Bayesian tip-dated phylogeny and biogeography of Cissampelideae (Menispermaceae): Mitigating the effects of homoplastic morphological characters
Abstract
The integration of morphological and molecular data is essential to understand the affinities of fossil taxa and spatio-temporal evolutionary processes of organisms. However, homoplastic morphological characters can mislead the placement of fossil taxa and impact downstream analyses. Here, we provide an example of how to mitigate effectively the effect of morphological homoplasy on the placement of fossil taxa and biogeographic inferences of Cissampelideae. We assembled three data types, morphological data only, morphological data with a molecular scaffold and combined morphological and molecular data. By removing high-level homoplastic morphological data or reweighting the morphological characters, we conducted 15 parsimony, 12 undated Bayesian and four dated Bayesian analyses. Our results show that the 14 selected Cissampelideae fossil taxa are placed poorly when based only on morphological data, but the addition of molecular scaffold and combination of morphological and molecular data greatly improve the resolution of fossil nodes. We raise the monotypic Stephania subg. Botryodiscia to generic status and discover that three fossils previously assigned to Stephania should be members of Diploclisia. The Bayesian tip-dated tree recovered by removing homoplastic morphological characters with a Rescaled Consistency Index <0.25 has the highest stratigraphic fit and consequently generates more reasonable biogeographic reconstruction for Cissampelideae. Cissampelideae began to diversify in Asia in the latest Cretaceous and subsequently dispersed to South America around the Cretaceous–Palaeogene boundary. Two dispersal events from Asia to Africa occurred in the Early Eocene and the Late Eocene–Late Oligocene, respectively. These findings provide guidelines and practical methods for mitigating the effects of homoplastic morphological characters on fossil placements and Bayesian tip-dating, as well as insights into the past tropical floristic exchanges among different continents.
Introduction
Understanding how spatiotemporal patterns of organisms on the planet are shaped through time is a central goal of biogeography (Lomolino et al., 2017). The recent explosion of molecular phylogenetic data for living taxa and the simultaneous developments of model-based molecular dating and ancestral range estimation methods have dramatically accelerated biogeographic studies (Hunt and Slater, 2016). Nevertheless, living taxa represent only a tiny subsample of all species that once existed; in particular, older lineages tend to have a higher level of extinction rates (Marshall, 2017). Fossils of each taxon, whether occurring within or outside the modern distribution area, provide additional data related to its phytogeographic history (Lieberman, 2003). Because fossils are rarely the same as or conspecific with living species, we cannot simply assign fossils to living groups based on overall similarity. It is now widely acknowledged that the integration of phylogenetic and palaeontological data is essential not only to understand the affinities of fossil taxa, but also to more reliably estimate divergence times, as well as to elucidate macroevolutionary dynamics (Doyle and Endress, 2010; Benton, 2015; Hunt and Slater, 2016; Varela et al., 2019; Yan et al., 2021). The integrated analysis of fossil and living taxa can only be done using morphological data (Wiens, 2004). However, universal morphological convergence in evolutionary history of organisms may lead to high levels of homoplasy, which can mislead phylogenetic inferences (Gaubert et al., 2005) or lead to the erroneous placement of fossil taxa (Pol and Escapa, 2009; Ksepka et al., 2017; Lee and Yates, 2018). In particular, owing to their frequent incompleteness relative to living taxa, morphological characters of fossil taxa are limited and are more susceptible to being homoplastic (Ksepka et al., 2017; Lee and Yates, 2018). Thus, mitigating the effect of homoplastic morphological characters on fossil placements is pivotal for obtaining an accurate picture of evolutionary history.
To build a tree containing extant and extinct species, three strategies can be adopted: only morphological data (e.g. Mao et al., 2021; Yin et al., 2021), morphological data with a molecular scaffold (e.g. Springer et al., 2001; Jacques et al., 2011; Ksepka et al., 2017) and combined morphological and molecular data (e.g. Wiens et al., 2010; Xiang et al., 2019). These strategies may be analysed using parsimony and Bayesian methods. Some studies show that the Bayesian method outperforms parsimony method for morphological data matrices, especially when the character number is relatively low (e.g. Wright and Hillis, 2014; O'Reilly et al., 2016, 2018; Puttick et al., 2017). However, the parsimony method can allow a posteriori reweighting of characters (Swofford, 2003) and accordingly improve phylogenetic inference (Goloboff et al., 2008). Bayesian tip-dating (TD) or the total-evidence dating method can co-estimate divergence ages and topology with fossil taxa as terminal tips (O’Reilly et al., 2015). This recently developed approach integrates morphological data from the fossil record and morphological and sequence data from living organisms, as well as stratigraphic information (Gavryushkina et al., 2017), and has been widely used in biogeographic studies (e.g. Varela et al., 2019; Yan et al., 2021; Bansal et al., 2022; Zhang et al., 2022). For tip-dating analyses, all coded morphological characters are usually used. However, few studies have been devoted to investigating whether morphological homoplasy affects fossil placements and divergence time estimation. Additionally, a simulation study suggests that stratigraphic information from fossils has a positive impact on tree topology (Mongiardino Koch et al., 2021). This needs to be tested using empirical data.
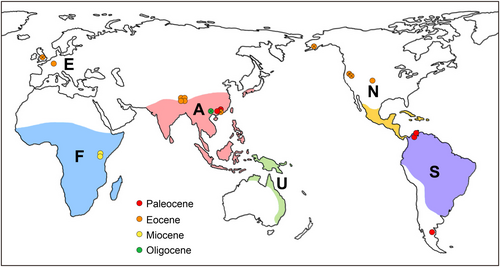
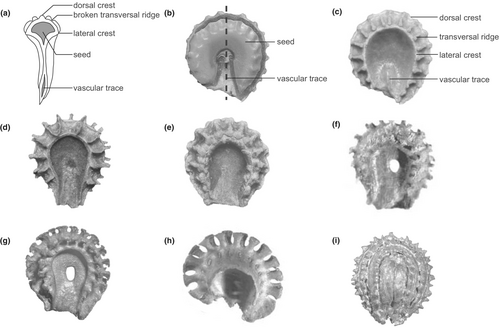
Menispermaceae, commonly known as the moonseed family, is a characteristic component in the physiognomy of modern tropical rainforests (Gentry, 1991; Richards, 1996; Wang et al., 2012). Because its distinctive endocarps are frequently preserved as fossils, the family has an abundant fossil record assigned to extant or extinct genera (Jacques, 2009a). As phylogenetic analyses indicate that endocarp characters are highly homoplastic in Menispermaceae (Ortiz et al., 2007; Hoot et al., 2009; Wefferling et al., 2013), extra caution in identifying affinities for fossil taxa is required. As the second species-richest tribe in this family, Cissampelideae contains five genera and c. 126 species (Ortiz et al., 2016), most of which are lianas and inhabit tropical rainforests. Antizoma with three species and the monotypic Perichasma are endemic to tropical Africa. Cyclea contains about 32 species that are entirely restricted to Southeast Asia. Stephania, consisting of about 68 species, is primarily distributed in Southeast Asia and tropical Africa, with a few species occurring in northeastern Australia. Cissampelos, containing about 23 species, is distributed throughout all tropical regions. During the past decade, the overwhelming majority of endocarp fossils reported from Menispermaceae have been placed in this tribe (Table 1). Evidence of fossil endocarps indicates that Cissampelideae were widely distributed in the Northern Hemisphere and South America in the Early Cenozoic (Fig. 1; Table 1). In terms of endocarp morphology, there is a significant amount of variation among the members of the tribe, such as ornamentation (number, shape and distribution of ribs or spines), perforation (presence or absence), endocarp shape (from obovate to rounded), number of dorsal crests (one or two) and dorsal and lateral crests (spiny or nonspiny) (Fig. 2; Jacques, 2009b; Herrera et al., 2011; Ortiz, 2012; Wefferling et al., 2013).
| Fossil taxon | Epoch | Age minimum (Ma) | Age maximum (Ma) | Locality | Reference |
|---|---|---|---|---|---|
| Cissampelos defranceschii C. Del Rio & T. Su | Middle Eocene | 37.71 | 47.8 | Xizang, China | Rio et al. (2021) |
| Cissampelos rusingensis Chesters | Burdigalian (Miocene) | 15.97 | 20.44 | Nyanza, Kenya | Chesters (1957) |
| Palaeoluna bogotensis Herrera, Manchester, Hoot, Wefferling, Carvalho et Jaramillo | Middle-Late Palaeocene | 56 | 61.6 | Cundinamarca, Colombia | Herrera et al. (2011) |
| Stephania auriformis (Hollick) Han & Manchester | Earlyl-Middle Eocene | 37.71 | 56 | London, UK; Alaska and Oregon, USA; Guangdong, China | Hollick (1936); Chandler (1961); Manchester (1994); Han et al. (2018, 2020) |
| Stephania bangorensis C. Del Rio & T. Su | Middle Eocene | 37.71 | 47.8 | Xizang, China | Rio et al. (2021) |
| Stephania geniculata Han & Jin | Middle Palaeocene | 59.2 | 61.6 | Guangdong, China | Han et al. (2020) |
| Stephania hootae Collinson | Middle Eocene | 37.71 | 47.8 | Hessen, Germany | Collinson et al. (2012) |
| Stephania jacquesii Han & Manchester | Late Eocene–Late Oligocene | 23.03 | 37.71 | Oregon, USA; Guangxi, China | Han et al. (2018) |
| Stephania miocenica Chesters | Burdigalian (Miocene) | 15.97 | 20.44 | Nyanza, Kenya | Chester (1957) |
| Stephania ornamenta Han & Jin | Middle Palaeocene | 59.2 | 61.6 | Guangdong, China | Han et al. (2020) |
| Stephania palaeosudamericana Herrera, Manchester, Hoot, Wefferling, Carvalho et Jaramillo | Middle–Late Palaeocene | 56 | 61.6 | Guajira, Colombia | Herrera et al. (2011) |
| Stephania psittaca Jud et Gandolfo | Early Danian (Palaeocene) | 61.6 | 66 | Chubut, Argentina | Jud et al. (2018) |
| Stephania shuangxingii C. Del Rio & T. Su | Middle Eocene | 37.71 | 47.8 | Xizang, China | Rio et al. (2021) |
| Stephania wilfii Han & Manchester | Palaeocene–Eocene boundary to Middle Eocene | 37.71 | 56 | Wyoming, USA; Xizang, China | Han et al. (2018); Rio et al. (2021) |
A molecular scaffold analysis with 25 morphological characters placed South American Palaeocene Stephania palaeosudamericana and Palaeoluna bogotensis in Cissampelideae (Herrera et al., 2011), but this backbone was later found to be incorrect (Wang et al., 2012; Wefferling et al., 2013). Importantly, during the past decade, nine endocarp fossils of the tribe have been reported from the Palaeocene to Oligocene of Eurasia and North America (Table 1; Han et al., 2018, 2020; Jud et al., 2018; Rio et al., 2021). However, their taxonomic assignments have never been assessed in a phylogenetic framework. In particular, as the largest genus within Menispermaceae, Stephania exhibits a significant amount of intrageneric variation in endocarp characters (Diels, 1910; Jacques, 2009b; Ortiz et al., 2016). The circumscription of this genus, as well as Cissampelos, has long been controversial (Xie et al., 2015; Ortiz et al., 2016), which further hampers the accurate placements of these fossils.
Here, for the first time, we use diverse strategies to exclude the coded endocarp characters with high-level homoplasy or to reweight the morphological characters, and then conduct a series of phylogenetic analyses to assess the placement of 14 fossil endocarps across the Cissampelideae phylogeny based on morphological data only, morphological data with a molecular scaffold and combined molecular and morphological data. Our aims were to explore how to effectively mitigate the impact of morphological homoplasy on the phylogenetic placement of fossil taxa of Cissampelideae, to examine the impact of fossil phylogenetic placement on the accuracy of age estimates and ancestral range reconstruction based on total evidence matrices, and to investigate how the current distribution and endemism of the tribe were shaped over time, hence providing new insights into the past tropical floristic exchanges among different continents.
Materials and methods
Molecular data
We sampled 37 species, representing all five of the currently recognized genera of Cissampelideae. Four individuals of the monotypic Stephania subg. Botryodiscia, whose systematic position is a key in determining the monophyly of Stephania, were included. Thirty additional species of Menispermoideae were selected as outgroups, representing all the other seven tribes of the subfamily (Lian et al., 2020). Our taxon sampling covers the endocarp diversity in Cissampelideae, as well as in Menispermoideae. Based on the results of Wang et al. (2012) and Ortiz et al. (2016), Menispermeae were used to root the tree. Seven DNA regions, including five chloroplast (rbcL, atpB, matK, ndhF and trnL-F) and two nuclear (26S ribosomal DNA (26S rDNA) and internal transcribed spacer (ITS)) loci were used in this study. The DNA extraction, amplification and sequencing procedures followed Wang et al. (2012, 2016). Voucher information and GenBank accession numbers are listed in the Table S1.
Molecular phylogenetic analyses
Sequences were aligned using Geneious v.10.1.3 (Kearse et al., 2012), and adjusted manually. The final alignments contained 70 living taxa with 1386 bp for rbcL, 1407 bp for atpB, 1251 bp for matK, 2073 bp for ndhF, 1127 bp for trnL-F (excluding two poly regions with 17 nucleotides), 1355 bp for 26S rDNA and 599 bp for ITS. Maximum likelihood (ML) non-parametric bootstrap (BS) analysis was carried out for each locus in RAxML v.8.0 (Stamatakis, 2014). No significant bootstrap support (exceeding ≥70%) for conflicting nodes was evident among individual chloroplast markers and among individual nuclear loci, and the five chloroplast DNA (cpDNA) (rbcL, atpB, matK, ndhF and trnL-F) and two nuclear ribosome DNA (nrDNA) (26S rDNA and ITS) loci were combined into two separate datasets. Phylogenetic analyses were carried out using ML, maximum parsimony (MP) and undated Bayesian inference (BI) methods for the cpDNA and nrDNA datasets, as well as for the seven-locus dataset (referred to hereafter as matrix 0).
For ML analyses, RAxML was run with the GTR + Γ substitution model for each DNA region, with clade support assessed using the fast bootstrap option and 1000 replicates. The MP analyses were performed in PAUP* v.4.0a152 (Swofford, 2003). Bootstrapping was conducted under a traditional search with 1000 replicates of random addition, 100 trees saved at each replicate and tree bisection reconnection swapping. The undated BI analyses were conducted in MrBayes v.3.2.5 (Ronquist et al., 2012). The best fit model of nucleotide substitution was assigned to each DNA region, as determined by the Akaike Information Criterion via jModeltest v.2.1.4 (Posada, 2008). Two independent runs, each consisting of four Markov Chain Monte Carlo chains, were conducted, sampling every 1000 generations for 50 million generations, starting with a random tree. Run stationarity was determined in Tracer v.1.6 (Rambaut et al., 2014). A majority rule (>50%) consensus tree was constructed after removing the burn-in (the first 25% of all sampled trees).
Fossil taxa and their placements
A total of 14 endocarp fossils assigned to Cissampelideae were included (Table 1). Twenty-four endocarp characters were obtained from the literature and personal observations (Appendix S1). The extant Antizoma angustifolia, Cissampelos madagascariensis and Haematocarpus validus were excluded because their endocarps are still unknown. The complete morphological character matrix (matrix 1) containing 78 fossil and living species is provided in Appendix S2.
To determine accurately the systematic positions of the 14 fossils, a series of phylogenetic analyses were carried out based on the morphological data only, morphological data with a molecular scaffold and combined molecular and morphological data (Fig. 3). The homoplastic level of each morphological character can be described using the consistency index (CI), the retention index (RI) or the rescaled consistency index (RC) (Farris, 1989). These indexes run from 0 to 1; an index of 1 indicates that the character fit the particular phylogeny 100% perfectly (i.e. it shows no homoplasy), while an index of 0 indicates that the character and cladogram are 0% consistent (i.e. it shows maximum amount of homoplasy) (Kitching et al., 1998). The CI, RI and RC values for each character that we coded were calculated in Mesquite v.3.61 (Appendix S1; Maddison and Maddison, 2019). Following Allaby (2020), we used the thresholds CI < 0.5 and RI < 0.5 as an indication of a high level of morphological homoplasy. We also used RC < 0.25 as a threshold as the RC value is generated by multiplying CI and RI. Based on these three thresholds, we subsampled morphological characters, respectively, and obtained three new matrices: matrix 2 (containing 11 morphological characters; CI ≥ 0.5), matrix 3 (containing 20 morphological characters; RI ≥ 0.5) and matrix 4 (containing 13 morphological characters; RC ≥ 0.25) (Fig. 3a). For the four morphological matrices (1–4), we first conducted separate phylogenetic analyses (phylogenetic analyses 1–4), and then used our molecular phylogenetic tree as a molecular scaffold, in which nodes with ML-bootstrap value (BS) <70% were collapsed, to run further analyses (phylogenetic analyses 5–8). Additionally, we used the reweighting function implemented in PAUP* v.4.0a152 to analyse the original morphological dataset (matrix 1). We assigned weights to characters based on the CI, RI and RC values, respectively, and ran analyses using a molecular scaffold (phylogenetic analyses 9–11).
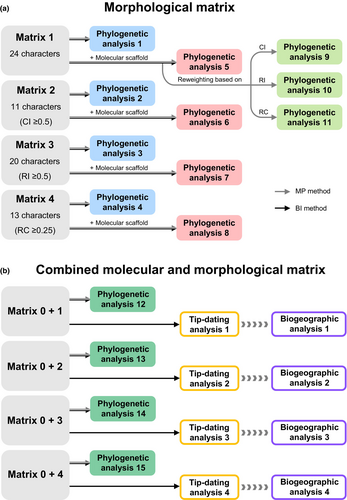
For the analyses of the combined molecular and morphological data, we only focused on Cissampelideae with Spirospermeae as an outgroup and excluded the three fossils that were not members of Cissampelideae (see the results below). The seven-locus dataset (matrix 0) was combined with the above four morphological datasets, respectively. Accordingly, we obtained four combined molecular and morphological matrices (0 + 1, 0 + 2, 0 + 3 and 0 + 4). The reweighting command must assign weights to all the characters in a matrix based on their fit to the tree currently in memory, which may reward informative molecular and morphological characters improperly (Swofford, 2003). Here, phylogenetic analyses were only performed without reweighting the homoplastic characters (phylogenetic analyses 12–15; Fig. 3b).
The reweighting function can be executed in MP and undated BI methods. However, for undated BI, the percentage of characters whose weight has to be increased or decreased is artificially assigned by replicating or removing characters (Huelsenbeck et al., 2015), which is not appropriate for dating analyses. Thus, three reweighting analyses (phylogenetic analyses 9–11) only were conducted using MP method, whereas the other phylogenetic analyses (1–8; 12–15) were conducted using MP and undated BI method in PAUP* v.4.0a152 and MrBayes v.3.2.5, respectively.
Divergence time estimation
To evaluate the impact of morphological homoplasy on divergence time estimation, four tip-dating analyses (1–4; Fig. 3b) were performed based on the above four combined molecular and morphological data matrices under a fossilized birth–death prior process (Heath et al., 2014). The GTR model with a gamma distribution was used for each DNA locus partition separately. For morphological data, the Mk model (Lewis, 2001) was used. We applied the uncorrelated lognormal relaxed-clock model to the molecular and morphological data (Drummond et al., 2006). The calibrated ages of the fossils were assigned a uniform prior distribution with the confidence interval of the deposition time of the fossil, defined as the interval from the maximum to the minimum possible age of the formation in which the fossil was found. We adopted the latest geological time scale of Cohen et al. (2013, updated). For comparative purposes, we also estimated divergence times using a node-dating method (Drummond et al., 2012). According to the results of our fossil placements, the oldest fossils of three clades (i.e. Stephania hootae†, Stephania miocenica† and Stephania psittaca†) were selected as calibration points and used with a uniform prior distribution. For all dating analyses, to avoid overestimation of root age, we set a uniform distribution with a maximum bound of 76 Ma, which is the stem group age of the Cissampelideae (Lian et al., 2020) and a minimum bound matching the age of the oldest sampled fossil for the root. Dating analyses were implemented in BEAST v.2.4.8 with the SA (sampled ancestor) package (Bouckaert et al., 2014). The Markov Chain Monte Carlo chains were run for 50 million generations, sampling every 5000 generations. Convergence and the adequate effective sample size values (>200) were checked in Tracer v.1.6 (Rambaut et al., 2014). After a burn-in of 25%, the maximum clade credibility (MCC) tree with mean heights and 95% highest posterior density (HPD) intervals on nodes was built using TreeAnnotator v.2.1.3 (part of the BEAST package).
Ancestral range reconstruction
Ancestral range reconstructions were carried out using the MCC trees generated from the four tip-dating (TD) analyses 1–4 (Fig. 3b), under the dispersal–extinction–cladogenesis model (DEC; Ree and Smith, 2008), implemented in RASP v.4.2 (Yu et al., 2020). In order to accommodate phylogenetic uncertainties, we used an empirical Bayesian approach inspired by Beaulieu et al. (2013), in which the likeliest biogeographic scenario was inferred across 1000 randomly chosen dated trees. To assess the effect of including fossil distributions on biogeographic analysis, we also ran two analyses using the MCC tree generated from the node-dating analysis under the extinction free model (DEC; Ree and Smith, 2008) and the lineage extinction model of ancestral distribution (LEMAD; Herrera-Alsina et al., 2022) that can model the geographic distribution of extinct species. Based on the floristic characteristics of Takhtajan (1986) and distributions of living and fossil species in Cissampelideae, six areas were defined: (A) tropical and subtropical Asia; (F) Africa; (U) Australasia; (E) Europe; (N) North America; and (S) South America. Following Mao et al. (2012), we specified dispersal probabilities between pairs of areas for four separate time slices. The maximum number of areas was restricted to two, as each sampled tip is restricted to no more than two areas.
Results
Phylogeny of the extant Cissampelideae
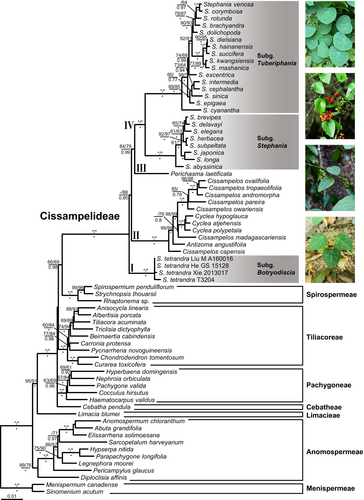
The topology of living Menispermoideae phylogeny generated based on the cpDNA dataset (Fig. S1) is highly congruent with the nrDNA tree (Fig. S2). Thus, we combined the plastid and nuclear datasets into a single dataset. The combined seven-locus dataset comprised 9198 characters, of which 2019 were variable. The ML and BI analyses resulted in identical trees that are highly consistent with the trees derived from the MP analysis except for the phylogenetic position of S. subg. Botryodiscia (Fig. 4). Eight clades, corresponding to the eight tribes of Menispermoideae, are recovered and strongly supported as monophyletic. Within the subfamily, Menispermeae is the first-branching lineage, followed by Anomospermeae, and then Limacieae. Spirospermeae is sister to Cissampelideae (MP-BS = 66%, ML-BS = 69%, posterior probabilities (PP) = 0.98). Within Cissampelideae, four major clades are recognized: clade I consists of subg. Botryodiscia; clade II contains Cissampelos, Cyclea and Antizoma (MP-BS = 100%, ML-BS = 100%, PP = 1.00); clade III contains Perichasma; and clade IV consists of subg. Stephania and subg. Tuberiphania (MP-BS = 100%, ML-BS = 100%, PP = 1.00). Both ML and BI analyses support subg. Botryodiscia (clade I) as the earliest-diverging lineage in Cissampelideae (ML-BS = 88%, PP = 0.85), while MP analysis supports clade II as basalmost in the tribe (MP-BS = 50%).
Placements of fossil taxa
Cladograms generated from the morphological data (phylogenetic analyses 1–4) are poorly resolved (Fig. S3a–d). Two and three nodes with fossil taxa were recovered by MP and BI analyses respectively (Table 2). Three phylogenetic reconstructions based on the subsampled datasets (matrices 2–4) tend to have both a lower proportion of resolved nodes and average support than those based on the original morphological dataset (matrix 1; Table 2). Among the three subsampled matrices, matrix 3 (containing the characters with RI ≥ 0.25) generated both a higher proportion of resolved nodes and average support. Compared with the original morphological dataset (matrix 1), the three subsampled matrices recovered fewer nodes with fossil taxa.
| Method | Analytical strategies | Phylogenetic analysis | Proportion of resolved nodes | Average support | Support of nodes with fossils | |||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | |||||
| MP | Morphological matrix | 1 | 12% | 68.30 | 70 | – | 53 | – |
| 2 | 5% | 63.25 | – | – | – | – | ||
| 3 | 7% | 75.00 | – | – | 58 | – | ||
| 4 | 5% | 63.00 | – | – | – | – | ||
| Morphological matrix + molecular scaffold | 5 | 81% | 89.72 | 66 | 41 | 87 | 73 | |
| 6 | 83% | 87.71 | 64 | – | 67 | 73 | ||
| 7 | 75% | 92.69 | – | 54 | 82 | 81 | ||
| 8 | 83% | 89.18 | – | 58 | 70 | 73 | ||
| Morphological matrix + molecular scaffold (reweighting) | 9 | 84% | 89.52 | 80 | 57 | 98 | 66 | |
| 10 | 80% | 91.07 | 73 | 50 | 89 | 67 | ||
| 11 | 84% | 90.57 | 79 | 65 | 92 | 63 | ||
| Combined molecular and morphological matrix | 12 | 73% | 76.93 | 64 | – | 84 | 76 | |
| 13 | 73% | 76.91 | 61 | – | 70 | 70 | ||
| 14 | 71% | 77.70 | – | – | 81 | 83 | ||
| 15 | 72% | 78.20 | – | 61 | 68 | 72 | ||
| Undated BI | Morphological matrix | 1 | 25% | 0.76 | 0.91 | – | 0.93 | 0.65 |
| 2 | 5% | 0.74 | – | – | 0.79 | – | ||
| 3 | 23% | 0.73 | – | – | 0.92 | 0.68 | ||
| 4 | 16% | 0.68 | – | – | 0.87 | 0.75 | ||
| Morphological matrix + molecular scaffold | 5 | 88% | 0.89 | 0.97 | 0.53 | 1.00 | 0.98 | |
| 6 | 77% | 0.93 | 0.98 | – | 0.93 | 0.99 | ||
| 7 | 81% | 0.92 | 0.57 | – | 1.00 | 1.00 | ||
| 8 | 80% | 0.93 | – | – | 0.99 | 0.99 | ||
| Combined molecular and morphological matrix | 12 | 85% | 0.93 | 1.00 | 1.00 | 1.00 | 1.00 | |
| 13 | 76% | 0.89 | 1.00 | – | 0.9 | 1.00 | ||
| 14 | 84% | 0.91 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| 15 | 83% | 0.91 | 0.7 | 0.8 | 1.00 | 1.00 | ||
- Nodal supports from maximum parsimony (MP) and Bayesian inference (BI) methods are bootstrap percentages and posterior probabilities, respectively.
The addition of the molecular scaffold significantly improves the proportion of resolved nodes and average support (Table 2; Fig. S3e–h). For parsimony analyses, removing (MP 6–8) and reweighting (MP 9–11) high-level homoplastic morphological characters generated a higher proportion of resolved nodes and lower average support, or a lower proportion of resolved nodes and higher average support, and the latter recognized all four nodes with fossils. Among the three reweighting analyses (MP 9–11), MP 11 (RC < 0.25 as a threshold) generated trees with both a higher proportion of resolved nodes and average support. For undated Bayesian analyses, the removal of homoplastic morphological characters slightly decreased the proportion of resolved nodes, but increased the average support and led to fewer nodes with fossil taxa being recovered (undated BI 6–8). The original morphological and reweighting analyses recovered four nodes with fossil taxa (phylogenetic analyses 5 and 9–11), whereas the analyses removing high-level homoplastic morphological characters only recovered two to three nodes with fossil taxa (phylogenetic analyses 6–8).
Trees generated from the combined molecular and morphological data (phylogenetic analyses 12–15; Fig. S3k–n) are better resolved than the trees generated based on morphological data only (phylogenetic analyses 1–4; Fig. S3a–d), but have a lower proportion of resolved nodes and average support than the trees built based on morphological data with a molecular scaffold (phylogenetic analyses 5–11; Fig. 5 and Fig. S3e–j; Table 2). For the combined molecular and morphological datasets, removing high-level homoplastic morphological characters had smaller effects on the trees, and compared with MP analyses, undated BI analyses recovered more nodes with fossils.
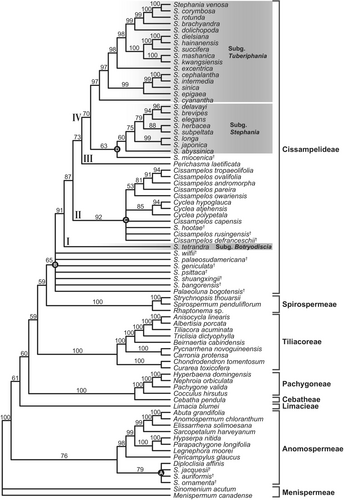
Weighting parsimony analysis against homoplasy according to the RC values (≥ 0.25; MP 11) yielded trees with both the highest proportion of resolved nodes and average support (Fig. 5; Table 2). Based on MP 11, the 14 sampled fossils were placed in four systematic positions. Stephania jacquesii†, Stephania auriformis† and Stephania ornamenta† are clustered with Diploclisia affinis of Anomospermeae (node A, BS = 79%). Seven fossil species of Stephania and Palaeoluna (i.e. S. bangorensis†, S. geniculata†, S. palaeosudamericana†, S. psittaca†, S. shuangxingii†, S. wilfii† and P. bogotensis†) are resolved as the stem group members of Cissampelideae (node B, BS = 65%), but their relationships were not resolved. Cissampelos defranceschii†, Cissampelos rusingensis† and S. hootae† are clustered with extant Cyclea and Cissampelos (node C, BS = 92%). Stephania miocenica† is clustered with extant subg. Stephania (node D, BS = 63%).
When focusing on Cissampelideae, compared with undated BI analyses, tip-dated BI analyses generated a higher proportion of resolved nodes (100%) (Table 3; Fig. S4a–d); in particular, the seven identified stem group members of Cissampelideae formed a clade (clade V in Fig. S4). Among the four tip-dated BI analyses, analysis 4 (RC < 0.25 as a threshold) placed the fossil taxa in the most consistent order as they appeared in the stratum (Fig. S4). Besides the fossil clade V, Cissampelideae contains four major clades: S. subg. Botryodiscia (clade I), clade II containing three fossil species (C. defranceschii†, C. rusingensis† and S. hootae†) and extant Cissampelos and Cyclea, clade III contains Perichasma (clade III) and clade IV consisting of extinct S. miocenica† and the extant subgenera Stephania and Tuberiphania of Stephania.
| Method | Phylogenetic analysis | Proportion of resolved nodes | Average support | Support of nodes with fossils | ||
|---|---|---|---|---|---|---|
| B | C | D | ||||
| Undated BI | 12 | 74% | 0.91 | 1.00 | 1.00 | 0.97 |
| 13 | 79% | 0.87 | 0.99 | 0.93 | 0.94 | |
| 14 | 74% | 0.89 | 1.00 | 1.00 | 0.99 | |
| 15 | 79% | 0.90 | 1.00 | 0.97 | 0.99 | |
| Tip-dated BI | Tip-dating analysis 1 | 100% | 0.83 | 0.97 | 0.99 | 0.38 |
| Tip-dating analysis 2 | 100% | 0.83 | 0.97 | 0.95 | 0.51 | |
| Tip-dating analysis 3 | 100% | 0.84 | 0.97 | 1.00 | 0.42 | |
| Tip-dating analysis 4 | 100% | 0.83 | 0.97 | 0.98 | 0.51 | |
- Nodal supports are posterior probabilities. BI, Bayesian inference.
Divergence time estimation and ancestral range reconstruction
Four tip-dating analyses generated highly congruent divergence time estimates for Cissampelideae (Fig. S4). A detailed comparison of the times estimated for selected nodes is shown in Table 4. Using the MCC trees generated from tip-dating analyses 1–4 as the input trees, our four ancestral range reconstructions are highly congruent except within the fossil clade V (biogeographic analyses 1–4; Fig. 6 and Fig. S8a–c). When the morphological characters of RC ≥ 0.25 are kept, all fossil species are placed in the tree in the same order as they appeared in the stratum. Thus, the results generated from the Matrix 0 + 4 are reported (Fig. 6) and used for the discussion. Cissampelideae probably started to diversify in Asia at 68.21 Ma (95% HPD 63.12–73.67; node 1). One dispersal event was inferred from Asia to the New World (between nodes 1 and 2). The age of node 2 was estimated at 65.97 Ma (95% HPD 61.95–71.08). The split of P. bogotensis† of South America and its Asian sister lineage was estimated at 57.66 Ma (95% HPD 56.01–61.90; node 3). The extant Cissampelideae date to 55.85 Ma (95% HPD 48.00–64.42; node 4). Two dispersal events from Asia to Africa occurred at ~52.78–48.52 Ma (between nodes 5 and 10) and at ~39.33–24.92 Ma (between nodes 6 and 7). The split of African Cissampelos capensis and Asian Cissampelos rusingensis† occurred at 21.18 Ma (95% HPD 16.28–29.13; node 8). The split of African Cissampelideae owariensis and its Asian-American sister group was estimated at 11.03 Ma (95% HPD: 8.93–15.33; node 9). African Stephania abyssinica and Stephania cyanantha separated from their Asian sisters at 13.22 Ma (95% HPD: 8.59–17.56; node 12) and 18.98 Ma (95% HPD: 13.13–25.60; node 13), respectively.
| Node | Node-dating method | Tip-dating method | |||
|---|---|---|---|---|---|
| Analysis 1 | Analysis 2 | Analysis 3 | Analysis 4 | ||
| 1 | – | 69.65 (64.25–74.11) | 68.50 (63.15–73.61) | 69.29 (63.74–73.84) | 68.21 (63.12–73.67) |
| 2 | – | 67.05 (62.52–71.74) | 65.98 (62.25–70.71) | 66.52 (62.36–71.66) | 65.97 (61.95–71.08) |
| 3 | – | 57.47 (56.00–60.63) | 62.73 (59.52–68.11) | 57.20 (56.00–60.32) | 57.66 (56.01–61.90) |
| 4 | 56.23 (42.03–69.94) | 59.82 (51.82–67.96) | 56.25 (49.05–65.81) | 59.72 (52.00–68.33) | 55.85 (48.00–64.42) |
| 5 | 52.04 (38.88–64.81) | 56.58 (49.42–64.55) | 53.09 (46.15–62.36) | 56.44 (48.98–64.53) | 52.78 (45.43–60.81) |
| 6 | – | 39.52 (37.71–45.00) | 39.43 (37.71–44.50) | 39.81 (37.72–45.40) | 39.33 (37.71–44.38) |
| 7 | 27.11 (17.69–37.95) | 25.28 (19.10–34.91) | 25.17 (17.57–36.89) | 25.76 (18.80–34.60) | 24.92 (17.52–35.84) |
| 8 | 17.35 (15.97–19.95) | 23.09 (15.04–31.64) | 17.27 (15.98–19.95) | 21.18 (16.28–29.13) | |
| 9 | 12.96 (7.96–18.18) | 12.80 (7.50–20.37) | 10.98 (7.32–16.20) | 12.64 (7.67–20.59) | 11.03 (8.93–15.33) |
| 10 | 48.05 (35.93–60.99) | 51.62 (41.88–60.24) | 48.98 (40.33–58.80) | 51.51 (42.08–60.76) | 48.52 (40.65–57.38) |
| 11 | 39.62 (28.71–51.75) | 41.69 (31.77–52.07) | 39.90 (31.07–49.23) | 41.78 (31.76–51.46) | 39.41 (30.93–48.74) |
| 12 | 15.23 (9.04–21.75) | 13.71 (8.82–19.64) | 13.06 (8.53–17.75) | 13.52 (8.89–19.20) | 13.22 (8.59–17.56) |
| 13 | 20.98 (14.09–29.23) | 21.06 (13.94–29.80) | 18.74 (12.88–25.86) | 20.48 (14.25–28.60) | 18.98 (13.13–25.60) |
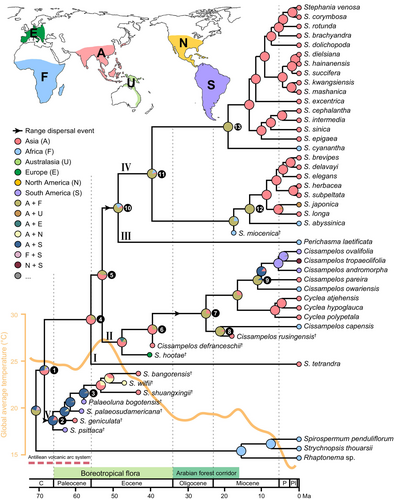
Divergence time estimates for Cissampelideae using node dating are shown in Fig. S5 and coincide with the estimates from the four tip-dating analyses (Table 4). Ancestral range reconstructions including only extant Cissampelideae under the DEC and LEMAD models are shown in Figs S7 and S8, respectively. Under the DEC model, the most recent common ancestor (MRCA) of extant Cissampelideae was present in Asia and Africa at 56.23 Ma (95% HPD 42.03–69.94; node 4). One dispersal event from Africa to Asia was inferred, which occurred c. 48.05–39.62 Ma (between nodes 10 and 11). Under the LEMAD model, the crown group of Cissampelideae probably occupied Asia (node 4), and subsequently dispersed into Africa at c. 52 Ma (node 5). Within Stephania s.str., two dispersal events were inferred from Asia to Africa at ~39.62–15.23 Ma (between nodes 11 and 12) and at ~39.62–20.98 Ma (between nodes 11 and 13).
Discussion
Phylogenetic relationships within extant Cissampelideae
- Botryodiscia (Diels) Lian Lian & Wei Wang, stat. nov. ≡ Stephania subg. Botryodiscia (Diels) Lo in Bull. Bot. Res. 2: 40. 1982. Type: Botryodiscia tetrandra (S. Moore) Lian Lian & Wei Wang.
- https://www.ipni.org/urn:lsid:ipni.org:names:77331191-1
- Botryodiscia tetrandra (S. Moore) Lian Lian & Wei Wang, comb. nov. Basionym: Stephania tetrandra S. Moore in Journ. Bot. 13: 225. 1875.
- Type locality: China, Chekiang, Kiukiang.
Effect of homoplastic morphological characters on fossil placements
The integrated phylogenetic analyses of fossil and living taxa are usually based on all available coded morphological characters. However, owing to frequent incompleteness relative to living taxa, especially lacking molecular data, the placement of fossil taxa is determined based on limited morphological characters that are more susceptible to being homoplastic (Ksepka et al., 2017; Lee and Yates, 2018). To test how to mitigate effectively the impact of morphological homoplasy on the fossil placements of Cissampelideae, we assembled three data types, i.e. morphological data only, morphological data with a molecular scaffold and combined morphological and molecular data. We used the thresholds CI < 0.5, RI < 0.5 and RC < 0.25 separately to describe high level of morphological homoplasy and consequently conducted 15 MP, 12 undated BI and four dated BI analyses (Fig. 3). Our results indicate that the 14 selected fossil taxa were poorly placed when only morphological data were considered, whereas the addition of molecular scaffold and combined morphological and molecular data might greatly improve the resolution of fossil nodes (Table 2). Ksepka et al. (2017) have suggested that a molecular scaffold has the potential to “rescue” the phylogenetic framework to different extents. In our study, for the same data type, removing homoplastic morphological characters usually decreased the resolution of fossil nodes as well as the proportion of resolved nodes and average support of the whole tree (Table 2; Fig. S3).
Among the 15 MP analyses, reweighting analyses generated the highest proportion of resolved nodes and average support, and recovered all four fossil nodes (Table 2). A recent study also suggests that reweighting parsimony analysis can improve fossil stability (Flores et al., 2021). Our analyses further indicate that compared with the original morphological data with a molecular scaffold, among the three reweighting strategies, only the reweighting analysis against homoplasy according to the RC value resulted in the increase of both the proportion of resolved nodes and average support (MP 5 vs. 11). The RC value is generated by multiplying CI and RI (Farris, 1989), and thus may integrate the advantages of both CI and RI values.
Our results show that the undated Bayesian method may outperform MP method for morphological matrices (1–4), as in other studies (e.g. Wright and Hillis, 2014; O'Reilly et al., 2016, 2018; Puttick et al., 2017). By a series of simulated analyses, Mongiardino Koch et al. (2021) also suggested that the undated Bayesian method significantly outperforms the MP method, especially when the proportions of fossil terminals and levels of missing data are high. When the molecular scaffold was used, trees generated by the MP analyses had a higher proportion of resolved nodes than the undated Bayesian analyses when homoplastic morphological characters were removed according to the CI and RC values (MP 6 vs. undated BI 6; MP 8 vs. undated BI 8). This implies that the undated Bayesian method does not always outperform the MP method when a molecular scaffold is added. Among the 12 undated BI analyses, the analysis based on all available morphological and molecular data performed best.
Our results indicate that compared with four undated Bayesian analyses, four tip-dated BI analyses containing stratigraphic information may greatly increase the proportion of resolved nodes (<80 vs. 100%; Table 3), implying that the stratigraphic information has a positive impact on tree topology, in agreement with the simulated result of Mongiardino Koch et al. (2021). In particular, all 14 selected Cissampelideae fossils were placed in the four tip-dating trees, supporting the viewpoint that the inclusion of stratigraphic age data can facilitate the placement of fossil taxa (King, 2021). Similarly, López-Antoñanzas and Peláez-Campomanes (2022) also demonstrated that tip-dated Bayesian analyses may yield trees that have higher stratigraphic congruence compared with trees from undated Bayesian and parsimony analyses. Besides, with the removal of morphological characters according to different indexes, the evolutionary relationships of the seven fossil taxa of clade V vary accordingly (Fig. S4). Based on all coded morphological characters, S. psittaca†, the oldest fossil in clade V, is recovered in a relatively derived position (Fig. S4a), whereas when the homoplastic morphological characters are removed according to the CI, RI or RC values, S. psittaca† is recovered as basalmost in clade V (Fig. S4b–d). Importantly, the TD tree recovered by removing homoplastic morphological characters with RC < 0.25 has the highest stratigraphic fit (Fig. S4d).
Phylogenetic analyses based on morphological data with a molecular scaffold (phylogenetic analyses 5–11) and on combined morphological and molecular data (phylogenetic analyses 12–15) show that three fossil species, i.e. S. jacquesii†, S. auriformis† and S. ornamenta†, have a distant relationship with extant Cissampelideae, and instead they group with Diploclisia of Anomospermeae (node A in Fig. 5). In fact, S. auriformis† was first described by Manchester (1994) as Diploclisia auriformis and then transferred to Stephania by Han et al. (2018), implying that endocarps of Diploclisia may be easily confused with that of Stephania. Whereas the remaining 11 fossil species formed a clade with extant Cissampelideae (node B in Fig. 5), Stephania bangorensis†, S. geniculata†, S. palaeosudamericana†, S. psittaca†, S. shuangxingii†, S. wilfii†, and P. bogotensis† are supported as the stem group members of Cissampelideae, S. hootae†, C. defranceschii† and C. rusingensis† are grouped with extant Cyclea and Cissampelos, i.e. clade II (node C in Fig. 5), and S. miocenica† clusters with S. subg. Stephania in clade IV (node D in Fig. 5). The tip-dated BI analyses further show that the seven stem group members of Cissampelideae form a clade (clade V in Fig. S4). Within clade II, S. hootae† is the earliest-diverging lineage, followed by C. defranceschii†, and C. rusingensis† and C. capensis formed a clade, sister to other extant Cissampelos and Cyclea. These fossil placements recovered here is a foundation for their future taxonomic revision.
Effect of homoplastic morphological characters on divergence time estimation and ancestral range reconstruction
Our results show that removing homoplastic morphological characters from the total evidence matrix has no obvious effect on divergence time estimation. The four tip-dating analyses generated similar time estimates for Cissampelideae, which is not surprising because for the four total evidence matrices, the number of morphological characters is much smaller than the number of variable molecular characters (13–24 ≤ vs. 2019). Tip-dated trees sometimes exhibit inflated divergence time estimates for deep nodes (O'Reilly et al., 2015; Ronquist et al., 2016). Based on our phylogenetic analyses with fossil and extant species, we used the early Palaeocene S. psittaca†, the middle Eocene S. hootae†, and the early Miocene S. miocenica† to constrain the stem group age of Cissampelideae, the stem group age of Stephania s.str.-Perichasma and the crown group age of Stephania s.str., respectively, and run the node-dating analysis. Our node-dating and tip-dating analyses resulted in highly congruent time estimates for Cissampelideae (Table 4), implying that these two dating methods can lead to convergent estimation when correct calibrating fossils are used. Nevertheless, tip-dating analyses produced narrower ranges of ages (Table 4), which suggests that tip-dating may estimate divergence times more accurately than node-dating. In this study, the crown group age of Cissampelideae is estimated to be ~56 Ma, which is much older than those of Wang et al. (2012; 38 Ma) and Lian et al. (2020; 46 Ma). Wang et al. (2012) only used S. miocenica† to constrain the stem group age of Stephania s.str., and Lian et al. (2020) only used the middle-late Palaeocene S. palaeosudamericana† to constrain the stem group age of Cissampelideae. Our data support the notion that internal calibrating fossils and their correct placements are vital to obtain accurate time estimates (Parham et al., 2012; Sauquet et al., 2012; Wang et al., 2016).
Our four biogeographic analyses generated largely congruent ancestral range reconstructions for Cissampelideae except within the fossil clade V (Fig. 6 and Fig. S7). This was expected because phylogenetic relationships within clade V were different among the tip-dating trees (Fig. S4). The tree recovered by removing homoplastic morphological characters with RC <0.25 (tip-dating analysis 4) has a better fit to stratigraphy than the trees recovered by other strategies. Thus, biogeographic analysis 4 using the MCC tree from tip-dating analysis 4 should more reliably reflect the evolutionary history of Cissampelideae than the other three biogeographic analyses (1–3), and hence it is used to discuss the spatio-temporal evolution of the tribe.
Spatio-temporal evolution of Cissampelideae
Without the inclusion of fossil taxa, our ancestral range reconstruction under the DEC model indicates that the MRCA of extant Cissampelideae probably occupied Asia and Africa around the Palaeocene–Eocene boundary, and only one dispersal from Africa to Asia occurred at ~48.05–39.62 Ma (between nodes 10 and 11; Fig. S7), whereas the reconstruction under the LEMAD model supports the MRCA of extant Cissampelideae probably being present in Asia and the two dispersal events from Asia to Africa occurring at ~39.62–15.23 Ma (between nodes 11 and 12) and ~ 39.62–20.98 Ma (between nodes 11 and 13) within Stephania (Fig. S8). Based on the ancestral range reconstruction integrating extant and fossil taxa (Fig. 6), we discover that the MRCA of extant Cissampelideae might also have occurred in Asia, but did not infer any dispersal event in Stephania. Instead within the crown group of Cissampelideae, we found two dispersal events from Asia to Africa at ~52.78–48.52 Ma (between nodes 5 and 11) and at ~39.33–24.92 Ma (between nodes 6 and 7). Moreover, the integrated reconstruction further recognized one dispersal event from Asia to South America that occurred in the stem group of the tribe (between nodes 1 and 2). These results suggest that the integration of fossil and extant taxa is a more powerful way to find accurate biogeographic signals than considering only extant taxa, as in other studies (e.g. Mao et al., 2012; Yan et al., 2021). Although it takes into extinction into account by modelling the geographic distribution of extinct species (Herrera-Alsina et al., 2022), the LEMAD model may still be unable to infer accurately the biogeographical history of Cissampelideae.
Here, we integrated fossil and extant taxa to examine the temporal and spatial patterns of Cissampelideae (Fig. 6). Our analyses indicate that Cissampelideae began to diversify in Asia in the latest Cretaceous (~68 Ma; node 1 in Fig. 6) and subsequently dispersed into South America at ~66 Ma (node 2 in Fig. 6). This period coincides with global vegetational upheaval and rapid ecosystem failure around the K–Pg boundary (Vajda et al., 2001; Nichols and Johnson, 2008). Cissampelideae, a liana group mainly found in tropical rainforests, could make use of new ecological niches generated by the K–Pg extinction event. Fossil and molecular data show that the global ecosystems gradually recovered and modern tropical rainforests developed after the mass extinction interval (Upchurch and Wolfe, 1987; Wing et al., 2009; Wang et al., 2012). Ashfall from the Chicxulub impact (Kring and Durda, 2002) and the rise of nitrogen-fixing taxa (Herrera et al., 2019) could increase soil fertility and stimulate forest productivity (Epihov et al., 2017). Different from the relatively open canopies in Late Cretaceous, the development of closed canopy rainforests in the Palaeocene created stronger vertical gradients in light and water use, providing ecological opportunities for lianas and other plants (Carvalho et al., 2021). During the Palaeocene, a relatively warm climate was prevalent in the Northern Hemisphere (Tierney et al., 2020). Meanwhile the boreotropical flora that spanned the low- and mid-latitudes of the Northern Hemisphere initiated and thermophilic plants could move between Eurasia and North America via the North Atlantic land bridge (Wolfe, 1975; Tiffney, 1985; Lian et al., 2020). Thus, the warm climate and widespread boreotropical flora may have facilitated exchanges of Cissampelideae between Asia and North America. Fossil evidence from hadrosaurs, condylarth and didelphimorph mammals suggests that a connecting land bridge, i.e. the Antillean volcanic arc system (Iturralde-Vinent, 2006), consisting of non-volcanic and volcanic islands, appeared in the shallow sea between North and South America during the latest Campanian/Maastrichtian (~75–66 Ma; Gayet et al., 1992; Goin et al., 2016). Thus, Cissampelideae might have dispersed southwards into South America from North America via the Antillean volcanic arc system. The time-calibrated tree of another megathermal family, Lauraceae (Chanderbali et al., 2001), suggests a similar pattern, supported by recently recovered fossils fruits closely related to Chlorocardium from the late Cretaceous of Central Cuba (Viñola-López et al., 2022). Palaeobotanical studies show that modern tropical rainforests in South America first developed in the Palaeocene (Wing et al., 2009; Jaramillo, 2023). In comparison with the Maastrichtian flora, the Palaeocene flora of the Neotropics was more analogous to modern rainforests in terms of family-level composition of angiosperms (Wing et al., 2009; Carvalho et al., 2021). Thus, a widespread, continuous megathermal rainforest belt might have covered the low- and mid-latitude regions of the Northern Hemisphere and have extended to South America in the Palaeocene. Our results further indicate that Cissampelideae was widely distributed in Asia and South America until a vicariance event occurred in the late Palaeocene (~58 Ma; node 3 in Fig. 6). During the late Palaeocene to early Eocene, the Antillean volcanic arc system gradually vanished (Iturralde-Vinent, 2006), and consequently, the exchange of megathermal plants between North and South America was disrupted.
Our results suggest two dispersal events from Asia to Africa in the crown group of Cissampelideae. The early dispersal took place in the Early Eocene (~53–49 Ma, between nodes 5 and 10 in Fig. 6), which is markedly before the collision between the Arabian and Eurasian plates (~35 Ma; Dewey et al., 1986; Rögl, 1998; Mouthereau et al., 2012). However, during this period, global temperatures peaked, i.e. the Early Eocene Climatic Optimum occurred (~52 Ma) (Fig. 6; Tierney et al., 2020), and many ‘stepping-stone’ chains existed in the Tethys Sea (Tiffney, 1985; Scotese, 2004). These may have facilitated Cissampelideae's dispersal southwards into Africa. A similar route of migration from southeastern Asia into Africa was found in some thermophilic taxa, such as Alangium (Cornaceae; Feng et al., 2009), Cucurbitaceae (Schaefer et al., 2009) and Hamamelidaceae (Xiang et al., 2019). Thus, the continuous megathermal rainforest belt in the Northern Hemisphere might have extended to Africa in the Early Eocene (Lian et al., 2023). The later dispersal occurred during the Late Eocene–Late Oligocene, ~39–25 Ma (between nodes 6 and 7 in Fig. 6), which temporally coincides with the Arabia–Eurasia collision (Dewey et al., 1986; Rögl, 1998; Mouthereau et al., 2012). During the land connections between Africa and Southwest Asia, a continuous tropical forest belt from eastern Africa to western India occurred throughout the Oligocene (Mandaville, 1984; Kürschner, 1998; Otero and Gayet, 2001). Thus, thermophilic Cissampelideae could have expanded their range via the Arabian forest corridor during the Late Eocene–Late Oligocene. Biogeographic analyses of several thermophilic taxa, such as Annonaceae (Thomas et al., 2015), Isodon (Lamiaceae; Yu et al., 2014), Searsia (Anacardiaceae; Yang et al., 2016) and Tinospora and Tiliacoreae (Menispermaceae; Lian et al., 2019, 2023), have documented the existence of widely floristic exchange between Africa and tropical Asia during this period, although the dispersal inferred in these taxa is in the opposite direction compared with the dispersal of Cissampelideae.
Our analyses show that Cissampelideae were probably widely distributed in Southeast Asia and Africa until the early Late Miocene. Four vicariance events occurred during the Early Miocene–early Late Miocene (~21 Ma, ~11 Ma, ~13 Ma and 19 Ma; nodes 8, 9, 12 and 13 in Fig. 6), which resulted in the divergences of the African Cissampelos capensis, C. owariensis, S. abyssinica and S. cyanantha from their Asian or Asian-American allies, respectively. The uplift of the Iranian Plateau started at ~25 Ma (Mouthereau et al., 2012). During the same period, the continued retreat of the Paratethys Sea occurred (Hrbek and Meyer, 2003). These events led to the change of land–sea distribution and triggered gradual aridification of Southwest Asia (Wu et al., 2015), and could thereby have hampered the exchange of megathermal plants between tropical Asia and Africa. In particular, after the mid-Miocene Climatic Optimum (~17–14 Ma), a steep and steady decline in global temperatures occurred (Fig. 6; Tierney et al., 2020), and the uplift, exhumation and shortening in the Zagros mountains and the Iranian Plateau region accelerated (Mouthereau et al., 2012; Xiang et al., 2021). Accordingly, aridification of Southwest Asia further intensified (Wu et al., 2015). Together, these events could have resulted in the final disappearance of the Arabian forest corridor and thereby could have entirely disrupted the exchange of megathermal plants between tropical Asia and Africa through Arabia in the early Late Miocene.
Conclusions
In this study, we took Cissampelideae as a case to explore how to mitigate effectively the effect of homoplastic morphological characters on the placement of fossil taxa and biogeographic inference. Our results show that the 14 selected Cissampelideae fossil taxa are placed poorly when based only on morphological data, whereas the addition of a molecular scaffold and combined morphological and molecular data may greatly improve the resolution of fossil nodes. For the morphological data with a molecular scaffold, the reweighting analysis against homoplasy according to the RC value can result in the increase of both the proportion of resolved nodes and average support. For the total evidence matrices, tip-dated BI analyses containing stratigraphic information can greatly increase the proportion of resolved nodes, and the Bayesian tip-dated tree recovered by removing homoplastic morphological characters with RC < 0.25 has the highest stratigraphic fit and consequently generates more accurate biogeographic reconstruction, especially for fossil clades. Cissampelideae began to diversify in Asia in the latest Cretaceous and subsequently dispersed to South America via the boreotropical flora and the Antillean volcanic arc system. Cissampelideae probably dispersed to Africa from Asia via ‘stepping-stone’ chains that existed in the Tethys Sea in the Early Eocene and via the Arabian forest corridor during the Late Eocene–early Late Miocene. Our Cissampelideae data suggest that reweighting/removing homoplastic morphological characters according to the RC value can mitigate effectively the effect of homoplastic morphological characters on the placements of fossil taxa, and removing homoplastic morphological characters with RC < 0.25 can generate a dated tree with the highest fit to stratigraphy, facilitating improved biogeographic inference. This study also contributes to our knowledge on the past tropical floristic exchanges among different continents.
Acknowledgements
We sincerely thank the associate editor Michael Pittman and two anonymous reviewers for their comments and invaluable suggestions that greatly improved our manuscript. This research was partially funded by the National Natural Science Foundation of China (32170210, 32300197 and 32361133549), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB31030000), the National Key Research and Development Program of China (2023YFF0805800), the K.C. Wong Education Foundation (GJTD-2020-05), the Russian Science Foundation (24-44-00027) and state assignments for CSBG SB RAS (AAAA-A21-121011290024-05).
Conflict of interest
None declared.
Open Research
Data availability statement
All data that support the findings of this study are available in the supplementary materials. Newly generated chloroplast and nuclear sequences have been uploaded to GenBank.




