||||
| Control: Plastic curettes | 15 | 25 |
ΔPPD: −1.37 ± 0.87 mm ΔMBL: −0.51 ± 0.17 mm Success: 22.0% |
B versus control ΔPPD: 1.04 mm ΔMBL: 0.61 mm |
||||
| Albaker et al. (2018) | Access flap | Test: Photodynamic Therapy | Curettes, saline soaked cotton gauze, systemic amoxicillin plus clavulanic acid | 11 | 11 | NR | NR | NR |
| Control: NA | 13 | 13 | NR | |||||
| Hallström et al. (2017) | Access flap | Test: Systemic azithromycin | Titanium curettes plus gauze soaked in saline | 15 | 15 | PPD ≤5 mm, no BoP/SoP, and no further bone loss ≥0.5 mm from baseline to 1y |
ΔPPD: −1.7 ± 1.1 mm Success: 46.6% |
ΔPPD: 0.1 mm ΔMBL: 0.5 mm (in favour of control group) Success: RR = 1.8 |
| Control: Placebo | 16 | 16 |
ΔPPD: −1.6 ± 1.5 mm Success: 25.0% |
|||||
| Carcuac et al. (2016) also reported in: Carcuac et al. (2017) | Access flap | Test A: Gauze soaked in 0.2% CHX + systemic amoxicillin | Titanium curettes | 27 | 47 | PPD ≤5 mm, no BoP/SoP, and no further bone loss ≥0.5 mm from baseline |
ΔwPPD: −2.80 ± 1.87 mm ΔMBL: −0.18 ± 1.15 mm Success: 40.4% |
Test A vs Control A ΔwPPD: 0.64 mm ΔMBL: 0.87 mm |
| Test B: Gauze soaked in saline + systemic amoxicillin | 25 | 46 |
ΔwPPD: −3.44 ± 1.66 mm ΔMBL: −0.51 ± 0.84 mm Success: 65.2% |
Test B vs Control B ΔwPPD: 1.75 mm ΔMBL: 1.47 mm |
||||
| Control A: Gauze soaked in 0.2% CHX | 23 | 48 |
ΔwPPD: −2.16 ± 1.79 mm ΔMBL: +0.69 ± 1.32 mm Success: 37.5% |
Test A vs Test B ΔwPPD: 0.64 mma ΔMBL: 0.33 mma a: in favour of B |
||||
| Control B: Gauze soaked in saline | 24 | 37 |
ΔwPPD: −1.69 ± 2.22 mm ΔMBL: +0.96 ± 1.42 mm Success: 35.1% |
Control A vs Control B ΔwPPD: 0.47 mm ΔMBL: 0.3 mm |
||||
| Isehed et al. (2016) also reported in: Isehed et al. (2018) | Access flap | Test: EMD | Ultrasonic scaler, titanium curettes | 12 | 12 | NR |
ΔPPD: −2.5 ± 2.0 mm ΔMBL: −0.7 ± 1.1 mm |
ΔPPD: 1.5 mm ΔMBL: 0.5 mm (in favour of control group) |
| Control: NA | 13 | 13 |
ΔPPD: −4.0 ± 2.9 mm ΔMBL: −0.2 ± 1.1 mm |
|||||
| Papadopoulos et al. (2015) | Access flap | Test: Diode laser | Plastic curettes | 8 | 8 | NR | NR | NR |
| Control: NA | 8 | 8 | NR | |||||
| Wang et al. (2020) | Combined | Test: Er:Yag laser | Ultrasonic scaler, stainless-steel curettes, systemic amoxicillin | 12 | 12 | NR |
ΔPPD: −2.65 ± 2.14 mm ΔMBL: −1.27 ± 1.14 mm |
ΔPPD: 0.80 mma ΔMBL: 0.19 mm |
| Control: Sham laser application | 12 | 12 |
ΔPPD: −1.85 ± 1.71 mm ΔMBL: −1.08 ± 1.04 mm |
|||||
| deTapia et al (2019) | Combined | Test: Titanium brushes | Ultrasonic scalers, 3% H2O2, systemic amoxicillin plus metronidazole | 15 | 15 | Absence of PPD ≥5 mm, with no BOP/SOP and no additional peri-implant bone loss |
ΔmPPD: −2.84 ± 0.93 mm ΔwPPD: −4.87 ± 1.55 mm ΔMBL: −2.51 ± 1.21 mm Success: 66.7% |
ΔmPPD: 1.29 mma ΔwPPD: 2.02 mma ΔmMBL: 1.78 mma ΔwMBL: 1.68 mma Success: RR = 2.6a |
|
Control: NA |
12 | 12 |
ΔmPPD: −1.55 ± 1.86 mm ΔwPPD: −2.85 ± 1.91 mm ΔMBL: −0.73 ± 1.26 mm Success: 23.0% |
|||||
| Schwarz et al., 2011 also reported in: Schwarz et al. (2012, 2013, 2017) | Combined | Test: Er:Yag Laser | Plastic curettes + cotton pellets soaked in saline | 15 | 15 | NR | ΔPPD: −1.7 ± 1.4 mm | ΔPPD: 0.7 mm (in favour of control group) |
| Control: NA | 15 | 15 | ΔPPD: −2.4 ± 1.5 mm | |||||
| Schlee et al. (2019) | Rec | Test: NA | Curettes and/or ultrasonic devices, pilot electrolytic method | 12 | 12 | NR | ΔMBL: −2.71 ± 1.70 mm | ΔMBL: 0.10 mm (in favour of control group) |
| Control: Powder spray | 11 | 11 | ΔMBL: −2.81 ± 2.15 mm | |||||
| Isler et al. (2018) | Rec | Test: Ozone therapy | Titanium curettes, saline irrigation, systemic amoxicillin plus metronidazole | 20 | 30 | PPD <5 mm without BOP and/or SoP, no further BL, and DF ≥1 mm |
ΔPPD: −3.5 ± 1.31 mm ΔMBL: −2.32 ± 1.28 mm Success: 50.0% |
ΔPPD: 1.1 mm ΔMBL: 1.15 mma |
| Control: NA | 21 | 30 |
ΔPPD: −2.42 ± 1.23 mm ΔMBL: −1.17 ± 0.77 mm Success: 36.6% |
|||||
| de Waal et al. (2015) | Res | Test: 2% CHX | Gauze soaked in saline | 21 | 48 | PPD ≤5 mm, no BoP/SoP, and no further bone loss ≥0.5 mm from baseline |
ΔPPD: −1.68 ± 1.06 mm ΔMBL: −0.24 ± 0.72 mm Success: 14.3% |
ΔPPD: 0.33 mm (in favour of control group) ΔMBL: 0.10 mm |
| Control: 0.12% CHX + 0.05% CPC | 20 | 54 |
ΔPPD: −2.01 ± 1.26 mm ΔMBL: −0.14 ± 0.49 mm Success: 28.8% |
|||||
| de Waal et al. (2013) | Res | Test: 0.12% CHX + 0.05% CPC | Gauze soaked in saline | 15 | 31 | PPD ≤5 mm, no BoP/SoP, and no further bone loss ≥0.5 mm from baseline |
ΔPPD: −2.21 ± 2.01 mm ΔMBL: −0.78 mm ± 0.93 mm Success: 3.2% |
ΔPPD: 0.57 mm ΔMBL: 0.18 mm |
| Control: NA | 12 | 38 |
ΔPPD: −1.64 ± 1.03 mm ΔMBL: −0.58 mm ± 0.86 mm Success: 2.1% |
|||||
| Romeo et al (2005) also reported in: Romeo et al. (2007) | Res | Test: Implantoplasty (diamond burs) | Gel of metronidazole, solution of tetracycline hydrochloride, systemic amoxicillin | 19 | 19 | NR |
ΔmMBL: 0 ± 0.14 mm ΔdMBL: −0.01 ± 0.13 mm |
ΔPPD: 2.48 mm ΔmMBL: 0.51 mm ΔdMBL: 0.57 mm |
| Control: No implantoplasty | 16 | 16 |
ΔmMBL: +0.51 ± 0.49 mm ΔdMBL: +0.56 ± 0.43 mm |
- Abbreviations: BoP, bleeding on probing; CHX, chlorhexidine; CPC, cetylpyridinium chloride; MBL, mean bone level (mMBL: mesial, dMBL: distal); PPD, probing pocket depth (mPPD: mean, wPPD: worst); Rec, reconstructive surgery; Res, resective surgery; RR, relative risk; SoP, suppuration on probing; wPPD, worst probing pocket depth.
- a Significant difference between the test and control group.
3.4.2 Meta-analyses
Data were pooled using WMEs and WMPs (comparisons against baseline). With regards to access flap procedures, the single use of curettes for surface decontamination resulted in a WME of 1.46 mm for PPD reduction (3 arms of 3 trials; 95% CI:1.17/1.74; I2 = 0.00%), in a negligible MBL change (2 arms of 2 trials; WME = -0.21 mm; 95% CI:--1.65/1.23; I2 = 97.35%), while the WMP for treatment success amounted to 28.6% (3 arms of 3 trials; 95% CI:18.5/38.7; I2 = 0.00%) (Figure 2a,b,C). The use of systemic antimicrobials in combination with local decontamination methods resulted in a WME of 2.46 mm for PPD reduction (5 arms of 3 trials; 95% CI:1.74/3.18; I2 = 86.01%), in a WME of 0.44 mm for MBL changes (4 arms of 2 trials; 95% CI:0.22/0.67; I2 = 70.16%) and in a WMP of 51% for treatment success (3 arms of 2 trials; 95% CI:33/69; I2 = 69.95%) (Figure 2d,e,f).
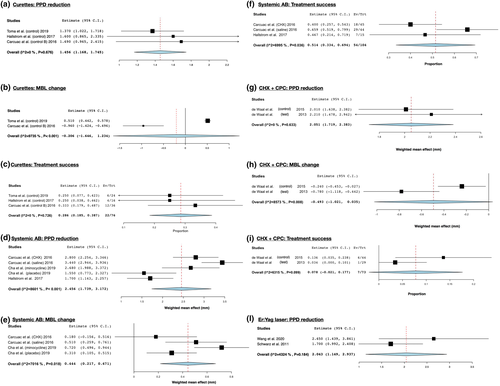
With regards to resective surgery, the use of 0.12% CHX + 0.05% CPC resulted in a WME of 2.05 mm for PPD reduction (2 arms of 2 trials; 95% CI:1.72/2.38; I2 = 0), a WME of −0.49 mm for MBL changes (2 arms of 2 trials; 95% CI:-1.02/0.04; I2 = 85.73) and a WMP of 7.8% for treatment success (2 arms of 2 trials; 95% CI:-2.1/17.7; I2 = 63.15) (Figure 2g,h,I).
With regards to reconstructive surgery, no meta-analysis was carried out since the studied decontamination protocols were only tested once.
With regards to combined surgery, the use of the Er:Yag laser resulted in a WME of 2.04 mm for PPD reduction (2 arms of 2 trials; 95% CI:1.15/2.94; I2 = 43.24) (Figure 2l).
3.5 Comparison among different decontamination protocols
3.5.1 Results of individual studies
The results of the included studies in terms of comparison between different decontamination protocols on the main outcomes considered in the present systematic review are reported in Table 2, while the results on the other considered outcomes are reported in Table S2 (“Comparison of the studied protocols” columns). Briefly, only 7 studies reported better clinical and/or radiographic results at 6- or 12-months examinations for one decontamination protocol over the others.
With regards to access flap surgery, Cha et al. (2019) reported 1.13 mm more PPD reduction, as well as 0.41 mm better MBL changes and 30.4% more treatment success using repeated local delivery of minocycline ointment over placebo. Toma et al. (2019) reported a 1.04 mm more mean PPD reduction and 0.61 more bone gain at the 6-months examination with the use of titanium brushes vs. the use of plastic curettes, as well as a 1.02 mm more mean PPD reduction and 0.43 mm more bone gain with the use of a glycine air-powder device versus the use of plastic curettes. Carcuac et al. (2016) reported a better efficacy of amoxicillin alone versus CHX soaked gauzes in terms of deepest PPD reduction (1.28 mm) and of bone level changes (1.20 mm). Moreover, amoxicillin alone showed 1.75 mm more deepest PPD reduction and 1.47 mm better MBL changes compared to the use of neither amoxicillin nor CHX soaked gauzes. In addition, amoxicillin combined with CHX soaked gauzes resulted in better MBL changes than the use of CHX alone (0.87 mm) and saline soaked gauzes (1.14 mm). Finally, the use of amoxicillin, but not of CHX soaked gauzes, has shown an added effect on treatment success, but only in implants with modified surfaces.
With regards to resective surgery, Romeo et al. (2004) studied the added effect of implantoplasty over the use of a 25% metronidazole gel followed by the use of a solution of 50 mg/mL tetracycline hydrochloride for 3 minutes. At the 12-months examination, 2.48 mm less PPD values were found at the site-level analysis in the implantoplasty group.
With regards to reconstructive surgery, Isler et al. (2018) reported an added effect of ozone therapy over the combined use of titanium curettes and sterile saline irrigation in terms of bone gain (1.15 mm) at the 12-months examination.
With regards to combined surgery, Wang et al. (2020) showed that Er:Yag laser resulted in an added effect of 0.8 mm in mean PPD reduction at 6-months follow-up than sham laser application, when both used in addition to ultrasonic scalers and steel curettes. De Tapia et al. (2019) reported an added effect of titanium brushes over the combined use of plastic ultrasonic scaler and 3% H2O2 at 12-months follow-up in terms of mean and deepest PPD reduction (added effect: 1.29 mm and 2.02 mm, respectively), mean and deepest bone level change (added effect: 1.78 mm and 1.68 mm, respectively) and treatment success (added effect: 33.6%).
Conversely, the remaining 9 studies showed no differences in clinical and radiographic parameters comparing two or more decontamination approaches (Albaker et al., 2018; de Waal et al., 2013, 2015; Hallström et al., 2017; Isehed et al., 2016; Lasserre et al., 2020; Papadopoulos et al., 2015; Schlee et al., 2019; Schwarz et al., 2011).
Considering the studies with longer follow-up periods, the 6–12 months results were confirmed in the majority of the reports (Romeo et al., 2007; Schwarz et al., 2012, 2013, 2017; Isehed et al., 2018), except for the study by Carcuac et al. (2017) in which the short-term benefits of systemic antibiotics at implants with modified surfaces were not sustained over 3 years.
3.5.2 Meta-analyses
Meta-analyses comparing clinical and radiographic outcomes of different decontamination protocols were only possible for the added effect of systemic antimicrobials in access flap surgery and for the added effect of Er:Yag laser in combined surgery.
The use of systemic antimicrobials in access surgery resulted in no added effect in terms of PPD reduction (2 trials; WMD = 0.63 mm; 95% CI: −0.69/2.55; I2 = 84%), whereas it provided added effect in terms of treatment success (2 trials; RR = 1.84; 95% CI: 1.17/2.91; I2 = 0%) (Figure 3a,b).
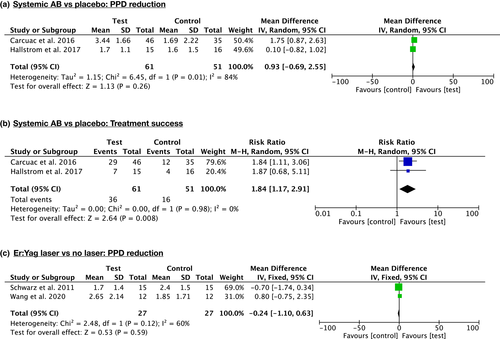
The use of the Er:Yag laser in combined surgery resulted in no added effect in terms of PPD reduction (2 trials; WMD = −0.24 mm; 95% CI: −1.10/0.63; I2 = 60%) (Figure 3C).
The “summary of findings” tables for the effect of systemic antimicrobials and Er:Yag laser are reported in Figure S2 and S3, respectively.
4 DISCUSSION
The findings from the present systematic review indicate that several decontamination protocols resulted in improved clinical and radiographic outcomes after surgical treatment of peri-implantitis. Owing to paucity of available trials, evidence regarding the superiority of some protocols over the others is mainly based on single RCTs. However, meta-analyses indicated a short-term added effect of systemic antimicrobials on treatment success but not on PPD reduction in access surgery, and a lack of added effect of Er:Yag laser on PPD reduction in combined surgery.
Ideally, implant surface decontamination should remove biofilm without causing surface damage not to render surfaces more conducive to bacterial colonization (Louropoulou et al., 2014). For such a purpose, mechanical, chemical and physical decontamination protocols have been tested in RCTs so far.
With regards to mechanical methods, in vitro studies indicated that non-metal curettes and rubber cups were minimally traumatic but ineffective to clean contaminated titanium surfaces; while ultrasonic scalers, metal curettes and rotating titanium brushes were effective particularly on modified titanium surfaces (John et al., 2014). The air abrasive system resulted effective in all types of implant surfaces, with glycine/erythritol powder causing less alterations than sodium bicarbonate (Cochis et al., 2013; Pranno et al., 2020). The included trials indicate a better clinical performance of titanium brushes over plastic curettes, ultrasonic scalers and air powder devices (Toma et al., 2019). Moreover, there is controversy regarding the role of implantoplasty. No clinical benefit of implantoplasty on implant survival rate was observed in a recent long-term retrospective study (Ravidà et al., 2020). The removal of threads and of the superficial portion of the implant surface may enhance intra-surgical decontamination, and the long-term recontamination may be potentially prevented, thanks to the reduced plaque-retention of the smoothed implant surface. The trials included in the present systematic review reported an added benefit of implantoplasty in terms of PPD reduction in both access flap and resective surgery (Lasserre et al., 2020; Romeo et al., 2004).
Coupling mechanical instruments with chemical/physical agents may improve the overall cleaning ability as the chemical agent may reach niches inaccessible mechanically (Carcuac et al., 2017). Different concentrations of CHX showed limited benefits both in in vitro models and in clinical trials, and a cytotoxic effect was reported (Schwarz et al., 2005). The available trials confirm that CHX alone and in combination with CPC does not provide clinical benefits when employed in access flap or resective surgery. EMD application on fixture surfaces switched subgingival microbiota to Gram+ aerobic populations (Isehed et al., 2016), and this ecological shift was linked in the only identified RCT with an increase in bone levels as compared with non-EMD controls. Moreover, in an exploratory analysis performed at 5 years, EMD application was associated with higher survival rates (Isehed et al., 2018). Being peri-implantitis an infection-driven disease, local and systemic antimicrobials have been proposed as an adjunctive method of decontamination. The only identified RCT on the added effect of local intra-surgical application of minocycline (and its subsequent sub-mucosal application 1- and 3-months after) resulted in a statistically significant bone gain compared to placebo, and in the highest rate of treatment success among all the included trials (66.7%) (Cha et al., 2019). With regards to their systemic administration, the meta-analyses reported in the present systematic review identified a greater probability of treatment success when they are employed (RR = 1.84). Nevertheless, a careful risk/benefit evaluation should be performed before systemic administration of systemic antimicrobials, in light of the potential onset of side effects and of the growing issue of antibiotic resistances (WHO, 2020).
Many different types of lasers have been proposed to decontaminate the implant surface and enhance the healing potential during treatment of peri-implantitis, with the Er:YAG laser having demonstrated a high degree of bactericidal effect at low-power intensity (Lin et al., 2018). However, the meta-analysis performed in the present systematic review showed that the adjunctive application of Er:Yag laser in combined surgery had no significant effect on PPD reduction over other mechanical and chemical decontaminating agents. The effect of photodynamic therapy was only evaluated in one RCT, showing also no additional benefits on clinical and radiographic results (Albaker et al., 2018; Chambrone et al., 2018).
To the best of the authors’ knowledge, this systematic review is the first providing a comprehensive qualitative and quantitative analysis on the decontamination protocols tested in RCTs as part of the surgical treatment of peri-implantitis. Limitations worth mentioning are mainly related to the available trials, since most of the evidence on decontamination protocols is based on single RCTs, and that half of the included trials were not considered at low risk of bias. Moreover, several factors such as implant surface characteristics (Berglundh, Wennström, & Lindhe, 2018) configurations of peri-implant defect (Schwarz et al., 2010) frequency and quality of supportive therapy (Heitz-Mayfield et al., 2018; Roccuzzo et al., 2018) can affect the results of surgical treatment of peri-implantitis, but their impact could not be analyzed due to the high heterogeneity among the included trials.
5 CONCLUSIONS
The present systematic review highlighted the absence of consistent evidence of superiority of any decontamination protocol over the others in the surgical treatment of peri-implantitis. Meta-analyses indicated an added benefit of systemic antimicrobials, but not of Er:Yag laser, in increasing treatment success rates. However, this effect was not present on PPD reduction, and it was only detected in the short-term.
Well-designed RCTs are needed to definitely identify the most effective mechanical decontamination method and to verify the added effect of adjunctive chemical/physical measures. When multiple implants per patient are included, the use of mixed models analysis should be implemented. In addition to implant survival and to PPD, BoP/SoP and MBL changes, researchers are encouraged to analyze also additional relevant outcomes which have been sparsely reported so far, including treatment success, soft tissue level changes, PROMs and rates of re-interventions and adverse events (e.g., implant fracture, emphysemas, side effects, etc.). Researchers are also suggested to employ a common comparator (i.e., titanium brushes) in order to favor future analyses of the literature, until clear evidence of superiority of a different protocol is identified.
AUTHOR CONTRIBUTIONS
GB, FC, NB and MA made substantial contributions to study conception. GB, FC, NB, FR and MA contributed to the study design. GB, FC and NB searched and collected the data. GB, FC, MR and FR performed data analysis and interpretation. GB, MR, FC, GMM and FR prepared the first draft of the manuscript. All authors have read, revised critically, and approved the final manuscript.
ACKNOWLEDGMENTS
The authors kindly thank S. Isler, J. Lasserre, E. Romeo, S. Toma, and Y. de Waal, for providing more information and estimates about their studies, regardless of the final decision to include or not include them in the present systematic review. The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article. Open Access Funding provided by Universita degli Studi di Torino within the CRUI-CARE Agreement. Open Access Funding provided by Universita degli Studi di Torino within the CRUI-CARE Agreement.
FUNDING INFORMATION
This study was funded solely by the institutions of the authors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
Ethics approval was not required for this systematic review.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article [and its supplementary information files].




